Open Access Article
Open Access ArticleFrequency of use of household products containing VOCs and indoor atmospheric concentrations in homes†
Aiden C.
Heeley-Hill
 a,
Stuart K.
Grange‡
a,
Stuart K.
Grange‡
 a,
Martyn W.
Ward
a,
Alastair C.
Lewis
a,
Martyn W.
Ward
a,
Alastair C.
Lewis
 *b,
Neil
Owen
c,
Caroline
Jordan
c,
Gemma
Hodgson
d and
Greg
Adamson
e
*b,
Neil
Owen
c,
Caroline
Jordan
c,
Gemma
Hodgson
d and
Greg
Adamson
e
aWolfson Atmospheric Chemistry Laboratories, University of York, York, YO10 5DD, UK
bNational Centre for Atmospheric Science, University of York, York, YO10 5DD, UK. E-mail: ally.lewis@ncas.ac.uk
cGivaudan UK Ltd, Kennington Road, TN24 0LT Ashford, UK
dQI Statistics, Overdene House, 49 Church Street, Theale, Berkshire RG7 5BX, UK
eGivaudan Fragrances Corp., 717 Ridgedale Ave, East Hanover, New Jersey 07936, USA
First published on 12th April 2021
Abstract
Volatile organic compounds (VOCs) are a key class of atmospheric emission released from highly complex petrochemical, transport and solvent sources both outdoors and indoors. This study established the concentrations and speciation of VOCs in 60 homes (204 individuals, 360 × 72 h samples, 40 species) in summer and winter, along with outdoor controls. Self-reported daily statistics were collected in each home on the use of cleaning, household and personal care products, all of which are known to release VOCs. Frequency of product use varied widely: deodorants: 2.9 uses home per day; sealant-mastics 0.02 uses home per day. The total concentration of VOCs indoors (range C2–C10) was highly variable between homes e.g. range 16.6–8150 μg m−3 in winter. Indoor concentrations of VOCs exceeded outdoor for 84% of households studied in summer and 100% of homes in winter. The most abundant VOCs found indoors in this study were n-butane (wintertime range: 1.5–4630 μg m−3), likely released as aerosol propellant, ethanol, acetone and propane. The cumulative use VOC-containing products over multiday timescales by occupants provided little predictive power to infer 72 hour averaged indoor concentrations. However, there was weak covariance between the cumulative usage of certain products and individual VOCs. From a domestic emissions perspective, reducing the use of hydrocarbon-based aerosol propellants indoors would likely have the largest impact.
Environmental significanceVOCs released from the domestic sector make up a significant fraction of national emission budgets in high-income countries. Large population-based studies that measure a full range of VOCs (e.g. C2–C10) indoors are rare because of experimental limitations. The cumulative use of VOC-containing products in homes provided little predictive power to infer time-averaged indoor concentrations, although weak covariance existed between the use of certain products and individual species. The high concentrations of butane indoors could be linked through occupant data to the widespread and frequent use of aerosol products. From both an emissions and indoor chemistry perspective a reduction in use of hydrocarbon-based propellants would appear to offer the most straightforward route to reducing domestic sector emissions. |
1. Introduction
Contemporary observations have indicated that, on average, people in high income countries spend up to 90% of their time in enclosed indoor spaces.1 This motivates the need to understand the chemistry of indoor environments, and to quantify any public health risk that may exist in the built environment where it may be a significant vector for exposure to air pollution.2–4Indoor chemistry and exposure science literature shows how multiple factors can influence indoor emissions and air quality.5 For volatile organic compounds (VOCs) specifically, air exchange rate is critical, as is the ingress of outdoor air, the internal combustion of fuels, cooking activities, off-gassing from building materials and furnishings, and the use of VOC-containing products. All potentially impact on indoor concentrations.6 Occupants themselves are also a living source of VOCs, from breath, skin, sweat and so on.7–10 The overall balance of human exposure to VOCs is therefore a blend of air inhaled indoors and when outside. Outdoor VOCs have been monitored routinely in many countries for decades and much is known about representative concentrations, variability and exposure. Indoor atmospheres are more difficult to representatively characterise for VOCs than outdoors as each built environment is unique. Detailed chemical inventories of indoor VOC concentrations are a developing aspect of research in the context of larger population studies.11–16 Existing studies suggest that concentrations, and therefore exposure to VOCs are very frequently greater indoors than outdoors.13–15
Indoor VOC measurements have historically used passive diffusion sampling tubes containing a chemical sorbent material. This can limit the range of VOCs detected and the sensitivity of that detection17 but has the practical advantage of being cheap, flexible and scaleable to large numbers of homes. Contemporaneous studies have utilised alternative analytical methods, such as proton-transfer-reaction mass spectrometry (PTR-MS) and chemical ionisation mass spectrometry (CIMS). These online methods provide chemical analysis in real-time, but this is often impractical to set-up in domestic environments.18–20 This highlights a key dilemma in studying VOCs indoors. Simple, scalable methods for population studies must rely on slow time integrated collection of samples over hours to many days, whilst advanced mass spectrometric methods can provide immense detail on second-by-second processes, but only for one or two test homes at a time. Neither method is ‘better’, insight emerges from the blending of information from both.
Often missing from on-line MS and adsorbent tubes used in indoor studies are measurements of the most volatile VOCs. Though more materials-intensive, an alternative is to deploy within homes internally silica-treated stainless-steel canisters, with flow restrictors as samplers; outlined in the United States Environmental Protection Agency Toxic Organic 15 Compendium Method.21 Offline laboratory analysis of canister-collected samples using, for example, combinations of both gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionisation detection (GC-FID) analysis thus broadens the range of gas-phase VOCs that can be screened.22,23
In recent years, there has been particular interest in the role of terpenoid VOCs within indoor settings. These are commonly released from consumer fragrances and are contained in personal care and cleaning products; these are mostly derived from plant oils.24–26 Terpenoids are also emitted indoors from natural sources: plants, flowers, fruit, herbs, and spices. Toxicological assessments show that monoterpene VOCs are not themselves harmful at typical part per billion concentrations that might be encountered indoors. For instance, D-limonene has been demonstrated to have a low order of toxicity potential at low inhalation exposure levels (ECHA REACH Registration,27), or when compared to REACH-compliant Derived No Effect Levels.28 Similar conclusions have been reached in other studies examining VOC emissions and indoor air exposures that were below critical exposure limits.29
An area of uncertainty has been the potential for these classes of relatively reactive VOCs to degrade to form secondary pollutants through indoor oxidation with ozone. Ozone can be drawn indoors from outside, and other possible oxidation routes include reactions with OH, Cl, and NO3 radicals that can be generated indoors.30 Gas phase by-products from the oxidation of VOCs indoors include formaldehyde, acetaldehyde – both species being formed as part of the atmospheric degradation of many different VOCs – and secondary organic aerosols (SOA).31,32 In this UK study the dominant fuel used in all homes was natural gas, comprising methane with ∼8% ethane and trace amounts of propane and C4 hydrocarbons. Other locations and countries can have different fuel blends often with higher amounts of propane and C4. We note that n-butane (a significant VOC in some of our later conclusions on indoor sources) comprises only ∼0.14% of typical UK natural gas, and so gas leakage in the home is not a significant indoor source.33
Undertaking broad, and ideally non-targeted, screening of the full range of VOCs present indoors is central to the attribution of observed abundances to their different contributing sources and to assess the relative balance of VOC exposure between indoors and outside. Whilst few VOCs are emitted by only one activity indoors, some do have distinctive contributing sources where it may be hypothesised that indoor speciation could be influenced by the consumption or usage patterns of the originating products albeit it with other factors such as air exchange rate possibly controlling absolute concentrations. For example, acetone, ethanol, dichloromethane, limonene and n-pentane are used as solvents within both professional and domestic cleaning products.34–37 Acetone and ethanol emissions can also be observed in human breath as a result of biological processes.38 Moreover, ethanol is emitted from food, such as bread.39 Iso-butane and n-butane are the major VOCs used as propellants within compressed gas products, often combined with propane and with ethanol as a cosolvent, dependent on manufacturer and product.37,40 Toluene, ethylbenzene and m, p and o xylene species are commonly associated with paints, glues and varnishes41 and ethane and propane are minor components of fossil methane gas,42 found indoors via small gas leaks. VOCs can be released indoors from leakage of the fuels used for heating and cooking, the speciation of these depending on the fuels used. In this UK study the dominant fuel used in all homes was natural gas, comprising methane with ∼8% ethane and trace propane and butane. Other locations and countries can have different fuel blends often comprising propane and butane.
