 Open Access Article
Open Access ArticleEnhanced polysaccharide nanofibers via oxidation over SiliaCat TEMPO
Rosaria
Ciriminna
 ,
Antonino
Scurria
,
Antonino
Scurria
 and
Mario
Pagliaro
and
Mario
Pagliaro
 *
*
Istituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: mario.pagliaro@cnr.it
First published on 29th June 2021
Abstract
Drawing on independent work carried out by academic and industrial researchers using the immobilized TEMPO catalyst SiliaCat TEMPO, in this study we show how shifting the carboxylation process mediated by TEMPO in solution to a process mediated by the above-mentioned hybrid sol–gel catalyst allows the synthesis of insoluble polysaccharide nanofibers of superior quality, eliminating waste. This will dramatically reduce the polysaccharide nanofiber production costs opening the route to large-scale production and uptake of these versatile nanofibers in a variety of functional products where their use has been limited by high cost. The results of this study will be useful for catalysis and biotechnology researchers as well as for chemistry educators teaching green chemistry, nanochemistry, and catalysis using the outcomes of recent research.
1. Introduction
The industrial production of cellulose nanofibers (CNFs) involves de Nooy's carboxylation reaction of the primary alcohol groups at the surface of the cellulose fibers1 mediated by 2,2,6,6-tetramethyl-1-piperidine-N-oxy radicals (TEMPO). The reaction is carried out in water using NaOCl (and catalytic bromide) as the primary oxidant at alkaline pH.2 Once synthesized, the fibrils are mechanically dispersed. In solution, the negatively charged carboxylate groups ensure mutual repulsion of the carboxylated fibers which remain stable in solution.3 First applied on an industrial scale by a large paper company in Japan at two different plants, the process currently affords CNFs at a cost of $90–100 kg−1.4The high cost is chiefly due to the relatively high cost of TEMPO and the cost of processing the solution containing the catalyst after the reaction. Separating nitroxyl radicals from other reactants in solution indeed is a multi-step expensive process.5 Furthermore, TEMPO is a genotoxic ingredient6 whose concentration in substances of biomedical use, such as the CNF, must be below a low threshold (4 ppm) of toxicological concern.7 As it happens with several labile molecules, furthermore, the removal efficiency rapidly decreases with lower TEMPO concentrations.7
In 2017, Renneckar and Patankar in Canada reported the green synthesis of CNFs in water by oxidising wood pulp fibres using the magnetically recoverable Karimi's catalyst (TEMPO@SiO2@Fe3O48) in place of TEMPO in solution, followed by mechanical disintegration of the oxidized cellulose fibers.9 The heterogeneously catalyzed process afforded 5 nm thick cellulose nanofibrils similar to those obtained in the oxidation mediated by TEMPO in solution, whereas the catalyst was easily recovered with a magnet and successfully reused in 4 successive reaction cycles.9
Noting that several immobilized TEMPO catalysts were available in 2018 to catalyze the reaction affording CNFs, one of us noted in 2018 that once stable and selective solid oxidation catalysts are available, the industrial uptake of the heterogeneously catalyzed route to CNF from paper companies would be rapidly scaled up.4
Drawing on recent work independently conducted by academic and industrial researchers using SiliaCat TEMPO, a commercial immobilized TEMPO catalyst,10 in this study we show how shifting the carboxylation process mediated by TEMPO in solution to a process mediated by the above-mentioned hybrid sol–gel catalyst allows the synthesis of insoluble polysaccharide nanofibers of superior quality, eliminating waste. This will dramatically reduce the polysaccharide nanofiber production costs opening the route to large-scale production and uptake of these versatile nanofibers in a variety of functional products where their use has been limited by high cost. The results of this study will be useful for catalysis and biotechnology researchers as well as for chemistry educators teaching green chemistry, nanochemistry,11 and catalysis using the outcomes of recent research.12
2. Enhanced polysaccharide nanofibers
In the following study, we use two examples of enhanced polysaccharide nanofibers synthesized over SiliaCat TEMPO: cellulose and sacchachitin nanofibrils. The former has been developed by academic researchers13 and further advanced by industrial research chemists,14 whereas the enhanced preparation of sacchachitin nanofibers has been lately reported by academic scholars.152.1 Early attempts
In a Japanese patent filed in 2012 Araki and co-workers reported that virtually any cellulose such as wood pulp, cotton linter, and algal and bacterial cellulose can be selectively oxidised over different supported TEMPO catalysts, from TEMPO@SiO2@Fe3O4 through silica-supported TEMPO.13 To ensure that a wide surface area of cellulose is modified during the heterogeneous reaction, they found, “it is desirable that the fiber has a width of 2 to 20 nm and a length of about 100 nm to several μm”.13Fig. 1 shows the suspension of untreated cellulose nanowhiskers in water with 1 M NaCl added (left) and that of cellulose nanowhiskers after oxidation over a silica-supported TEMPO catalyst (right). The untreated nanowhiskers form aggregates and precipitates, but oxidised cellulose nanowhiskers do not form aggregates and remain well dispersed. Furthermore, when the latter suspension was observed between crossed polarizers, the oxidised nanowhiskers showed significant flow birefringence, indicating the presence of rod-like molecules.
