Controlled O2 reduction at a mixed-valent (II,I) Cu2S core†
Jordan
Mangue
a,
Clément
Gondre
a,
Jacques
Pécaut
b,
Carole
Duboc
 c,
Stéphane
Ménage
c,
Stéphane
Ménage
 a and
Stéphane
Torelli
a and
Stéphane
Torelli
 *a
*a
aUniv. Grenoble Alpes, CNRS, CEA, IRIG, Laboratoire de Chimie et Biologie des Métaux, 17 rue des Martyrs, 38054 Grenoble Cedex 9, France. E-mail: stephane.torelli@cea.fr
bUniv. Grenoble Alpes, CEA, CNRS, IRIG, SYMMES, UMR 5819 Equipe Chimie Interface Biologie pour l’Environnement, la Santé et la Toxicologie, 38054 Grenoble Cedex 9, France
cUniv. Grenoble Alpes, Département de Chimie Moléculaire, 301 rue de la chimie, 38054 Grenoble Cedex 9, France
First published on 16th July 2020
Abstract
Inspection of Oxygen Reduction Reactions (ORRs) using a mixed-valent Cu2S complex as a pre-catalyst revealed a tuneable H2O2vs. H2O production under mild conditions by controlling the amount of sacrificial reducer. The fully reduced bisCuI state is the main active species in solution, with fast kinetics. This new catalytic system is robust for H2O2 production with several cycles achieved and opens up perspectives for integration into devices.
With the increase in the world population and the shrinkage of unsustainable fossil fuels that we depend on, there is a crucial need to explore carbon-free alternatives to ensure a safe and sustainable future. In line with this, the so-called Oxygen Reduction Reactions (ORRs) are important processes in fuel cell technology1 for achieving a hydrogen-based society.2 However, this cathodic event remains the limiting step regarding the efficiency of a complete device.3 ORRs find their essence in Nature with biological respiration4 including laccases that catalyse the 4e−/4H+ reduction of O2 to H2O.5 The other important product formed upon O2 reduction is hydrogen peroxide (H2O2) via a 2e−/2H+ process. H2O2 is important for living organisms where it is, for instance, biosynthesized by the immune system to kill microbes,6 a signal molecule under oxidative stress conditions7 or used for metabolic purposes by copper metalloenzymes such as galactose oxidase.5b It is a staple in the industry with a ranking in the top 100 most important reactants with more than 3 million tons produced per year8 and is widely used in pharmaceuticals, cosmetics and electronics.9 H2O2 has recently emerged as a potent latent energy carrier through its O–O bond (Gibbs free energy of formation of ΔG0f = −120 kJ mol−1 from H2 and O2) and is thus a suitable candidate for energy storage for fuel cell technology.10 However, given that its production mainly relies on the energy-consuming and precarious anthraquinone process,11 new eco-friendly methodologies for controlled H2O2 production from O2 reduction are yet to be discovered.
The conception of efficient catalysts for homogeneous ORRs based on noble and also non-noble metal ions such as Fe, Co or Mn has stimulated intensive research.12 In these cases, the electron source for the catalytic activity comes from sacrificial reducers such as metallocenes or via electrocatalysis. With respect to homogenous Cu-based catalysts, mono-,13 di-14 and trinuclear15 copper complexes have been studied and relevant activities reported. Interestingly, the presence of a Lewis acid (Sc3+) was shown to induce selective catalysis for two-electron O2 reduction.16 Under heterogeneous conditions, immobilized Cu complexes are particularly efficient for O2 reduction into H2O.17
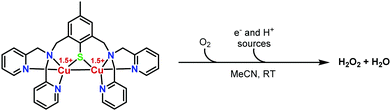
With the aim of targeting ORRs with original di-copper systems, we report here the activity of our previously described mixed-valent (MV) copper complex 118 possessing a N6Cu2S environment (Fig. 1). We evidenced a tuneable chemical H2O2vs. H2O selectivity in acetonitrile using controlled amounts of ferrocene derivatives as electron sources and in the presence of an organic acid. This study represents, to the best of our knowledge, the first example of a selective ORR involving a Cu2S core under homogeneous and mild conditions.