Another consideration, though not within the scope of this study, is further chemical interactions, such as the formation of secondary organic aerosols (SOAs) and the influence of surface reservoirs. Heterogeneous surface chemistry is an emerging topic in indoor chemistry and is covered in the recent literature.43–45 SOA production is driven predominantly by the oxidants OH and O3.46 Though indoor data on these species were not collected in this study, it is likely that species with a short indoor residence time will be affected by different oxidant concentrations between seasons.47,48
Domestic usage of VOC-containing products can be simplistically placed into one of two classifications. ‘Large dose – low frequency’ emissions are those arising from infrequent activities such as painting and decorating, or the installation of new furniture. These have relatively well-described effects in the research literature.3,49 The contribution of these sources is reflected in efforts to reduce VOC content in building products and paints, for example in the EU via the Construction Products Directive 89/106/EEC and Paints Directive 2004/42/EC.
By contrast, the effects of ‘small dose – high frequency’ emissions are much more uncertain contributors to both indoor air quality and as a source of outdoor VOC pollution as well. Whilst many different products contain trace amounts of VOCs, the connections between the use of small dose – high frequency products, and overall domestic VOC emissions and concentrations is uncertain in real-world settings. These products are diverse in their applications and are used, potentially, multiple times per day and by multiple occupants. This source classification can include personal care and household products.49–52 In the public reporting and general discussion of the relationships between VOCs and indoor air quality there is often anecdotal linkage made between particular types of consumer products and adverse indoor air quality outcomes. Fragranced candles, for example, are frequently cited in the context of personal indoor VOC exposure.28 There is however little direct evidence showing a quantitative and causal relationship between frequency of use of a specific product and the observed concentrations of a particular VOC indoors, rather it is inferred from product formulation. We note however the work of Adgate (2004)13 which did suggest a correlation between indoor VOC concentrations and the use of cleaning products.
1.1 Study objectives
In this study we set out to evaluate the potential association between real-world indoor VOC concentrations, the speciation of the VOCs found indoors, and the consumption patterns of consumer products. An association between the cumulative frequency of use of an individual product (over a period of three days), or use of many products, and changes in indoor VOC speciation and concentrations would potentially provide an attractive predictive method to estimate VOCs more widely, should consumption statistics be known. We focus on the metric of culminative ‘frequency of recorded uses’ of products, since it is simple and reliable data to collect in a population study. We readily acknowledge that other, more difficult to quantify factors such as the size of dose in each use, and the differences in product-to-product formulation from different manufacturers will also be very important controlling variables that influence VOC emissions. By collecting both indoor and outdoor samples simultaneously, we have been able to then assess the relative significance of indoors versus outdoors as locations for exposure to VOCs for this study cohort. Since we use only simple methods we do not have data on real-time activities such as ventilation rates, or wider environmental conditions such as in-room photolysis. We do however collect some proxy data such as building, age, type, occupancy and so on that allows some of these aspects to be explored further.2. Methods
2.1 Experimental methodology
A cohort of 204 volunteer participants was drawn from an existing and well-characterised panel of naïve consumer product testers, based in Ashford, United Kingdom. All the homes are located within the Ashford town region, meaning the homes here should be typically characterised as experiencing suburban UK background conditions for outdoor pollutants The study used 60 individual homes (all primary residences) with a median occupancy of 4 people per home. The demographics of the participants and information of the property types are shown in the ESI Tables A and B.† Of the participants in the first winter sampling experiments, 91.7% also participated in the summer experiment. Five new replacement homes were added in the summer experiment to maintain a constant sample size, since a small number of participants were unavailable for both seasons. The broader purpose and hypothesis of the study was not divulged to the participants, who were asked only to place the canister samplers in their homes and record statistical information daily on a tablet-based information system. Study participant identities and home locations were known to Givaudan UK, but these were not divulged to the University of York. Households were given a unique household ID, to which canister IDs were assigned during the experimental periods. These actions were performed to preserve participant and home anonymity.A total of 360 indoor air samples and 55 outdoor background control samples were collected over two, nine-week sampling periods between February and April 2019 (defined as winter), and July and September 2019 (summer). Feb–April 2019 – period average minimum outdoor temperature 4.7 °C; max 11.4 °C. July–Sept 2019 – period average minimum outdoor temperature 14.9 °C; max 20.5 °C. Three indoor samples were taken in each house per sampling campaign, giving a total of six samples per house for the study. Three households were randomly selected each week to collect a control outdoor sample, placing a sampler in a back garden away from the home.
Samples were collected indoors over three days into 6 L internally silica-treated stainless-steel canisters. These canisters were evacuated initially to 300 Pa. They used 72 hour equivalent flow controllers to create a linearly averaged 48 hour sampling time (Entech, CA, USA and Restek, PA, USA), and then a reduced flow rate for the final 24 hours. A sampling period of 72 hours allowed the capture of VOC concentration spikes accompanying product use, in addition to the longer decay attendant to product evaporation, such as from skin or hair. Canisters were evacuated, in the laboratory, on a high-vacuum rig before use. Field and laboratory blank canisters were interspersed randomly amongst the samples during the automated laboratory analysis. Samplers were only placed in a living room or kitchen-living room if the property was open plan. Guidance was given to avoid placing samplers directly near sources of VOCs such as flowers, diffusers, plug-ins and so on. The most common location for samplers was on the floor which, when the inlet restrictor is included, meant a sampling height of ∼50 cm above the floor level. The sampling gas flow profile of a typical sampler is shown in ESI Fig. A.†
Following sample collection in homes participants returned their canisters to a central collection point in Ashford and these were couriered to the University of York. Samples were analysed within seven days of collection, with canisters then evacuated for re-use and returned to Ashford. Each canister sample was pressurised to 179 kPa using highly purified air, whereupon they were connected to autosamplers. Field blanks and calibration standards were included in the sample sequence. Two separate instruments were used in this study and samples run on both instruments: (1) a thermal desorption GC-FID-FID system used to quantify C2–C8 non-methane hydrocarbons and short chain oxygenates, based on the method of Hopkins et al.53 This used two PLOT columns connected to a Markes Unity (Markes International, Llantrisant, UK) thermal desorption/autosampler system. (2) Thermal desorption GC-TOF-MS based on the methods in Shaw et al.54 using a Markes Unity 2 thermal desorption system, Agilent 6890 (Agilent Technologies, Santa Clara, CA, USA) with volatility-based GC separation on methyl siloxane GC column and ALMSCO TOF detector (ALMSCO International, Llantrisant, UK). This provided quantification of C4–C12 VOCs. Per 6 L sample, a total of 1 L was taken (500 ml for each analytical system). The species quantified in this study are listed in Table 1; in some cases, the same VOC was measured on both analytical systems, providing a further crosscheck of analytical performance.
| Median concentration | 5th percentile | 95th percentile | Standard deviation | |
|---|---|---|---|---|
| n-Butane | 107 | 2.3 | 1180 | 547 |
| Propane | 44.2 | 1.2 | 609 | 456 |
| Acetone | 43.8 | 4.2 | 156 | 53.8 |
| Iso-butane | 40.4 | 1.5 | 597 | 227 |
| Ethanol | 40.1 | dl | 283 | 184 |
| α-Pinene | 8.0 | dl | 56.7 | 24.4 |
| D4 siloxane | 6.6 | dl | 96.1 | 33.7 |
| Ethane | 4.3 | 0.9 | 45.9 | 41.6 |
| Limonene | 3.8 | 0.3 | 24.0 | 10.0 |
| Iso-pentane | 3.7 | 0.6 | 40.8 | 38.1 |
| Toluene | 1.5 | 0.2 | 28.1 | 72.6 |
| m/p-Xylene | 1.5 | 0.2 | 10.4 | 54.0 |
| Iso-butene | 1.2 | 0.1 | 10.8 | 23.4 |
| o-Xylene | 1.2 | dl | 15.2 | 54.6 |
| n-Pentane | 1.1 | 0.4 | 10.3 | 102 |
| Isoprene | 1.0 | 0.1 | 3.1 | 17.7 |
| Ethene | 0.8 | 0.2 | 2.8 | 2.6 |
| Ethylbenzene | 0.8 | 0.07 | 6.7 | 6.3 |
| cis-2-Butene | 0.8 | 0.06 | 6.7 | 15.5 |
| p-Cymene | 0.7 | 0.05 | 4.1 | 2.6 |
| Benzene | 0.5 | 0.2 | 1.8 | 28.8 |
| 2-Methylpentane | 0.4 | 0.06 | 3.0 | 881 |
| 1-Pentene | 0.7 | 0.03 | 5.1 | 2.3 |
| n-Hexane | 0.4 | 0.06 | 1.6 | 21.6 |
| Propene | 0.4 | 0.10 | 1.1 | 1.9 |
| n-Heptane | 0.3 | 0.06 | 2.4 | 9.9 |
| Acetylene | 0.3 | 0.05 | 1.1 | 0.4 |
| Methanol | 0.3 | dl | 18.8 | 32.6 |
| 1-Butene | 0.3 | 0.04 | 1.2 | 0.7 |
| n-Octane | 0.2 | 0.03 | 3.7 | 5.8 |
| trans-2-Pentene | 0.2 | 0.01 | 10.7 | 5.8 |
| Dichloromethane | 0.2 | dl | 1.9 | 5.5 |
| 1,3,5-Trimethylbenzene | 0.2 | dl | 4.4 | 1.8 |
| 1,3-Butadiene | 0.2 | 0.03 | 2.9 | 6.7 |
| β-Pinene | 0.1 | dl | 12.4 | 7.4 |
| 2,2,4-Trimethylpentane | 0.1 | 0.01 | 3.2 | 30.5 |
| trans-2-Butene | 0.07 | dl | 0.4 | 0.2 |
| Tetrachloroethylene | 0.03 | dl | 0.4 | 2.1 |
| γ-Terpinene | dl | dl | 0.7 | 3.0 |
Calibration was based on gravimetrically prepared high pressure (10 MPa) standards, a combination of a 4 ppb, 30 component NMHC ozone precursor non-methane hydrocarbon standard (National Physical Laboratory, Teddington UK) and custom-blended multicomponent standard including terpenes and oxygenated VOCs based on in-house dilution of part per million gravimetric standards into secondary high pressure passivated cylinders with individual VOCs in the part per billion range. In all cases the calibration standard balance gas was high purity nitrogen (chromatograms in ESI Fig. B and C†). The limit of detection for individual VOCs on both systems was typically in the 5–50 parts per trillion range. On appropriate molecular weight conversion at 25 °C to VOC-specific mass concentrations, this equated to detection limits (defined as 3 times S/N) for individual VOCs typically in the range 0.015–0.2 μg m−3. The range of different detection limits reflects differing carbon responses by FID and differing fragmentation patterns and ionisation efficiency in the MS.