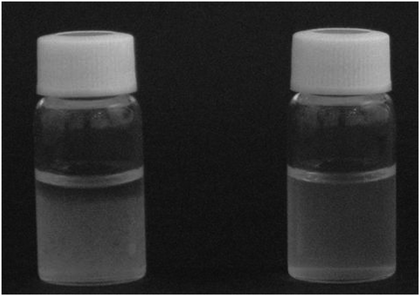 | ||
| Fig. 1 Suspension of untreated cellulose nanowhiskers in water added with 1 M NaCl (left) and cellulose nanowhiskers after oxidation over a silica gel-supported TEMPO catalyst (right). [Reproduced from ref. 13, with kind permission]. | ||
Perhaps as it was published as a patent publication, this remarkable discovery of surface carboxylation of cellulose nanowhiskers remained virtually ignored. For instance, the authors of the 2017 paper describing the use of Karimi's catalyst in cellulose nanofiber synthesis wrote that “there has been no report on use of such heterogeneous magnetic catalyst to modify solid biopolymer substrates such as wood pulp fibre”.9 Actually, Ariki's and co-workers patent13 even includes a photograph of a beaker containing the cellulose nanowhiskers after surface oxidation over Karimi's catalyst in which all the catalyst particles were collected and nearly fully recovered using an external magnet. The latter catalyst, in addition, was reusable whereas the silica-supported catalyst recovered by centrifugation was reported to rapidly lose its original high activity due to “a decrease in the nitrogen content and TEMPO group content of the regenerated silica gel-supported TEMPO” suggesting “the possibility that the silica gel is lost due to surface peeling due to physical breakage of the silica gel when stirring”.13 However, according to the same scholars, the silica-supported catalyst was “preferred because of its availability and ease of molecular introduction of the modifying reagent onto the surface”.
Aiming to increase the reusability of a solid catalyst, in 2016 Ariki's team described the surface carboxylation of cellulose nanowhiskers mediated by mPEG2000-TEMPO, a monomethoxy poly(ethylene glycol) 2000 polymer grafted with TEMPO.16 After the oxidation step, the used mPEG2000-TEMPO was recovered (by extraction with dichloromethane) and reused in three consecutive reaction cycles. Again, the degree of oxidation in the second and third cycles reduced to 60% of that observed in the first cycle. The team ascribed this loss in activity to the incomplete recovery of mPEG2000-TEMPO after every cycle (∼80%) due to the irreversible adsorption of the catalyst on the surface of the carboxylated cellulose nanowhiskers.