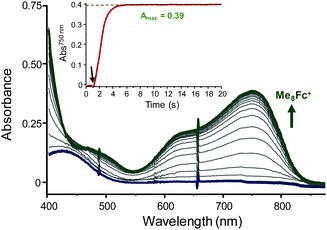
Catalytic ORRs by 1 (0.05 mM, final concentration) were evaluated at room temperature (298 K) in air-saturated MeCN solutions. 2,6-Lutidinium tetrafluoroborate (LutHBF4, 400 molar equiv.) was used as an innocent proton donor (weak coordinating ability of both the conjugate base and the BF4− counter-anion) and sacrificial electrons (10 to 100 molar equiv.) were provided by means of ferrocene (Fc, E1/2 Fc+/0 = 0 V vs. Fc+/0), dimethylferrocene (Me2Fc, E1/2 Me2Fc+/0 = −0.10 V vs. Fc+/0), octamethylferrocene (Me8Fc, E1/2 Me8Fc+/0 = −0.42 V vs. Fc+/0) or decamethylferrocene (Me10Fc, E1/2 Me10Fc+/0 = −0.49 V vs. Fc+/0). Fc and Me2Fc were not compatible with the in situ reduction of any redox form of 1 (E1/21 = −0.44 V vs. Fc+/0, ΔE = 0.07 V, Cu2I,II → Cu2I,I; E1/22 = −0.30 V vs. Fc+/0, ΔE = 0.08 V, Cu2I,II → Cu2II,II),18 whereas Me8Fc and Me10Fc were suitable for these processes. Monitoring the reaction by UV-Visible (UV-vis) spectrophotometry unambiguously showed the formation of Fc+, Me2Fc+, Me8Fc+ or Me10Fc+ at 614 nm (ε = 410 M−1 cm−1), 650 nm (ε = 290 M−1 cm−1), 750 nm (ε = 390 M−1 cm−1) and 778 nm (ε = 495 M−1 cm−1), respectively (Fig. S1–S8 and Table S1, ESI†) and attested to the O2 reduction in all cases. Interestingly, only 2 molar eq. of Fc+ and 30 molar eq. of Me2Fc+ (Fig. S3 and S4, ESI†) were detected when going up to 100 molar eq., whereas the maximum possible turnover numbers (TONs) are reached with Me8Fc and Me10Fc (denoted as Me8–10Fc when compared in the following) regardless of the excess (Fig. 2 and Fig. S5–S8, ESI†).
Quantitative Me8–10Fc+ formation allowed the use of the H2O2-specific TiO-type procedure (Fig. S9, ESI†) in order to discriminate between H2O2 and H2O production.19 This method is extremely precise compared to iodine titration. H2O production was calculated considering the amount of Me8–10Fc+ not involved in the H2O2 formation (see the ESI† for more details).
A key result is the change in selectivity, H2O2vs. H2O, observed by varying the amount of Me8–10Fc (Table 1): almost exclusive H2O2 formation occurs with 10 molar eq., whereas going to 100 molar eq. mainly leads to H2O production (entries 1 and 6, Table 1). The selectivity is moderately affected by either the kinetics or the difference in the reducing abilities of Me8–10Fc (ΔE1/2 = 0.07 V). As control experiments, no O2 or H2O2 reduction by Me8–10Fc occurs under the same conditions (and reaction times) in the absence of 1 (Fig. S10, ESI†). This strongly supports the H2O formation process originating from a catalysed two-electron reduction of a coordinated (hydro)peroxide at high Me8–10Fc concentrations. The inactivity of low-valent [Cu(Tol)2](OTf) (OTf = trifluoromethanesulfonate anion and Tol = toluene) finally reinforces the remarkable effect of the S/N coordination spheres in 1 on the reactivity and excludes solvated CuI ions as activators.