Measurement uncertainty was dominated by uncertainties carried forward in calibration from the gravimetric primary gas standards. These were quoted by manufacturers as 5% uncertainty. Further uncertainty arises from run to run analytical reproducibility, itself a function of VOC concentration. For measurements of VOCs more than 10 times the detection limit, reproducibility of analysis was typically better than 1% for GC-FID. When other components of the sampling system are considered, such as variability in inlet flow rate and blank canister artefacts, an expanded uncertainty of ∼7% results. For measurements of VOCs closer to the detection limit uncertainties are considerably greater, rising to 50% for chromatographic peaks that are 3 times signal to noise. Our measurements cover a very wide range concentrations, often high values relative to detection limits. We report concentrations by default to three significant figures, unless the concentration was sufficiently low that the third figure decade was equivalent to or greater than the estimated uncertainty, in which case values were truncated to fewer significant figures to avoid artificial precision being inferred.
2.2 Survey methodology
A participant and activity survey was developed to place the chemical data in the context of property information, residence occupancy, and resident demographics. A daily log was then completed to obtain information about the use of VOC-containing products by residents in each home. The survey was based on pre-existing panel study methodologies used by Givaudan UK, and was digitised for user inputs on a supplied tablet computer. Products included in the survey were selected to cover a wide range of different VOC-emitters commonly found in the home. The survey considered only VOCs likely to be conventionally used within the main domestic living space of the home (see ESI Tables C and D†). In combination, complete data log records and matching chemical analysis were generated for 92% of the deployed samplers. Around 8% of sampling opportunities were lost due to participant sampling errors, failure to complete diary logs, or the sample analysis not meeting the required laboratory QA/QC standards.The study was limited to recording occupants' frequency of use of products as a numerical value of number of times per day. Frequency of use is clearly only part of the overall behaviour that defines VOC emissions from a particular product when in use. The size of dose used will also be a factor in determining emissions, but this is complex to estimate in a self-led diary study. A further important influence is individual product composition, though participants were not asked to record manufacturer or brand. We discuss this further in the conclusions section.
2.3 Statistical methodology
Data analysis was performed using R v.4.02 “Taking off Again” and the RStudio environment v.1.3.1073 “Golden Rod”, data manipulation was performed using the dplyr (v.1.0.2) package. The majority of the methods used in this manuscript utilise descriptive statistics, with attendant visualisation therein. 25th and 75th and 5th and 95th percentiles were used to ascertain high and low concentrations where appropriate. Median values were favoured over mean values so as not to confound outlier influence and concentration values when considering averages. Correlation analysis was performed using the cor function of the stats (v. 4.0.2) package in R. Visualisation of the correlation matrix was achieved using the corrplot function of the corrplot package (v. 0.84). Correlation is displayed as follows: a narrow, forward-slanting straight line represents a strong correlation, a full circle represents no correlation and a backward-slanting straight line represents an anti-correlation. Darker blues indicate greater correlation, darker reds represent lesser correlation. Numbers are on a scale of −1 to 1, with −1 being anticorrelated and 1 being fully correlated. Covariance analysis was performed after rescaling the raw concentration data on a scale of 0–1, and rescaling the covariance values from 0–100 using the normalize function of the BBmisc package in R (v.1.11). Data normality was tested using the Anderson–Darling test. The Wilcoxon Rank Sum Test was performed to test statistical difference between the mean of two groups of data. The test is non-parametric so assumes non-normal data distribution. Regression analysis was performed using the lm function in the stats R package.Total indoor VOC concentrations, henceforth referred to as TVOC, is a widely used metric in the literature to measure total VOC mass indoors. TVOC is typically measured by dedicated sensors which make an operationally defined determination of concentrations. There is no absolute traceable methodology for TVOC; total carbon by FID is the closest approximation, often yielding similar values to the summation of the individual parts as quantified by GC-MS or GC-FID. Here we use the sum concentration of all VOCs analysed by GC-FID and GC-MS a methodology common to other studies.55–58
3. Results
3.1 Product use statistics
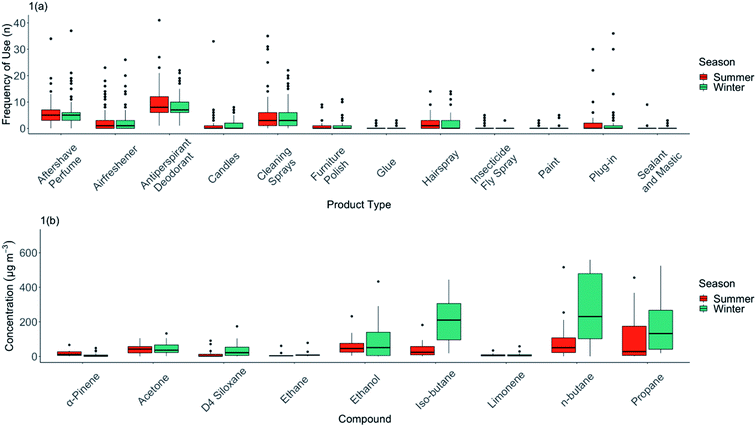
An initial analysis was performed on the frequency of use of individual classes of VOC-containing products, and a summary of total recorded uses in each home is shown in Fig. 1(a). Many of these products are typically listed in review literature as being contributors to indoor VOCs. We note that there are, in practice, a very wide range of frequencies of actual use in real-world settings, something that is rarely quantified or discussed in reviews. VOC sources such as paints are only used infrequently in homes, as would be expected from a likely large dose – low frequency product; 72% of homes never used any paints during this study. We do recognise however that decorating products, such as paints, will continue to emit VOCs at some level for an extended period after initial application and may contribute to what is measured.59The most commonly-used consumer product source of VOCs indoors were aerosol antiperspirant deodorants. These were used in all 60 homes that were studied and with an average frequency across the cohort of 2.9 uses per home per day. Some VOC-containing products such as plug-in air fresheners were used in relatively few of the UK homes studied, but frequency of their use varied widely from only occasional use to up to >35 uses per sampling 72 hour period. This very wide variability in types of products used, and the frequency of use of any given product, highlights the inappropriateness of generalising about the contributions of particular product types as contributors to indoor VOC concentrations. Little commonality existed in VOC product usage, or frequency of use between homes, beyond the almost universal use of deodorants, cleaning sprays and perfumes.
There were some modest differences in the seasonal use of different product types (Fig. 1(a) and Table 1). For instance, personal care products (i.e. antiperspirant/deodorants) were reported as being in greater use during the summer than in the winter (frequency of use median = 8 per sampling period in summer, 7 in winter). Usage of other product types remained largely constant between seasons.
3.2 VOC concentrations across the study cohort and comparison with outdoors
A summary of the VOCs found indoors is shown in Table 1. As has been reported in many previous studies, the variability between homes was very large. A small number of VOCs do, however, stand out as being dominant in terms of contribution to the overall VOC concentration indoors. n-Butane had the highest median concentration in the homes measured, with multiple homes having 72 hour averages exceeding 1000 μg m−3. Two other commonly used solvents (and with other indoor sources), ethanol and acetone, were also observed in significant concentrations. The distribution statistics for the most abundant VOCs by season are shown in Fig. 1(b).TVOC was calculated by season for each home shown in ESI Fig. D.† Median TVOC in summer was 370 μg m−3, and 426 μg m−3 in winter; this was a statistically insignificant difference (Wilcoxon Rank Sum Test, W = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356, p = 0.126). Notable in Fig. 1(b) was the difference in median n-butane concentrations between winter and summer (summer = 69.4 μg m−3, winter = 185 μg m−3). Although frequency of use in this product category was lower in winter, the higher concentrations observed in winter may reflect lower ventilation rates, and its accumulation indoors given it is a relatively unreactive VOC. This was also in evidence for iso-butane, a linked emission from aerosols propellants.