2.2 Cellulose nanofiber over SiliaCat TEMPO
Following early attempts with silica-supported TEMPO,13 in 2019 research chemists at a large household, health and personal care company based in South Korea reported that the carboxylation of bacterial cellulose mediated by SiliaCat TEMPO, without using catalytic bromide, affords CNFs of vastly superior quality when compared to carboxylation mediated by homogeneous TEMPO in the presence of catalytic (and metal corrosive) bromide (Scheme 1).14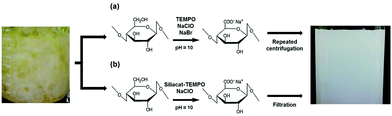 | ||
| Scheme 1 Synthesis of cellulose nanofibers via the (a) conventional homogeneously catalyzed process mediated by TEMPO and (b) the one-pot homogeneously catalyzed process mediated by SiliaCat TEMPO. [Reproduced from ref. 14, Creative Commons Attribution (CC BY) license]. | ||
Furthermore, the reaction can be carried out in one-pot because after the reaction the solid catalyst is separated and recovered by simple filtration instead of centrifugation. The residual hypochlorite in solution (present as aqueous Cl2, HOCl, and OCl− ions) is completely removed by the addition of 0.3% of ascorbic acid, without washing (Scheme 1).
The properties of the CNFs obtained after the one-pot synthesis (O–CNF) are different when compared to those of the nanofibers obtained via the conventional synthesis (C–CNF). Pointing to less pronounced oxidation of the C6-primary hydroxyls, the carboxyl content was higher in the homogeneously oxidised CNFs, while the aldehyde content was higher for the heterogeneously oxidised CNFs (Table 1). On the other hand, the scanning electron microscopy (SEM) investigation clearly showed that that the average diameter of the O–CNFs is 80 nm, close to the 100 nm diameter of the original BC nanofibers. The diameter of the conventionally synthesized C–CNFs, in turn, is reduced to just 30–50 nm (Fig. 2).
| Polymer | Carboxyl content (mmol g−1) | Aldehyde content (mmol g−1) |
|---|---|---|
| a Measured via conductivity titration; BC = bacterial cellulose; C–CNF = cellulose nanofibers obtained after the conventional homogeneous synthesis; O–CNF = cellulose nanofibers obtained after the one-pot heterogeneous synthesis. | ||
| BC | 0.02 ± 0.01 | 0 |
| C–CNF | 1.12 ± 0.10 | 0.08 ± 0.04 |
| O–CNF | 1.02 ± 0.08 | 0.12 ± 0.03 |
 | ||
| Fig. 2 SEM images of (a) pure BC, (b) conventional TEMPO-oxidised CNF and (c) CNF obtained in one-pot over SiliaCat TEMPO. Scale bar = 500 nm. [Reproduced from ref. 14, Creative Commons Attribution (CC BY) license]. | ||
The team ascribed this finding to the fact that the SiliaCat TEMPO microparticles comprising the catalyst do not penetrate the cellulose fiber bundle but only act on the surface of the bacterial cellulose fiber. Indeed, the O–CNFs observed via FE-SEM high-resolution imaging along with other water-soluble bio-polymers commonly used in skincare such as carbopol (polyacrylic acid), xanthan gum, and carboxymethyl cellulose (CMC) have fiber structures in which bundles of nanofibers are entangled (Fig. 3). This fact, the team noted, suggests that the one-pot synthesized CNFs will maintain their characteristics for example in skincare applications.