| Entry | [Me8–10Fc] (mM) | Cat/e−/H+ | % H2O2 Me8Fc/Me10Fc | % H2O Me8Fc/Me10Fc | TON | TONmax | k obs (s−1) Me8Fc/Me10Fc | t (s) Me8Fc/Me10Fc |
|---|---|---|---|---|---|---|---|---|
| a See the ESI for further experimental details. | ||||||||
| 1 | 0.5 | 1/10/400 | 90/82 | 10/18 | 10 | 10 | 1.06 ± 0.02/0.47 ± 0.02 | 4.1 ± 0.2/5.6 ± 0.3 |
| 2 | 1.0 | 1/20/400 | 83/72 | 17/28 | 20 | 20 | 0.61 ± 0.02/0.39 ± 0.02 | 6.5 ± 0.3/5.9 ± 0.3 |
| 3 | 2.0 | 1/40/400 | 57/58 | 43/42 | 40 | 40 | 0.28 ± 0.04/0.52 ± 0.03 | 15.0 ± 0.4/6.0 ± 0.5 |
| 4 | 3.0 | 1/60/400 | 51/37 | 49/63 | 60 | 60 | 0.13 ± 0.01/0.62 ± 0.04 | 28.1 ± 0.5/5.9 ± 0.3 |
| 5 | 4.0 | 1/80/400 | 38/10 | 62/90 | 80 | 80 | 0.15 ± 0.01/0.60 ± 0.03 | 32.1 ± 1/6.2 ± 0.3 |
| 6 | 5.0 | 1/100/400 | 10/5 | 90/95 | 100 | 100 | 0.12 ± 0.01/0.41 ± 0.02 | 41.2 ± 2/10.6 ± 0.4 |
Kinetically speaking, the fact that the reactions with Fc and Me2Fc are relatively slow and not complete compared to those with Me8–10Fc (Table 1 and Table S2, ESI†) suggests that 1 or its protonated form 1H (see below for the behaviour of 1 with LutHBF4) could initiate the reaction. However, its rather modest efficiency indicates that MV states cannot be considered as the most active forms. This also demonstrates that the redox potentials of the oxidized species generated along the reaction course are not (or partially) thermodynamically compatible with the reducing abilities of Fc and Me2Fc to reach the total consumption of the electron source.
Consequently, from now on, only the case of Me8–10Fc is discussed since quantitative and fast conversions were obtained. For 10 and 20 molar eq., the kinetic traces (Fig. S5 and S7, ESI†) display pseudo-first-order profiles and comparable reaction times. Starting with 40 molar eq. and above, the reaction times are significantly shorter with Me10Fc compared to Me8Fc, for which successive steps are identifiable (Fig. S5–S8, ESI†). The overall comparison of the kinetics traces clearly indicates the involvement of more active species when using Me10Fc compared to Me8Fc. It is worth noting that the kobs values are inversely proportional or quasi-independent with respect to [Me8Fc] (to a certain extent) or [Me10Fc], respectively (Fig. S11, ESI†). This suggests that the gradual accumulation of H2O results in competitive reaction pathways for Me8Fc (with a global steady state starting from 60 molar eq.) that are not present for Me10Fc (its consumption not being the rate-determining step).
This new catalytic system has proven to be robust for H2O2 production, especially under the most favourable conditions. For instance, at least four consecutive cycles were achieved with successive addition of 10 molar eq. Me8Fc and its quantitative consumption after each injection (Fig. S12, ESI†). An overall selectivity of 85% in H2O2, similar to that observed for a single run, was determined. This result demonstrates that H2O2 accumulation neither (i) affects the selectivity/efficiency nor (ii) poisons the catalyst.
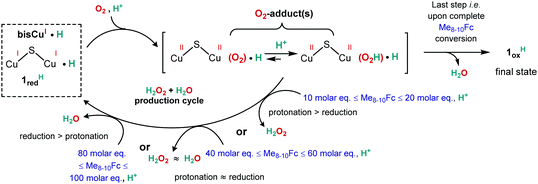
The behaviour of 1 in the presence of Me8–10Fc and LutHBF4 prior to exposure to air was then investigated in order to gain insights into the nature of the putative relevant copper species involved during the O2 reduction process. For solubility reasons, as the concentrations required to perform such experiments are different from those used for catalysis, only one condition (i.e. 10 molar eq. Me8–10Fc and 400 molar eq. LutBF4) was tested. Since identical results were obtained whatever the nature of the electron source, only the data obtained with Me8Fc are discussed. Under strict anaerobic conditions, the UV-vis/NIR spectrum of 1 is modified upon addition of LutHBF4 and/or Me8Fc (Fig. S13, ESI†). The presence of protons leads to the formation of a new 1H species with conservation of a dinuclear delocalized MV state (absorbance in the NIR region). As expected, Me8Fc in excess can reduce 1 to form 1red. Addition of both the electron and proton sources results in the formation of Me8Fc+, the loss of the MV signature of 1 and the generation of the reduced/protonated form 1redH. In this case, the spectrum of the final mixture can be adjusted by combining 10% of the remaining 1H, and 0.9 molar eq. Me8Fc+ and 9.1 molar eq. Me8Fc. 1redH should weakly contribute to the final spectrum due to the presence of d10 CuI ions. In the meantime, the EPR spectra showed modifications of the hyperfine when 1 is converted into 1H, followed by a decrease of the signal intensity consecutive to the reduction to 1redH (10% remaining intensity, Fig. S14, ESI†). An identical spectrum was obtained by adding a mixture of protons and electrons to a solution of 1. Finally, the low-valent state was trapped by ESI-MS under similar experimental conditions (Fig. S15, ESI†). All these experiments provide solid evidence for a mono-electronic reduction/protonation process of 1 into 1redHvia1H. Independently, exposure of a solution of 1redH to air leads to full Me8Fc consumption and the H2O2/H2O ratio is in line with that reported in Table 1 (entry 1). These results clearly indicate that low-valent 1redH is highly reactive and a key actor during catalysis.