356, p = 0.126). Notable in Fig. 1(b) was the difference in median n-butane concentrations between winter and summer (summer = 69.4 μg m−3, winter = 185 μg m−3). Although frequency of use in this product category was lower in winter, the higher concentrations observed in winter may reflect lower ventilation rates, and its accumulation indoors given it is a relatively unreactive VOC. This was also in evidence for iso-butane, a linked emission from aerosols propellants.
Statistically significant seasonal differences in indoor concentrations were observed for certain species. For α-pinene, the summer median concentration was considerably higher than winter (summer = 11.9 μg m−3, winter = 2.9 μg m−3), the median concentration of α-pinene indoors was 8.0 μg m−3 and outdoors was only 0.8 μg m−3, suggestive of more significant possible sources of emissions from outgassing of wood products from within the fabric of the house.20 In contrast, limonene had lower median concentrations indoors in summer: 3.6 μg m−3, winter: 4.7 μg m−3, potentially reflective of its accumulation in winter from use of cleaning and fragranced products, and other food sources. The median concentration indoors was 3.8 μg m−3 and outdoors was only 0.2 μg m−3, again indicative of a potent inside source, rather than significant ingress from outdoors.
There are relatively few comprehensively speciated indoor studies in the literature to compare these new observations against. A study of a broadly similar nature was the European EXPOLIS study of VOC emissions in Helsinki by Edwards et al. (2001).60 This reported concentrations of aromatic, halocarbon, and monoterpenes that were, in general, higher than seen in this study. More recent changes in legislation and product composition could have led to lower emissions, ergo lower concentrations in 2019, given the significant near 20 years gap between studies. Seasonal differences in concentrations were reported as negligible, though the EXPOLIS study incorporated spring and autumn measurements when temperatures were broadly similar. A study of the indoor quality of apartments by Schlink et al. (2010)61 reported higher concentrations of aromatics and monoterpene species than were found in this study. Jia et al. (2008)62 also reported higher concentrations of several VOCs than in this study, with the exception of α-pinene, with samples collected from a number of individual residences over winter and summer. In accordance with this study, seasonality had little influence on indoor concentrations, and correlations between individual species were limited.
3.3 VOC concentrations and building age/type
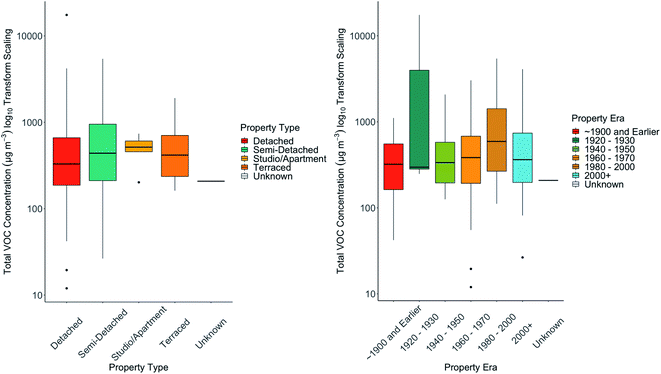
Air exchange rates (AER) are a critical factor in controlling indoor VOC concentrations, whether through allowing the ingress of outdoor VOCs, or through increased concentrations accumulating from sources indoors due to lower dilution.63 AER is not straightforwardly measured in large numbers of homes simultaneously and could not be directly measured in these homes due to the practicalities involved. Instead, property age and type, and glazing were considered as possible proxies for ventilation – it might be assumed that older buildings (e.g. older than 1900) would have the poorest insulation and highest rates of ventilation than modern buildings (e.g. post 2000) built to higher energy efficiency standards. Each house in the study was placed into one of six age categories and four building types. In Fig. 2 (right hand panel) we show the concentration statistics (median, interquartile, 95th percentile values) for total VOC (TVOC) as a function of building age, and on the left hand panel for building type. In our dataset there were no statistically significant differences between TVOC and building age. We would note that for all building ages a wide range of concentrations were observed in each class. The highest median TVOC was found in buildings in the era 1960–1979. Similarly, no substantial differences were seen in the median TVOC of homes of different type. Slightly higher values were seen in studio and apartments, although again the differences were not statistically significant. Whilst building type and ventilation are without doubt critical factors that influence indoor TVOC concentrations, no systematic differences emerged in this dataset suggesting that factors such as ventilation do not provide an overwhelming degree of control on concentrations.3.4. Balance of VOCs between indoor and outdoor air
Indoor/outdoor ratios for the ten most abundant species by season can be seen in Fig. 3. This data can largely be rationalised by consideration of the indoor sources. N-Butane for example had a high indoor to outdoor ratio (indoor median = 107 μg m−3, outdoor median = 5.2 μg m−3) reflecting the frequently use of aerosols in the study, whereas a long-lived VOC such as ethane from widespread natural gas leakage had broadly similar concentrations both indoors and out. Of these ten species, only pentane had higher abundance outdoors, which likely reflects its dominant emission from gasoline evaporation and relatively limited use in household products.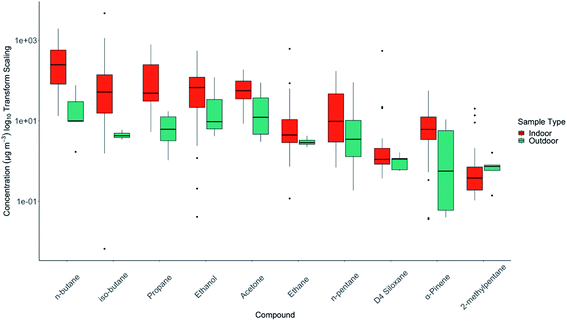 | ||
| Fig. 3 Rank order plot of the indoor/outdoor ratios for ten most abundant species across both campaigns and all households. The y-axis has been transformed to a log10 scale to aid visualisation. | ||
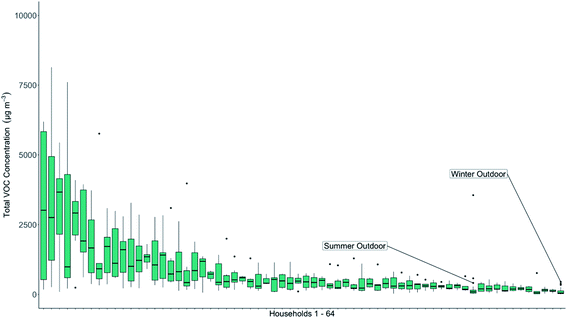
The TVOC concentrations measured in each household are shown as a rank order plot in Fig. 4, along with the winter and summer outdoor concentrations. The mean value for each home is shown with a black bar. The mean winter outdoor TVOC concentration was recorded at 102 μg m−3, the lowest recorded group mean TVOC value in the data set shown in Fig. 4. The mean summer outdoor value was 261 μg m−3.