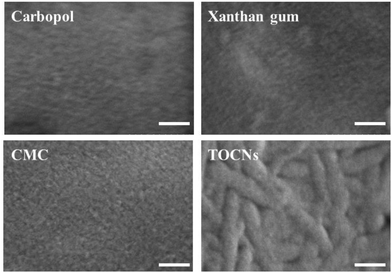 | ||
| Fig. 3 Nanometer-resolution structures of CNFs obtained in one-pot over SiliaCat TEMPO and other water-soluble polymers obtained via high resolution imaging using FE-SEM. Scale bar = 100 nm. [Reproduced from ref. 14, Creative Commons Attribution (CC BY) license]. | ||
Indeed, the one-pot synthesized CNFs form a network of nanofibers on the skin surface, blocking for example particulate matter such as carbon black from entering into the microgrooves on the skin's surface, whereas the high CNF water-absorbing capacity allows easy removal from the skin surface of the nanofibers blocking carbon black by simple washing with tap water.14 The large size of the organosilica microparticles (63–250 μm), much larger than the diameter of the CNFs (80 nm), enables quick filtration (under vacuum) through a nylon mesh of 50 μm pore size. The cellulose nanofiber solution easily passes through the filter affording a pure CNF solution whereas the catalyst beads remain on the nylon mesh from which they are recovered and made available for reuse in a subsequent oxidation run. “Due to its simplicity, efficiency, and ease of use” the team concluded, “the proposed one-pot synthesis method will be employed in production scenarios to prepare production quantities of bio-based polymer nanofibers in various potential industrial applications in the fields of skincare and biomedical research”.14
The trade named “Aqua Cellulose Solution”, a formulation comprising 1.5% CNFs, 95.5% deionized water, and 3% hexandiol, was commercialized shortly afterwards by a leading natural cosmetic company in South Korea.17 The latter formulation has been recently successfully used by the same team to produce semi-dissolving microneedle patches for effective transdermal drug delivery without loading the drugs themselves.17
2.3 Sacchachitin nanofibers
Consisting of about 40% chitin and 60% β-1,3-glucan, sacchachitin is a water-insoluble heteropolysaccharide extracted from the medicinal fungus Ganoderma tsugae fruiting body after the removal of the water-soluble medicinal compounds via digestion in concentrated NaOH (1 N) at 85 °C for 24 h followed by depigmentation with 0.1% hypochlorite.18 Due to their ability to form three-dimensional (3D) hydrogels at low (2% w/v) concentrations, TEMPO-oxidised sacchachitin nanofibers (SCNFs) are currently evaluated in diabetic wound healing and tissue regeneration because the 3D gel structure, the lack of toxicity and high biocompatibility of this polysaccharide are ideally suited for utilization as a biomaterial scaffold.19 In the healing of chronic wounds, such as, for instance, diabetic wounds, sacchachitin hydrogels incorporated in 2-acrylamide-2-methyl-propane sulfonate-based wound dressing accelerate healing, and further provide the healed wound with growth of sweat glands and hair follicles (i.e., wound healed as functional tissue).19The researchers ascribed the observed acceleration of clotting time to the nanofiber structure providing attachments for fibrin and reducing the secretion of exudate. Hence, in order to streamline the oxidation process, eliminating the need to purify the SCNFs from residual genotoxic TEMPO, in 2021 the same scholars based in Taiwan reported the first successful one-pot synthesis of SCNFs mediated by SiliaCat TEMPO.15 The team showed that when using increasing amounts of the primary oxidant (1.6, 5.0, and 10.0 mmol NaClO g−1 polysaccharide) in the presence of the sol–gel catalyst, surface carboxylation smoothly occurs. After quenching the residual hypochlorite and lyophilizing, all SCNF hydrogel samples showed a highly porous amorphous structure. In detail, after a 2% (w/v) SCNF suspension was mechanically disintegrated using a high pressure homogenizer for three, five, and 10 cycles at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi, each sample observed by the vial-inversion method was found to be in the gel form, similar to what happens with SCNF hydrogels obtained via homogeneously catalyzed oxidation mediated by TEMPO (Fig. 4). The results of the MTT assay of WS-1 human fibroblasts and MC3T3E1 cell viability test showed no cytotoxicity and high biocompatibility also for SCNFs obtained over the sol–gel hybrid catalyst. It is also relevant in sight of practical applications that the two-step sacchachitin extraction process has been lately advanced by replacing NaOH with KOH and using NaClO2 in place of NaOCl as the bleaching agent.20 This allows the two subsequent steps to be carried out in the same pot thereby increasing the extraction yield to 35% of the initial fungus weight.
000 psi, each sample observed by the vial-inversion method was found to be in the gel form, similar to what happens with SCNF hydrogels obtained via homogeneously catalyzed oxidation mediated by TEMPO (Fig. 4). The results of the MTT assay of WS-1 human fibroblasts and MC3T3E1 cell viability test showed no cytotoxicity and high biocompatibility also for SCNFs obtained over the sol–gel hybrid catalyst. It is also relevant in sight of practical applications that the two-step sacchachitin extraction process has been lately advanced by replacing NaOH with KOH and using NaClO2 in place of NaOCl as the bleaching agent.20 This allows the two subsequent steps to be carried out in the same pot thereby increasing the extraction yield to 35% of the initial fungus weight.