O2 oxidation of 1 is also considered part of the catalytic event. The corresponding 1ox form was generated by exposing a MeCN solution of 1 to air. Its crystal structure shows the presence of a double-bridged di-copper(II) unit involving the S atom from the ligand and an additional hydroxyl anion (Fig. S16, ESI†). 1ox has a significantly different UV-vis/NIR spectrum compared to its precursor with the loss of the NIR band (Fig. S17, ESI†). The extinction of the EPR signal (>90%, Fig. S18, ESI†) is in agreement with a strong antiferromagnetic coupling between the two metal ions leading to the S = 0 ground state. Such magnetic behaviour was already observed with a similar scaffold20 or in the case of a phenolate spacer with identical Me(N,N-bis(methylpyridyl))amine pendant arms.21 The ESI-MS spectrum of 1ox (Fig. S19, ESI†) shows two prominent peaks at m/z = 344.3 (Δm/z = 0.5) and 839.3 (Δm/z = 1.0) consistent with the solved solid-state structure. Catalytic experiments with 1ox, 10 molar eq. Me8Fc and 400 molar eq. LutHBF4, lead to quantitative conversion within a reaction time scale close to that of 1 (kobs = 1.17 s−1, Fig. S20 in the ESI†). H2O2 titration indicates a selectivity similar to that of 1 (88/12 for H2O2/H2O). The activity of 1ox is in line with its facile protonation (to 1oxH) and reduction (Fig. S21, ESI†) under anaerobic conditions. Such species could thus be part of the reactivity.
These results suggest that the H2O2/H2O production cycle mainly relies on 1redH and allow the proposition of a reaction scheme (Scheme 1). 1redH is generated by reduction/protonation of 1 or alternatively by air oxidation/protonation/reduction of 1via1oxH (Fig. S22, ESI†). O2 activation at 1redH followed by protonation leads to the key O2-adduct(s) from which the selectivity can be explained by the inevitable competition between its protonation and/or reduction. Taking into account the composition of the mixture at the end of the reaction (the presence of O2, LutHBF4 and Me8Fc+), 1oxH is certainly the final fate of the catalyst, as observed by ESI-MS (Fig. S23, ESI†). This proposition is also reinforced by the ability of performing several cycles, necessarily through an oxidized species, with no loss in selectivity and efficiency. Nevertheless, 1oxH cannot be considered as a predominantly active species during catalysis at that point, since H2O would be the main product under any experimental condition.
To conclude, we demonstrate here that copper/sulphur assemblies such as 1 are very efficient for ORRs at room temperature with fast kinetics. By controlling the amount of Me8-10Fc, a significant and tuneable H2O2vs. H2O selectivity can be achieved. H2O accumulation results from peroxide ligation and reduction at an active species since H2O2 is not reduced in situ in the absence of 1. The reductive power of the reaction mixture is a factor that helps explain the selectivity. With rather “low excess” Me8-10Fc, the two-electron reduction into H2O2 is favoured. When the concentration of the electron source increases, a competition between H2O2 release and its subsequent reduction is set up and becomes gradually predominant. Independently, a similar study with 1ox will be of great interest to compare its reactivity with a related phenolate-bridged di-copper II complex, whose ability for the ORR was reported in 2012.14c The impact of the thiophenolate vs. phenolate moiety could be appreciated (at first approximations 1ox seems faster) and correlated to electronic properties. In a different light, even if H2O2 is quite an aggressive molecule in solution, the observation that several cycles can be performed with successive Me8Fc injection attests to the robustness of the system. These results are encouraging for further applications in heterogeneous catalysis upon grafting air stable 1ox whether onto an inert surface in the presence of an external electron source or on electroactive materials for electrocatalysis. Finally, a global reaction sequence for the activity of 1 and its derivatives is proposed and is now under dissection with complementary kinetic experiments and characterization of the pivotal O2-adduct(s).