TVOC concentrations indoors exceeded outdoors in 84% of households when compared to the mean summertime outdoor concentration and in 100% of households when compared to the wintertime mean outdoor concentration. A small number (seven) of high indoor concentration households were detected in the study, but with a long tail of homes where indoor air concentrations were within a factor of two of outdoors. Across the cohort as a whole the median indoor TVOC concentration was 413 μg m−3, approximately 1.5 and 4 times higher than outdoors in summer and winter respectively. Whilst the number of outdoor samples collected in this study was smaller than those collected indoors, and not every home had a matching control outdoor sample, it is clear that the more significant route for VOC exposure in this study group would be from inhalation of indoor air, rather than outdoors when considered solely on a like-for-like concentration basis. If a weighting for the greater time typically spent indoors compared to outdoors was applied then the differential between the two possible routes for exposure for an individual grows further, although in this study we did not collect data on individual time in each environment. Time spent indoors, daily is typically cited as 90%, so we can confidently assume that people will experience the majority of their VOC exposure indoors. Of the many species found indoors, recent studies have identified toluene, hexane and formaldehyde as priority chemicals for further study as they promote respiratory irritation and an inflammatory response.64
3.5. Relationships between individual VOCs indoors
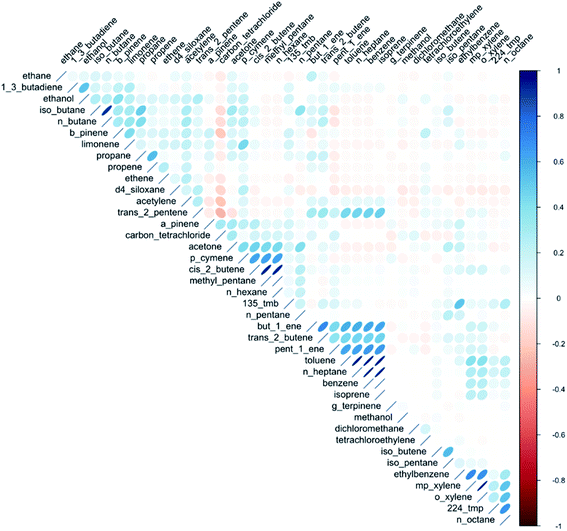
Since VOCs come from many sources, the relationships between them are complex, but speciation may carry with it information that provides insight into the contributing sources. The relationships between VOCs, correlated/uncorrelated etc., is a variable that is somewhat independent of AER, if one assumes that dilution is generally with outdoor air that is much lower in VOCs than the indoor air. Some VOCs are closely linked to one another in terms of their abundance and variability, whilst others have behaviours that is completely decoupled. Significant correlations between VOCs were evident between indoor concentrations of some alkanes, likely due to their common use as solvents in different types of household and personal care products. Correlations were also seen between benzene, toluene, ethylbenzene, and the xylene isomers (BTEX) again consistent with them having common sources. These VOCs are often combined together in refined solvent materials such as paints and glues. Weaker correlations were observed between different monoterpenes, or between different functional group classes.A matrix correlation plot is shown in Fig. 5 and provides a visual indicator that indoor VOCs do not behave as a single pollutant. There are many complex relationships between the different VOCs within this matrix, from the very highly correlated e.g. benzene and n-heptane (r = 0.98), iso-butane and n-butane (r = 0.91), to fully uncorrelated. The significance of the relationships between individual VOCs was found to be broadly similar between seasons, although some relationships became stronger in the summer months, such as those between the individual BTEX species.
Literature surrounding the correlations between VOC concentrations indoors is sparse, though Esplugues et al. (2010)65 identified strong correlations between BTEX species indoors. Current literature has apportioned emissions to large-scale sources because of the similar VOCs released, e.g. use of paints, renovation work, traffic etc.,14 but there is a dearth of literature attributing particular VOC emissions to the use of specific household product types.
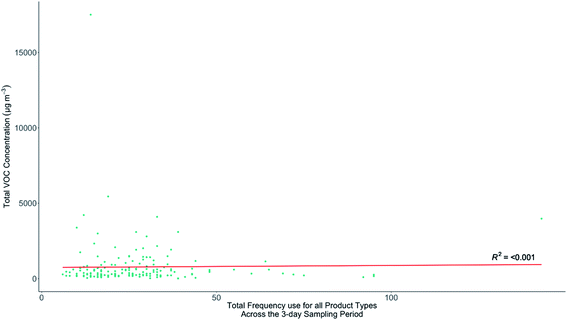
3.6 Indoor VOC concentrations and frequency of product use
Section 3.3 showed there were no clear links between TVOC and building age or type, and this lack of systematic connection also extended to other factors such as occupant number, age, or bedroom count. Given these factors did not provide significant predictive power for indoor VOCs a hypothesis in this study was that the combined frequency of use of all VOC-containing products in the home could be reflected in the indoor speciation and possibly concentrations of VOCs observed. Homes that had similar building characteristics (and therefore AER), and that frequently used VOC-containing products, might show on average higher indoor VOC concentrations than the homes of infrequent users. A secondary hypothesis was that frequent users of specific VOC-containing products may also, on average, have distinctive distributions of VOCs (a speciation) that could be linked to particular products. An initial analysis of the relationships between TVOC concentrations and the total number of household recorded uses of all products for the duration of each sample is shown in Fig. 6. No statistically significant relationship between these two variables was found, likely confirming that other factors such as AER variability overwhelm any signal remaining from household product use.Given that there was no canonical distribution in the speciation of indoor VOCs, TVOC would be expected to be a poor metric to use when attempting to link indoor concentrations with product use. For example, TVOC may be overly sensitive to contributions from a dominant indoor VOC source that may not have any association or emissions from household products. ESI Fig. E† explores how the concentrations of individual VOCs vary as a function of total frequency of all products used for the duration of each sample. As with TVOC, there is no statistically significant relationship between the concentrations of individual VOCs and the total frequency of recorded uses of all products in each home. Using a metric of combined frequency of use of VOC-containing products in a home is therefore not a predictor of indoor VOC concentrations in that home, either expressed as a TVOC value, or for the concentration of any individual VOC.
The differences in the nature and variance of the two datasets (e.g. unit integer vs. continuous) may mean that x, y correlation and linear interpolation of product use frequency against VOC concentration may lead to a poor fit. Covariance, however, provides an alternative measure of the degree of relationship between the two data sets, scaled to be independent of unit of measurement. Covariance is determined as the product of deviations of data points from their respective mean values.
Each dataset was rescaled from 0 to 100, and the covariance between a selected range of parameter pairs then shown as a matrix plot in Fig. 7. To simplify the figure, we select six of the most frequently used product types and six of the more abundant VOCs. Using this methodology, some weak relationships between variables begin to emerge. There is covariance in the frequency of use of different product types (e.g. the frequency of use of household cleaning sprays co-varies with insecticides). Some of these inter-product covariance relationships can be rationalised as being a consequence of occupant preferences and behaviours. Some weak but statistically significant covariance also emerged between frequencies of individual product usage and indoor concentrations of specific individual VOCs. For example, there was weak covariance between indoor limonene concentrations and the frequency of use of insecticides and plug-in air fresheners. The relationships are plausible based on the known composition of the products themselves.
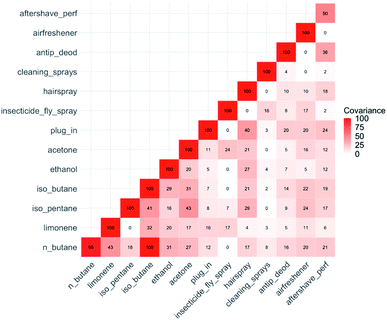 | ||
| Fig. 7 Covariance values for selected VOC and product use frequency pairs. Covariance values are derived from concentration and product usage data, all data rescaled from 0 to 100. | ||
The conclusions drawn here linking concentrations with usage of products have no direct comparators in the literature. However, the general outcomes can be compared to Rösch et al. (2014)14 who assessed associations between VOC emissions and pattern scenarios (common activities that release VOCs). The authors noted that for patterns where VOC emission profiles are similar, it was impossible to apportion a particular profile to a particular source. As mentioned earlier, Adgate (2004),13 reported that use of cleaning products was associated with higher concentrations of D-limonene and lower concentrations of β-pinene. They also indicate that room deodoriser use was associated with higher α-pinene concentrations.
3.7 Comparison with literature
There are relatively few contemporary comparator studies in existing literature, however, some larger population studies do exist and will be discussed here. Adgate et al. (2004)13 examined three exposure scenarios for 153 school-aged children in Minneapolis, MN, USA, namely outdoors, indoors at school, indoors at home and personal exposure. Organic vapour monitors were used to measure 15 common VOCs in these different scenarios in winter and spring 2000. Sexton et al. (2004)15 measured outdoor, indoor and personal exposure concentrations of 15 VOCs for 71 adults in three urban areas of the Minneapolis–Saint Paul metropolitan area, MN, USA, again using passive air samplers. Finally, Rösch et al. (2014)14 measured 60 VOCs in 622 apartments in Leipzig, Germany. Median concentrations for VOCs that are common to this and at least one other study are shown in Table 2.| This study | Adgate et al. (2004)13 | Sexton et al. (2004)15 | Rösch et al. (2014)14 | |
|---|---|---|---|---|
| 1,3,5-Tmb | 0.2 | — | — | 0.21 |
| α-Pinene | 8.0 | 2.4 | 15.53 | |
| β-Pinene | 0.1 | 2.5 | 1.2 | 1.84 |
| Benzene | 0.5 | 2.2 | 1.9 | 1.09 |
| Dichloromethane | 0.2 | 0.4 | 1.1 | — |
| Ethylbenzene | 0.8 | 1 | 1.40 | 0.9 |
| Limonene | 3.8 | 28.6 | 9 | 13.03 |
| m/p-Xylene | 1.5 | 3.7 | 1.6 | 1.84 |
| n-Heptane | 0.3 | — | — | 1.2 |
| n-Hexane | 0.4 | — | — | 1.12 |
| n-Octane | 0.2 | — | — | 0.56 |
| o-Xylene | 1.2 | 1.2 | 1.6 | 0.61 |
| Tetrachloroethylene | 0.03 | 0.5 | 0.6 | — |
| Toluene | 1.5 | 8.2 | 12.3 | 8.06 |
The concentrations of monoterpenes were consistently lower in this work than those recorded in the other studies. With regards to BTEX species, benzene and toluene were in lower concentrations here, whilst the xylenes and ethylbenzene were broadly similar. Alkanes were considerably lower here and 1,3,5-trimethylbenzene concentrations broadly similar. Results in other studies, such as the National Human Exposure Assessment Survey (NHEXAS) and the Toxics Exposure Assessment Columbia–Harvard (TEACH) studies also report similar values to existing literature.11,66
We note however the rather limited range of species where a direct comparison between studies can be made. Table 2 is in a sense misleading, since it does not include the four most abundant VOCs that we observe, since they were not measured in these other studies, likely because of incompatibility with the sampling and/or analytical methods used. There is potential therefore for a literature bias towards discussing those particular VOCs which are commonly measured in indoor population studies e.g. mid-volatility, Tenax-compatible compounds that can be quantitatively collected using either pumped or diffusive sampling tubes. When more universal ‘whole air’ sampling methods are used a different set of VOCs come to the fore as most abundant, such as butane, ethanol, acetone, cyclic siloxanes etc.