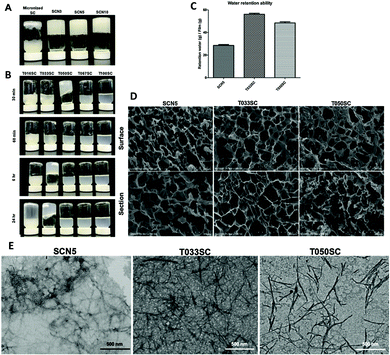 | ||
Fig. 4 (A) Evaluation of the gel-forming properties by the vial-inversion method for 2% (w/v) mechanically disintegrated SCNFs obtained by passing through a NanoLyzerat 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi for three, five, and 10 cycles (SCN3, SCN5, and SCN10). (B) TEMPO-oxidized SCNFs treated with various amounts of NaOCl (T016SC, T033SC, T050SC, T067SC, and T100SC). (C) Water-retention ability of 2% (w/v) lyophilized SCN5, T033SC, and T050SC. (D) SEM images of 2% (w/v) lyophilized SCN5, T033SC, and T050SC. (E) TEM images of SCN5, T033SC, and T050SC 2% suspensions. [Reproduced from ref. 19, with kind permission]. 000 psi for three, five, and 10 cycles (SCN3, SCN5, and SCN10). (B) TEMPO-oxidized SCNFs treated with various amounts of NaOCl (T016SC, T033SC, T050SC, T067SC, and T100SC). (C) Water-retention ability of 2% (w/v) lyophilized SCN5, T033SC, and T050SC. (D) SEM images of 2% (w/v) lyophilized SCN5, T033SC, and T050SC. (E) TEM images of SCN5, T033SC, and T050SC 2% suspensions. [Reproduced from ref. 19, with kind permission]. | ||
3. Perspective and conclusions
The study on the use of hybrid sol–gel catalyst SiliaCat TEMPO in the carboxylation of water-insoluble polysaccharide fibers affording cellulose or sacchachitin nanofibers suggests four main research and one educational outcomes of general relevance to catalysis and biochemistry researchers, as well as to scholars teaching green chemistry, nanochemistry and catalysis. One major outcome concerns also the chemical industry, particularly its paper segment.First, mesoporous solid catalysts can be used to selectively convert water-insoluble polymeric nanofibers by a reaction taking place between two solid reactants, namely the polysaccharide fibril surface and the immobilized TEMPO moieties. This was originally shown by Ariki's team who demonstrated that it is enough to have cellulose fibers with a 2–20 nm width to observe cellulose carboxylation over mesoporous silica-supported TEMPO.13 In the case of the ORMOSIL (organically modified silica) SiliaCat TEMPO catalyst, the organosilica sol–gel cages allow the access of the primary hydroxyl groups of the bound sugar units to the TEMPO radicals entrapped in the large and broadly distributed (8–30 nm) inner mesoporosity.21
Second, the fact that the original report in a 2012 patent13 of the heterogeneously catalyzed carboxylation remained virtually ignored until scholars in Canada five years later reported cellulose nanofiber carboxylation9 provides another relevant example of the need to increase knowledge of research reported in chemical patents amid academic and industry researchers. As unveiled by Di Renzo and co-workers,22 a similar outcome in materials research occurred with the discovery of mesoporous silica reported in a patent filed in 1969,23 which remained ignored until its rediscovery by industrial researchers in 1991.24
Third, contrary to silica which is unstable at alkaline pH, including at pH 10 typically used in the de Nooy's polysaccharide selective carboxylation,3 the organic modification of silica with the methyl groups in SiliaCat TEMPO dramatically reduces the number of silanol groups at the surface of unmodified SiO2, preventing dissolution in the alkaline solution and allowing prolonged reuse of the catalyst.25
Fourth, as shown by scholars in South Korea along with the enhanced chemical stability (leach-proof nature), the oxidation of cellulose nanofibrils mediated by SiliaCat TEMPO affords nanofibers of distinctly lesser polymeric degradation, and thus of higher quality.14 This fact shows evidence that also for polymeric molecules the use of the sol–gel entrapped catalyst improves the catalytic process when compared to the homogeneously catalyzed conversion. Besides enabling the one-pot conversion for both small and large molecules, in the case of a small alcohol substrate, the use of SiliaCat TEMPO allows significantly faster reaction rates.21 In the case of cellulose or sacchachitin nanofiber synthesis, the reaction is slower when compared to the reaction mediated by TEMPO in solution, but polymeric degradation and fiber width reduction are dramatically diminished.