This work was supported by the Labex ARCANE and the CBH-EUR-GS (ANR-17-EURE-0003) fund via the CNRS, CEA and the Grenoble-Alpes University.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Boudghene Stambouli and E. Traversa, Renewable Sustainable Energy Rev., 2002, 6, 295–304 CrossRef; (b) K. Mase, M. Yoneda, Y. Yamada and S. Fukuzumi, Nat. Commun., 2016, 7, 11470 CrossRef CAS PubMed; (c) Y. Isaka, S. Kato, D. Hong, T. Suenobu, Y. Yamada and S. Fukuzumi, J. Mater. Chem. A, 2015, 3, 12404–12412 RSC.
- W. Zhang, W. Lai and R. Cao, Chem. Rev., 2017, 117, 3717–3797 CrossRef CAS PubMed.
- O. Gröger, H. A. Gasteiger and J.-P. Suchsland, J. Electrochem. Soc., 2015, 162, A2605–A2622 CrossRef.
- G. T. Babcock and M. Wikström, Nature, 1992, 356, 301–309 CrossRef CAS PubMed.
- (a) E. I. Solomon, D. E. Heppner, E. M. Johnston, J. W. Ginsbach, J. Cirera, M. Qayyum, M. T. Kieber-Emmons, C. H. Kjaergaard, R. G. Hadt and L. Tian, Chem. Rev., 2014, 114, 3659–3853 CrossRef CAS PubMed; (b) E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Chem. Rev., 1996, 96, 2563–2605 CrossRef CAS PubMed.
- S. F. Erttmann and N. O. Gekara, Nat. Commun., 2019, 10, 3493 CrossRef PubMed.
- H. Sies, Redox Biol., 2017, 11, 613–619 CrossRef CAS PubMed.
- R. L. Myers, The most 100 Most Important Chemical Compounds, Greenwood Press, London, 2007 Search PubMed.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem. Int. Ed., 2006, 45, 6962–6984 CrossRef CAS PubMed.
- S. Fukuzumi, Y. Yamada and K. D. Karlin, Electrochim. Acta, 2012, 82, 493 CrossRef CAS PubMed.
- R. Kosydar, A. Drelinkiewicz and J. P. Ganhy, Catal. Lett., 2010, 139, 105–113 CrossRef CAS.
- (a) M. L. Pegis, C. F. Wise, D. J. Martin and J. M. Mayer, Chem. Rev., 2018, 118, 2340–2391 CrossRef CAS PubMed; (b) C. W. Machan, ACS Catal., 2020, 10, 2640–2655 CrossRef CAS; (c) P. T. Smith, Y. Kim, B. P. Benke, K. Kim and C. J. Chang, Angew. Chem. Int. Ed., 2020, 59, 4902–4907 CrossRef CAS PubMed; (d) Y.-M. Zhao, G.-Q. Yu, F.-F. Wang, P.-J. Wei and J.-G. Liu, Chem. – Eur. J., 2019, 25, 3726–3739 CrossRef CAS PubMed; (e) L. Wang, M. Gennari, F. G. Cantú Reinhard, J. Gutiérrez, A. Morozan, C. Philouze, S. Demeshko, V. Artero, F. Meyer, S. P. de Visser and C. Duboc, J. Am. Chem. Soc., 2019, 141, 8244–8253 CAS; (f) M. Gennari, D. Brazzolotto, J. Pécaut, M. V. Cherrier, C. J. Pollock, S. DeBeer, M. Retegan, D. A. Pantazis, F. Neese, M. Rouzières, R. Clérac and C. Duboc, J. Am. Chem. Soc., 2015, 137, 8644–8653 CrossRef CAS PubMed.