4. Discussion
This study has surveyed the indoor concentrations of a wide range of VOCs (∼C2–C10) in 60 UK homes alongside collecting contextual information and a diary of frequency of household use of VOC-containing consumer products. Using whole air sampling as the collection methodology has allowed for a comprehensive screening of VOCs without any biases associated with the upper-limit of compound volatility, and has included infrequently measured very volatile species such as ethane, ethene, acetylene, methanol, ethanol, propane and butane. Whilst physical factors such as air exchange rate might be anticipated to exert a significant control over indoor VOCs, our study showed no systematic differences between TVOC and different eras and construction type, despite covering a range from pre 1900 to post 2000. Each property type group included homes that spanned a very wide range of indoor VOC concentrations, from below 100 ppb TVOC to in excess of 1000 ppb.Whilst VOC-containing domestic products are undoubtedly a source of emissions of VOCs in the home, the cumulative frequency of their use is not, in isolation, a predictor of overall abundance of VOCs indoors when averaged over a three day period. The total recorded uses of VOC-containing products varied widely across 60 homes, but this was not reflected systematically in the resulting time-averaged indoor concentrations of either the total amount of VOC present, or the concentrations of individual VOCs. Whilst many different consumer products contain VOCs, the frequency with which those products are used in real-life varied widely. This behavioural component of indoor air quality emissions is not well-understood or widely reported in the research literature. Whilst this study is likely only directly reflective of UK habits, products and behaviour, it shows that some VOC-containing products are used only very infrequently, whilst others such as deodorant aerosols are used in virtually all homes and at high frequencies. Even for commonly-used products such as deodorants which have simple and distinctive chemical formulations, no strong relationships were found between their frequency of use and the indoor concentrations of the key ingredients, n-butane or iso-butane.
The release of VOCs from consumer products is often cited as having links to adverse indoor air quality, however in this study we find few statistically robust connections between concentrations and the frequency of use of those products which contain VOCs. This is not to suggest that these products are not contributors to emissions and indoor concentrations – they clearly are – however, other factors such as the size of dose of product used, product-to-product variability between manufacturers, persistent indoor VOC emissions from other sources (like off-gassing from wood, furniture etc.), episodic emissions from food and cooking and physical factors such as ventilation, exert greater influence over indoor concentrations over longer averaging periods. The limitations of time-averaged measurements are acknowledged, and no doubt if followed at higher time resolution (e.g. by PTR-MS) linkage between transient concentrations of VOCs and product use would be clearer, as has been seen in many highly instrumented test homes.
Whilst the vast majority of VOCs are emitted directly from sources within the homes, such as consumer products, from cooking, furnishings and so on, some VOCs may be generated as secondary by-products following gas phase oxidation. Most VOCs reported in this study are primary hydrocarbons, halocarbons or siloxanes, and so by their nature are not secondary. It is possible however that some fraction of alcohols and ketones measured could derive from oxidation of those primary hydrocarbon-like VOCs, although the strength of that source is very uncertain.
VOCs such as n-butane are linked to a relatively limited number of possible indoor sources. The very high concentrations seen in some homes will almost certainly have arisen from the use of compressed aerosols, where product composition between manufacturers and brands is reasonably consistent and therefore largely discountable as a confounding variable. Recording only frequency of use, and not dose size, is possibly a confounding influence. We note the very limited information available on consumer use of aerosols, beyond overall national consumption statistics (in the UK ∼10 aerosol cans per person per year, National Atmospheric Emissions Inventory, 2017 (ref. 67)). Reducing frequency of use of aerosols containing n-butane would appear to be the most effective intervention to reduce the overall total indoor concentrations of VOCs and overall emissions of VOCs arising from domestic product use.
TVOC may be an inadequate metric to use when attempting to link indoor concentrations with VOC product use, but it does provide an interesting insight into potential exposure routes for VOCs overall. Concentrations of VOCs in this study were higher indoors than outdoors for all homes in winter and for 84% of homes during the summer. A small number of homes had high concentrations, but the majority were within around a factor of two of outdoor concentrations. Exposure to ambient concentrations up to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg m−3 have previously been reported to be unlikely to cause any ill-effects beyond sensory irritation.55
000 μg m−3 have previously been reported to be unlikely to cause any ill-effects beyond sensory irritation.55
No households in this study reached this threshold on a mean concentration basis, but this TVOC value was exceeded in one three-day sample in one household. Those very high concentrations were driven by hydrocarbons from aerosol sources. From a study of this limited sample size robust statistics are therefore not available on the likely population prevalence of homes routinely exceeding the 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg m−3 value, but it is clearly possible, and may occur perhaps at the frequency >1 in 100 homes.
000 μg m−3 value, but it is clearly possible, and may occur perhaps at the frequency >1 in 100 homes.
VOCs released indoors are not limited in their effects to the indoor environment. Since indoor oxidation rates are relatively slow compared to outside, the fate for a fraction of indoor-released VOCs is for them to be ventilated outdoors where they contribute, as other VOC sources do, to tropospheric ozone and SOA formation.68 Domestic and industrial solvents are now thought to comprise the largest component of the urban VOC emissions budget in high-income countries,69 overtaking VOC emissions from road transport. This emissions sector may be subject to further controls to support attainment of obligations in international treaties such as the UNECE Convention on long-range Transboundary Air Pollution and EC National Emissions Ceiling Directive.70,71
Indoor observations shed some light on the scale of VOC emissions from domestic consumer products, an area with widely acknowledged uncertainties in international reporting and national emission inventories. The high concentrations of VOCs that derive from aerosol propellants seen in virtually all the homes studied here, and that are used with high frequencies, highlights that there may be particular policy value in considering reformulation or removal of this specific source of emissions. Measured purely as mass of VOC emissions, iso and n-butane from aerosols appear to form the largest contribution from indoor emissions as assessed from real-life behaviours. This is also borne out by estimates of VOCs in emissions inventories that are resolved in sufficient sectoral and speciated detail. From the UK National Atmospheric Emissions Inventory in 2017, ∼34 k per tonnes of VOCs were estimated to be emitted from aerosols in the source categories of ‘cosmetics and toiletries’ and ‘household products’, representing around 4% of total UK VOC emissions. Placed in context, VOC emissions originating from domestic use of aerosols within the home are broadly similar in magnitude to the total estimated VOC emissions from all road transport sources in the UK (2017 data: ∼49 ktonnes).
The air quality impacts of VOCs released indoors are not equal between different species, and we note that many of the most abundant VOCs seen here are relatively unreactive in the context of indoor oxidation chemistry. Translation of mass concentrations into metrics that reflect the formation of atmospheric by-products, such as secondary product creation potential is one means to evaluate this effect.46 Although it is beyond this study, it is likely that the air quality role and influence of alkenes, monoterpenes and aromatic compounds would be elevated, relative to their contributions when expressed only in mass terms.
Funding
Funding for in-home observations and measurements was provided by Givaudan UK Ltd. ACL receives support from the National Centre for Atmospheric Science NERC National Capability research programme in air pollution.Conflicts of interest
NO, CJ and GA are employees of Givaudan UK Ltd and Givaudan Fragrances Corp. who are industrial suppliers of chemicals used in household and personal care products. To support full independence and transparency in this study, all analytical work and data analysis was undertaken externally by University of York and no restrictions placed on freedoms to publish. All data collected in this study is freely available from the Centre for Environment Data and Analysis (https://www.ceda.ac.uk).Acknowledgements
This work has been enabled by a range of underpinning research support from the Natural Environment Research Council. ACL, NO, CJ, and GA designed the original experiment. AHH and MWW performed the laboratory measurements of VOCs using GC-FID and GC-MS. AHH, GH and SKG undertook the analysis and visualisation of data presented here. All authors contributed to the writing of the manuscript and the development of its conclusions. Data from this study is lodged at the Centre for Environment Data and Analysis (https://www.ceda.ac.uk), a public data repository for the environmental sciences.References
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41, 1164–1179 CrossRef CAS.
- C. A. Redlich, J. Sparer and M. R. Cullen, Sick-building syndrome, Lancet, 1997, 349, 1013–1016 CrossRef CAS.