An important educational outcome of this study for chemistry and catalysis educators engaged in advancing catalysis education26 is that both academic and industry researchers need to improve their knowledge of the “rigorously reviewed novel work”27 reported in chemical patents. The patent literature is openly accessible via the internet using numerous comprehensive patent databases (including Espacenet, Patentscope, Google Patents, Dimensions, etc.). Still, in mid-2018 the monthly magazine of the Royal Society of Chemistry in Great Britain hosted an article noting that research chemists continued “to overlook patents as sources of chemical information,… losing a valuable source of knowledge in terms of chemical purpose, completeness and a competitive intellectual property strategy”.28 Indeed, patents are a specific source of literature, and one has to have great experience in extracting reliable information from them. Compared to chemical knowledge shared in the international peer reviewed literature, however, the descriptions given in patents are often incomplete and difficult to understand because the legal professionals (and not the chemists) who write patents make them deliberately ambiguous (since patents need to protect, and not fully disclose, new chemical products and new chemical processes). On the other hand, data scientists have developed and made available to research chemists new digital tools to uncover chemical compounds hidden in patents,29 as well as new chemical conversions.
The final outcome of this study concerns the paper industry which finds nanocellulose as one of the key new products with which to compensate the dramatic fall in paper demand following the advent of the internet.30 As mentioned in the introduction, a few years ago a large paper company in Japan was among the first to commercialize CNFs manufactured via the homogeneously catalyzed process using TEMPO. In detail, since 2017, the company operates the large-scale production of nano-dispersed CNFs of uniform fiber width (3–4 nm) from wood pulp chemically fibrillated and oxidised with NaOCl in the presence of catalytic amounts of TEMPO and bromide at two new CNF production facilities with a 560 t a-1 overall capacity.31 From effective antibacterial and deodorant sheets through mouth gels and enhanced tires, the Cellenpia ingredient resulting from CNF production is included in several high-value-added products.32
As shown by Ariki with silica-supported TEMPO,13 by Renneckar using Karimi's catalyst,9 and by researchers at the aforementioned large household and personal care company using SiliaCat TEMPO,14 companies willing to manufacture CNFs via the TEMPO-mediated oxidation followed by homogenisation can now switch to the one-pot heterogeneously catalyzed process. At the end of the reaction, residual hypochlorite is quenched with 0.3% ascorbic acid (ascorbic acid is oxidized to dehydroascorbic acid and hypochlorite is reduced to chloride), CNF is readily separated from the solid catalyst via simple filtration, and the hybrid sol–gel catalyst is reused in a subsequent oxidation run.14 Eventually, as further shown by scholars based in Taiwan,15 the use of mechanically robust and easy to handle SiliaCat TEMPO sol–gel glass will streamline the carboxylation process preliminary to the formation of polysaccharide nanofibers, including that of sacchachitin nanofibers, holding great applicative potential in biomedicine.19,20 This, in conclusion, will dramatically reduce the polysaccharide nanofiber production costs opening the route to mass-scale production and uptake in a variety of products where the use of these versatile nanofibers has been limited by their high cost.4
Funding information
The authors did not receive any funds for this study.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank Professor David Avnir (The Hebrew University of Jerusalem), Dr Valerica Pandarus and Dr François Béland (SiliCycle, Canada), and Professors Laura M. Ilharco and Alexandra Fidalgo (Universidade de Lisboa, Instituto Superior Técnico) for many years of research collaboration on catalytic sol–gel materials.References
- A. Isogai and Y. Kato, Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation, Cellulose, 1998, 4, 153–164, DOI:10.1023/A:1009208603673.