- (a) M. Langerman and D. G. H. Hetterscheid, Angew. Chem. Int. Ed., 2019, 58, 12974–12978 CrossRef CAS PubMed; (b) D. Das, Y.-M. Lee, K. Ohkubo, W. Nam, K. D. Karlin and S. Fukuzumi, J. Am. Chem. Soc., 2013, 135, 2825–2834 CrossRef CAS PubMed; (c) M. A. Thorseth, C. S. Letko, T. B. Rauchfuss and A. A. Gewirth, Inorg. Chem., 2011, 50, 6158–6162 CrossRef CAS PubMed; (d) S. Fukuzumi, H. Kotani, H. R. Lucas, K. Doi, T. Suenobu, R. L. Peterson and K. D. Karlin, J. Am. Chem. Soc., 2010, 132, 6874–6875 CrossRef CAS PubMed; (e) S. Kakuda, R. L. Peterson, K. Ohkubo, K. D. Karlin and S. Fukuzumi, J. Am. Chem. Soc., 2013, 135, 6513–6522 CrossRef CAS PubMed.
- (a) L. Tahsini, H. Kotani, Y.-M. Lee, J. Cho, W. Nam, K. D. Karlin and S. Fukuzumi, Chem. – Eur. J., 2012, 18, 1084–1093 CrossRef CAS PubMed; (b) C. Liu, H. Lei, Z. Zhang, F. Chen and R. Cao, Chem. Commun., 2017, 53, 3189–3192 RSC; (c) S. Fukuzumi, L. Tahsini, Y.-M. Lee, K. Ohkubo, W. Nam and K. D. Karlin, J. Am. Chem. Soc., 2012, 134, 7025–7035 CrossRef CAS PubMed.
- X. Engelmann, E. R. Farquhar, J. England and K. Ray, Inorg. Chim. Acta, 2018, 481, 159–165 CrossRef CAS.
- S. Kakuda, C. J. Rolle, K. Ohkubo, M. A. Siegler, K. D. Karlin and S. Fukuzumi, J. Am. Chem. Soc., 2015, 137, 3330–3337 CrossRef CAS PubMed.
- (a) S. Gentil, D. Serre, C. Philouze, M. Holzinger, F. Thomas and A. Le Goff, Angew. Chem. Int. Ed., 2016, 55, 2517–2520 CrossRef CAS PubMed; (b) M. S. Thorum, J. Yadav and A. A. Gewirth, Angew. Chem. Int. Ed., 2009, 48, 165–167 CrossRef CAS PubMed; (c) C. C. L. McCrory, A. Devadoss, X. Ottenwaelder, R. D. Lowe, T. D. P. Stack and C. E. D. Chidsey, J. Am. Chem. Soc., 2011, 133, 3696–3699 CrossRef CAS PubMed; (d) F.-F. Wang, Y.-M. Zhao, P.-J. Wei, Q.-L. Zhang and J.-G. Liu, Chem. Commun., 2017, 53, 1514–1517 RSC.
- S. Torelli, M. Orio, J. Pécaut, H. Jamet, L. Le Pape and S. Ménage, Angew. Chem. Int. Ed., 2010, 49, 8249–8252 CrossRef CAS PubMed.
- (a) C. Matsubara, N. Kawamoto and K. Takamura, Analyst, 1992, 117, 1781–1784 RSC; (b) K. Takamura, C. Matsubara and T. Matsumoto, Anal. Sci., 2008, 24, 401–404 CrossRef CAS PubMed.
- C. Esmieu, M. Orio, S. Torelli, L. Le Pape, J. Pecaut, C. Lebrun and S. Menage, Chem. Sci., 2014, 5, 4774–4784 RSC.
- (a) C. Belle, C. Beguin, I. Gautier-Luneau, S. Hamman, C. Philouze, J. L. Pierre, F. Thomas and S. Torelli, Inorg. Chem., 2002, 41, 479–491 CrossRef CAS PubMed; (b) S. Torelli, C. Belle, I. Gautier-Luneau, J. L. Pierre, E. Saint-Aman, J. M. Latour, L. Le Pape and D. Luneau, Inorg. Chem., 2000, 39, 3526–3536 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2014256. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc03987j |
| This journal is © The Royal Society of Chemistry 2020 |