- J. D. Spengler and K. Sexton, Indoor air pollution: a public health perspective, Science, 1983, 221, 9–17 CrossRef CAS PubMed.
- J. A. J. Stolwijk, Risk Assessment of Acute Health and Comfort Effects of Indoor Air Pollution, Ann. N. Y. Acad. Sci., 1992, 641, 56–62 CrossRef CAS PubMed.
- D. Y. C. Leung, Outdoor-indoor air pollution in urban environment: challenges and opportunity, Front. Environ. Sci., 2015, 2, 1–7 Search PubMed.
- A. P. Jones, Indoor air quality and health, Atmos. Environ., 1999, 33, 4535–4564 CrossRef CAS.
- P. R. Veres, P. Faber, F. Drewnick, J. Lelieveld and J. Williams, Anthropogenic sources of VOC in a football stadium: assessing human emissions in the atmosphere, Atmos. Environ., 2013, 77, 1052–1059 CrossRef CAS.
- A. Wisthaler and C. J. Weschler, Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6568–6575 CrossRef CAS PubMed.
- Z. Zou, J. He and X. Yang, An experimental method for measuring VOC emissions from individual human whole-body skin under controlled conditions, Build. Sci., 2020, 181, 107137 Search PubMed.
- M. Gallagher, C. J. Wysocki, J. J. Leyden, A. I. Spielman, X. Sun and G. Preti, Analyses of volatile organic compounds from human skin, Br. J. Dermatol., 2008, 159, 780–791 CrossRef CAS PubMed.
- S. M. Gordon, P. J. Callahan, M. G. Nishioka, M. C. Brinkman, M. K. O'Rourke, M. D. Lebowitz and D. J. Moschandreas, Residential environmental measurements in the national human exposure assessment survey (NHEXAS) pilot study in Arizona: preliminary results for pesticides and VOCs, J. Exposure Anal. Environ. Epidemiol., 1999, 9, 456–470 CrossRef CAS PubMed.
- Y. S. Lin, P. P. Egeghy and S. M. Rappaport, Relationships between levels of volatile organic compounds in air and blood from the general population, J. Exposure Sci. Environ. Epidemiol., 2008, 18, 421–429 CrossRef CAS PubMed.
- J. L. Adgate, T. R. Church, A. D. Ryan, G. Ramachandran, A. L. Fredrickson, T. H. Stock, M. T. Morandi and K. Sexton, Outdoor, indoor, and personal exposure to VOCs in children, Environ. Health Perspect., 2004, 112, 1386–1392 CrossRef CAS PubMed.
- C. Rösch, T. Kohajda, S. Röder, M. v. Bergen and U. Schlink, Relationship between sources and patterns of VOCs in indoor air, Atmos. Pollut. Res., 2014, 5, 129–137 CrossRef.
- K. Sexton, J. L. Adgate, G. Ramachandran, G. C. Pratt, S. J. Mongin, T. H. Stock and M. T. Morandi, Comparison of Personal, Indoor, and Outdoor Exposures to Hazardous Air Pollutants in Three Urban Communities, Environ. Sci. Technol., 2004, 38, 423–430 CrossRef CAS PubMed.
- S. M. Rappaport and L. L. Kupper, Variability of environmental exposures to volatile organic compounds, J. Exposure Anal. Environ. Epidemiol., 2004, 14, 92 CrossRef CAS PubMed.
- E. Woolfenden, Monitoring VOCs in Air Using Sorbent Tubes Followed by Thermal Desorption-Capillary GC Analysis: Summary of Data and Practical Guidelines, J. Air Waste Manage. Assoc., 1997, 47, 20–36 CrossRef CAS.
- D. J. Price, D. A. Day, D. Pagonis, H. Stark, L. B. Algrim, A. V. Handschy, S. Liu, J. E. Krechmer, S. L. Miller, J. F. Hunter, J. A. de Gouw, P. J. Ziemann and J. L. Jimenez, Budgets of Organic Carbon Composition and Oxidation in Indoor Air, Environ. Sci. Technol., 2019, 53, 13053–13063 CrossRef CAS PubMed.
- T. Schripp, S. Etienne, C. Fauck, F. Fuhrmann, L. Märk and T. Salthammer, Application of proton-transfer-reaction-mass-spectrometry for indoor air quality research, Indoor Air, 2014, 24, 178–189 CrossRef CAS PubMed.
- Y. Liu, P. K. Misztal, J. Xiong, Y. Tian, C. Arata, R. J. Weber, W. W. Nazaroff and A. H. Goldstein, Characterizing sources and emissions of volatile organic compounds in a northern California residence using space- and time-resolved measurements, Indoor Air, 2019, 29, 630–644 CAS.
- W. A. McClenny and M. W. Holdren, Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air –Second Edition – Compendium Method TO-15: Determination Of Volatile Organic Compounds (VOCs) In Air Collected In Specially-Prepared Canisters And Analyzed By Gas Chromatography/Mass Spectrometry (GC/MS), 1999, 1–63.
- J. S. Herrington, Rapid Determination of TO-15 Volatile Organic Compounds (VOCs) in Air, 2016 Search PubMed.
- M. de Blas, M. Navazo, L. Alonso, N. Durana and J. Iza, Automatic on-line monitoring of atmospheric volatile organic compounds: gas chromatography–mass spectrometry and gas chromatography–flame ionization detection as complementary systems, Sci. Total Environ., 2011, 409, 5459–5469 CrossRef CAS PubMed.
- M. Matura, M. Sköld, A. Börje, K. E. Andersen, M. Bruze, P. Frosch, A. Goossens, J. D. Johansen, C. Svedman, I. R. White and A.-t. Karlberg, Selected oxidized fragrance terpenes are common contact allergens, Contact Dermatitis, 2005, 52, 320–328 CrossRef CAS PubMed.
- B. C. Singer, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids, Indoor Air, 2006, 16, 179–191 CrossRef CAS PubMed.
- A. Stepanyuk and A. Kirschning, Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase, Beilstein J. Org. Chem., 2019, 15, 2590–2602 CrossRef CAS PubMed.
- D. T. Kirkpatrick, unpublished work, undertaken as WIL Research Laboratories, now Charles River Laboratories, http://criver.com.
- T. Petry, D. Vitale, F. J. Joachim, B. Smith, L. Cruse, R. Mascarenhas, S. Schneider and M. Singal, Human health risk evaluation of selected VOC, SVOC and particulate emissions from scented candles, Regul. Toxicol. Pharmacol., 2014, 69, 55–70 CrossRef CAS PubMed.
- M. Trantallidi, C. Dimitroulopoulou, P. Wolkoff, S. Kephalopoulos and P. Carrer, EPHECT III: health risk assessment of exposure to household consumer products, Sci. Total Environ., 2015, 536, 903–913 CrossRef CAS PubMed.
- C. M. Wang, B. Barratt, N. Carslaw, A. Doutsi, R. E. Dunmore, M. W. Ward and A. C. Lewis, Unexpectedly high concentrations of monoterpenes in a study of UK homes, Environ. Sci.: Processes Impacts, 2017, 19, 528–537 RSC.
- M. E. Jenkin, L. A. Watson, S. R. Utembe and D. E. Shallcross, A Common Representative Intermediates (CRI) mechanism for VOC degradation. Part 1: gas phase mechanism development, Atmos. Environ., 2008, 42, 7185–7195 CrossRef CAS.
- M. S. Waring, Secondary organic aerosol in residences: predicting its fraction of fine particle mass and determinants of formation strength, Indoor Air, 2014, 24, 376–389 CrossRef CAS PubMed.
- Environment Agency, Material comparators for end-of-waste decisions – fuels: natural gas – report: SC130040/R15, ShARE 25, 2016 Search PubMed.
- W. W. Nazaroff and C. J. Weschler, Cleaning products and air fresheners: exposure to primary and secondary air pollutants, Atmos. Environ., 2004, 38, 2841–2865 CrossRef CAS.
- G. Vincent, P. M. Marquaire and O. Zahraa, Abatement of volatile organic compounds using an annular photocatalytic reactor: study of gaseous acetone, J. Photochem. Photobiol., A, 2008, 197, 177–189 CrossRef CAS.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Tools and techniques for solvent selection: green solvent selection guides, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- T. Salthammer, Very volatile organic compounds: an understudied class of indoor air pollutants, Indoor Air, 2016, 26, 25–38 CrossRef CAS PubMed.
- P. R. Galassetti, B. Novak, D. Nemet, C. Rose-Gottron, D. M. Cooper, S. Meinardi, R. Newcomb, F. Zaldivar and D. R. Blake, Breath ethanol and acetone as indicators of serum glucose levels: an initial report, Diabetes Technol. Ther., 2005, 7, 115–123 CrossRef CAS PubMed.