- T. Saito, Y. Nishiyama, J.-L. Putaux, M. Vignon and A. Isogai, Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose, Biomacromolecules, 2006, 7, 1687–1691, DOI:10.1021/bm060154s.
- In the early 1990s, de Nooy was the first to adapt the Anelli-Montanari biphasic oxidation of alcohols to carbonyls to the selective oxidation of polysaccharide primary alcohol groups to carboxylates in water only. For an account, see: A. E. de Nooy, A. C. Besemer and H. van Bekkum, Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans, Carbohydr. Res., 1995, 269, 89–98, DOI:10.1016/0008-6215(94)00343-E.
- M. Pagliaro, Cellulose nanofiber: An advanced biomaterial soon to become ubiquitous, Chim. Oggi, 2018, 36(4), 61–62 Search PubMed.
- J. Jetten and A. Besemer, Process for the recovery of nitroxy compounds from organic solutions and oxidation process, US20050154206A1, 2005 Search PubMed.
- G. Szekely, M. C. Amores de Sousa, M. Gil, F. Castelo Ferreira and W. Heggie, Genotoxic impurities in pharmaceutical manufacturing: sources, regulations, and mitigation, Chem. Rev., 2015, 115, 8182–8229, DOI:10.1021/cr300095f.
- H. E. Strohmeyer and G. W. Sluggett, Determination and control of TEMPO, a potentially genotoxic free radical reagent used in the synthesis of filibuvir, J. Pharm. Biomed. Anal., 2012, 62, 216–219, DOI:10.1016/j.jpba.2011.12.036.
- B. Karimi and E. Farhangi, A highly recyclable magnetic core-shell nanoparticle-supported TEMPO catalyst for efficient metal- and halogen-free aerobic oxidation of alcohols in water, Chem. – Eur. J., 2011, 17, 6056–6060, DOI:10.1002/chem.201100047.
- S. C. Patankar and S. Renneckar, Greener synthesis of nanofibrillated cellulose using magnetically separable TEMPO nanocatalyst, Green Chem., 2017, 19, 4792–4797, 10.1039/c7gc02383a.
- A. Michaud, G. Gingras, M. Morin, F. Béland, R. Ciriminna, D. Avnir and M. Pagliaro, SiliaCat TEMPO: an effective and useful oxidizing catalyst, Org. Process Res. Dev., 2007, 11, 766–768, DOI:10.1021/op700050j.
- M. Pagliaro, Advancing nanochemistry education, Chem. – Eur. J., 2015, 21, 11931–11936, DOI:10.1002/chem.201501042.
- M. Pagliaro, Chemistry education fostering creativity in the digital era, Isr. J. Chem., 2019, 59, 565–571, DOI:10.1002/ijch.201800179.
- J. Araki, M. Tsukahara, K. Iwamoto and K. Okada, Surface modification method of polysaccharide solid, JP2013177569A, 2013 Search PubMed.
- S.-H. Jun, S.-G. Park and N.-G. Kang, One-pot method of synthesizing TEMPO-oxidized bacterial cellulose nanofibers using immobilized TEMPO for skincare applications, Polymers, 2019, 11, 1044, DOI:10.3390/polym11061044.
- Y.-H. Tsao and L.-C. Chen, Development and characterization of SiliaCat-TEMPO-oxidized sacchachitin, 2021 Across the Taiwan Strait and International Student Academic Conference, Yuanpei University Institutional Repository http://120.106.195.12/handle/310904600Q/18994.
- J. Araki and I. Maiko, Surface carboxylation of cellulose nanowhiskers using mPEG-TEMPO: its recovery and recycling, Polym. J., 2016, 48, 1029–1033, DOI:10.1038/pj.2016.65.
- J. E. Song, S.-H. Jun, S.-G. Park and N.-G. Kang, A semi-dissolving microneedle patch incorporating TEMPO-oxidized bacterial cellulose nanofibers for enhanced transdermal delivery, Polymers, 2020, 12, 1873, DOI:10.3390/polym12091873.