- E. Gorgus, M. Hittinger and D. Schrenk, Estimates of Ethanol Exposure in Children from Food not Labeled as Alcohol-Containing, J. Anal. Toxicol., 2016, 40, 537–542 CrossRef CAS PubMed.
- R. L. Elder, Cosmetic Ingredient Review: Final report of the safety assessment of isobutane, isopentane, n-butane, and propane, J. Am. Coll. Toxicol., 1982, 1, 127–142 CrossRef.
- M. Słomińska, P. Konieczka and J. Namieśnik, The Fate of BTEX Compounds in Ambient Air, Crit. Rev. Environ. Sci. Technol., 2014, 44, 455–472 CrossRef.
- Y.-M. Chang, W.-H. Hu, W.-B. Fang, S.-S. Chen, C.-T. Chang and H.-W. Ching, A Study on Dynamic Volatile Organic Compound Emission Characterization of Water-Based Paints, J. Air Waste Manage. Assoc., 2011, 61, 35–45 CrossRef CAS PubMed.
- C. J. Weschler and W. W. Nazaroff, Semivolatile organic compounds in indoor environments, Atmos. Environ., 2008, 42, 9018–9040 CrossRef CAS.
- C. Wang, D. B. Collins, C. Arata, A. H. Goldstein, J. M. Mattila, D. K. Farmer, L. Ampollini, P. F. DeCarlo, A. Novoselac, M. E. Vance, W. W. Nazaroff and J. P. D. Abbatt, Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents, Sci. Adv., 2020, 6, eaay8973 CrossRef CAS PubMed.
- D. M. Lunderberg, K. Kristensen, Y. Tian, C. Arata, P. K. Misztal, Y. Liu, N. Kreisberg, E. F. Katz, P. F. DeCarlo, S. Patel, M. E. Vance, W. W. Nazaroff and A. H. Goldstein, Surface Emissions Modulate Indoor SVOC Concentrations through Volatility-Dependent Partitioning, Environ. Sci. Technol., 2020, 54, 6751–6760 CrossRef CAS PubMed.
- N. Carslaw and D. Shaw, Secondary product creation potential (SPCP): a metric for assessing the potential impact of indoor air pollution on human health, Environ. Sci.: Processes Impacts, 2019, 21, 1313–1322 RSC.
- C. J. Weschler, Ozone in Indoor Environments: Concentration and Chemistry, Indoor Air, 2000, 10, 269–288 CrossRef CAS PubMed.
- K. Pytel, R. Marcinkowska and B. Zabiegała, Investigation of the Dynamism of Nanosized SOA Particle Formation in Indoor Air by a Scanning Mobility Particle Sizer and Proton-Transfer-Reaction Mass Spectrometry, Molecules, 2020, 25, 1–23 CrossRef PubMed.
- M. Wormuth, M. Scheringer and K. Hungerbühler, Linking the Use of Scented Consumer Products to Consumer Exposure to Polycyclic Musk Fragrances, J. Ind. Ecol., 2005, 9, 237–258 CrossRef CAS.
- X. M. Wu, D. H. Bennett, B. Ritz, D. L. Cassady, K. Lee and I. Hertz-Picciotto, Usage pattern of personal care products in California households, Food Chem. Toxicol., 2010, 48, 3109–3119 CrossRef CAS PubMed.
- N. Carslaw, L. Fletcher, D. Heard, T. Ingham and H. Walker, Significant OH production under surface cleaning and air cleaning conditions: impact on indoor air quality, Indoor Air, 2017, 27, 1091–1100 CrossRef CAS PubMed.
- E. Garcia-Hidalgo, N. von Goetz, M. Siegrist and K. Hungerbühler, Use-patterns of personal care and household cleaning products in Switzerland, Food Chem. Toxicol., 2017, 99, 24–39 CrossRef CAS PubMed.
- J. R. Hopkins, A. C. Lewis and K. A. Read, A two-column method for long-term monitoring of non-methane hydrocarbons (NMHCs) and oxygenated volatile organic compounds (o-VOCs), J. Environ. Monit., 2003, 5, 8–13 RSC.
- J. T. Shaw, R. T. Lidster, D. R. Cryer, N. Ramirez, F. C. Whiting, G. A. Boustead, L. K. Whalley, T. Ingham, A. R. Rickard, R. E. Dunmore, D. E. Heard, A. C. Lewis, L. J. Carpenter, J. F. Hamilton and T. J. Dillon, A self-consistent, multivariate method for the determination of gas-phase rate coefficients, applied to reactions of atmospheric VOCs and the hydroxyl radical, Atmos. Chem. Phys., 2018, 18, 4039–4054 CrossRef CAS.
- European Commission, EUR 17675 EN European Collaborative Action Indoor Air Quality and Its Impact on Man – Environment and Quality of Life – Report No. 19: Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations, 1997 Search PubMed.
- M. De Bortoli, H. Knöppel, E. Pecchio, A. Peil, L. Rogora, H. Schauenburg, H. Schlitt and H. Vissers, Concentrations of selected organic pollutants in indoor and outdoor air in Northern Italy, Environ. Int., 1986, 12, 343–350 CrossRef CAS.
- L. Mølhave, Volatile Organic Compounds, Indoor Air Quality and Health, Indoor Air, 1991, 1, 357–376 Search PubMed.
- M. Noguchi, A. Mizukoshi, Y. Yanagisawa and A. Yamasaki, Measurements of Volatile Organic Compounds in a Newly Built Daycare Center, Int. J. Environ. Res. Public Health, 2016, 13, 736 CrossRef PubMed.
- L. E. Sparks, Z. Guo, J. C. Chang and B. A. Tichenor, Volatile Organic Compound Emissions from Latex Paint – Part 1. Chamber Experiments and Source Model Development, Indoor Air, 1999, 9, 10–17 CrossRef CAS PubMed.
- R. D. Edwards, J. Jurvelin, K. Saarela and M. Jantunen, VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland, Atmos. Environ., 2001, 35, 4531–4543 CrossRef CAS.
- U. Schlink, A. Thiem, T. Kohajda, M. Richter and K. Strebel, Quantile regression of indoor air concentrations of volatile organic compounds (VOC), Sci. Total Environ., 2010, 408, 3840–3851 CrossRef CAS PubMed.
- C. Jia, S. Batterman and C. Godwin, VOCs in industrial, urban and suburban neighborhoods, part 1: indoor and outdoor concentrations, variation, and risk drivers, Atmos. Environ., 2008, 42, 2083–2100 CrossRef CAS.
- G. Hernandez, S. L. Wallis, I. Graves, S. Narain, R. Birchmore and T.-A. Berry, The effect of ventilation on volatile organic compounds produced by new furnishings in residential buildings, Atmos. Environ.: X, 2020, 6, 100069 CAS.
- M. Dezest, M. Le Bechec, L. Chavatte, V. Desauziers, B. Chaput, J.-L. Grolleau, P. Descargues, C. Nizard, S. Schnebert, S. Lacombe and A.-L. Bulteau, Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail, Sci. Rep., 2017, 7, 10707 CrossRef PubMed.
- A. Esplugues, F. Ballester, M. Estarlich, S. Llop, V. Fuentes-Leonarte, E. Mantilla and C. Iñiguez, Indoor and outdoor air concentrations of BTEX and determinants in a cohort of one-year old children in Valencia, Spain, Sci. Total Environ., 2010, 409, 63–69 CrossRef CAS PubMed.
- S. N. Sax, D. H. Bennett, S. N. Chillrud, P. L. Kinney and J. D. Spengler, Differences in source emission rates of volatile organic compounds in inner-city residences of New York City and Los Angeles, J. Exposure Sci. Environ. Epidemiol., 2004, 14, S95–S109 CrossRef CAS PubMed.
- National Atmospheric Emissions Inventory, Data access viahttps://naei.beis.gov.uk/data/data-selector.
- J. P. D. Abbatt and C. Wang, The atmospheric chemistry of indoor environments, Environ. Sci.: Processes Impacts, 2020, 22, 25–48 RSC.
- B. C. McDonald, J. A. de Gouw, J. B. Gilman, S. H. Jathar, A. Akherati, C. D. Cappa, J. L. Jimenez, J. Lee-Taylor, P. L. Hayes, S. A. McKeen, Y. Y. Cui, S.-W. Kim, D. R. Gentner, G. Isaacman-VanWertz, A. H. Goldstein, R. A. Harley, G. J. Frost, J. M. Roberts, T. B. Ryerson and M. Trainer, Volatile chemical products emerging as largest petrochemical source of urban organic emissions, Science, 2018, 359, 760–764 CrossRef CAS PubMed.
- European Union, Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the reduction of national emissions of certain atmospheric pollutants, amending Directive 2003.35/EC and repealing Directive 2001/81/EC, 2016 Search PubMed.
- United Nations, 1979 Convention on Long-Range Transboundary Air Pollution, 1979 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0em00504e |
| ‡ Now at Empa, Swiss Federal Laboratories for Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland |
| This journal is © The Royal Society of Chemistry 2021 |