- C.-H. Su, C.-S. Sun, S.-W. Juan, C.-H. Hu, W.-T. Ke and M.-T. Sheu, Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes, Biomaterials, 1997, 18, 1169–1174, DOI:10.1016/S0142-9612(97)00048-3.
- F.-C. Chao, M.-H. Wu, L.-C. Chen, H.-L. Lin, D.-Z. Liu, H.-O. Ho and M.-T. Sheu, Preparation and characterization of chemically TEMPO-oxidized and mechanically disintegrated sacchachitin nanofibers (SCNF) for enhanced diabetic wound healing, Carbohydr. Polym., 2020, 229, 115507, DOI:10.1016/j.carbpol.2019.115507.
- M.-H. Wu, W. Wang, F.-C. Chao, C.-M. Hsieh, L.-C. Chen, H.-L. Lin, H.-O. Ho, T.-J. Huang and M.-T. Sheu, One-pot fabrication of sacchachitin for production of TEMPO-oxidized sacchachitin nanofibers (TOSCNFs) utilized as scaffolds to enhance bone regeneration, Carbohydr. Polym., 2021, 254, 117270, DOI:10.1016/j.carbpol.2020.117270.
- R. Ciriminna, C. Bolm, T. Fey and M. Pagliaro, Sol-Gel ormosils doped with TEMPO as recyclable catalysts for the selective oxidation of alcohols, Adv. Synth. Catal., 2002, 344, 159–163, DOI:10.1002/1615-4169(200202)344:2%3C159::AID-ADSC159%3E3.0.CO;2-Q.
- F. Di Renzo, H. Cambon and R. Dutartre, A 28-year-old synthesis of micelle-templated mesoporous silica, Microporous Mater., 1997, 10, 283–286, DOI:10.1016/S0927-6513(97)00028-X.
- V. Chiola, J. E. Ritsko and C. D. Vanderpool, Process for producing low-bulk density silica, US3556725A, 1971 Search PubMed.
- J. S. Beck, C. T.-W. Chu, I. D. Johnson, C. T. Kresge, M. E. Leonowicz, W. J. Roth and J. W. Vartuli, Synthetic porous crystalline material: its synthesis and use, WO1991011390, 1991 Search PubMed.
- A. Fidalgo, R. Ciriminna, L. M. Ilharco and M. Pagliaro, Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol-gel catalysts, Chem. Mater., 2005, 17, 6686–6694, DOI:10.1021/cm051954x.
- M. Pagliaro, «Catalysis: A unified approach»: A new course in catalysis science and technology, J. Flow Chem., 2021, 11, 53–58, DOI:10.1007/s41981-020-00100-x.
- D. Weaver and C. Barden, Don't overlook the rigorously reviewed novel work in patents, Nature, 2009, 461, 340, DOI:10.1038/461340b.
- PatSnap, The dark side of chemical patents, Chemistry World, 14 May 2018. https://www.chemistryworld.com/sponsored-content/the-dark-side-of-chemical-patents/3008998.article (last accessed on June 28, 2021).
- L. Goodchild van Hilten, How data scientists are uncovering chemical compounds hidden in patents, Elsevier Connect, November 28, 2019. https://www.elsevier.com/connect/how-data-scientists-are-uncovering-chemical-compounds-hidden-in-patents (last accessed on June 28, 2021).
- G. Latta, A. J. Plantiga and M. R. Sloggy, The effects of Internet use on global demand for paper products, J. For., 2016, 114, 433–440, DOI:10.5849/jof.15-096.
- Nippon Paper Group, Cellulose nanofiber manufacturing technology and application development, 2018. https://www.nipponpapergroup.com/english/research/organize/cnf.html (last accessed on June 28, 2021).
- Nippon Paper Group, Products that contribute to building a sustainable society – the Cellenpia® series, 2020. https://www.nipponpapergroup.com/csr/npg_csrr2020_e_CNF.pdf (last accessed on June 28, 2021).
| This journal is © The Royal Society of Chemistry 2021 |
