A dual-ratiometric fluorescent probe for individual and continuous detection of H2S and HClO in living cells†
Xiuxiu
Yue‡
a,
Jingpei
Wang‡
a,
Jinliang
Han
a,
Benhua
Wang
 *a and
Xiangzhi
Song
*a and
Xiangzhi
Song
 *ab
*ab
aCollege of Chemistry & Chemical Engineering, Central South University, Changsha 410083, Hunan Province, China. E-mail: benhuawang@csu.edu.cn; song@rowland.harvard.edu
bKey Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, Changsha 410083, Hunan Province, China
First published on 30th January 2020
Abstract
HClO and H2S are crucial for maintaining the homeostasis in cells and play vital roles in many physiological and pathological processes. Herein, we present a fluorescent probe that can respectively and simultaneously detect H2S and HClO in a dual-ratiometric manner with good linearity. Utilizing this probe, the imaging of intracellular H2S or/and HClO in living cells in a ratiometric manner was achieved.
Hypochlorous acid (HClO), an important reactive oxygen species (ROS), possesses antibacterial activities and plays a vital role in killing pathogens and microorganisms.1 HClO can be endogenously produced from hydrogen peroxide and chloride ions with the catalyzation of myeloperoxidase enzyme (MPO) in leukocytes.2 A high level of intracellular HClO can cause serious tissue damage to induce a series of diseases, such as neurodegenerative diseases, atherosclerosis, cancer, ischemia, reperfusion injury, rheumatoid rheumatism, and so on.3 Hydrogen sulfide (H2S), one of the important gaseous signaling molecules in organs and cells, has a prominent function in the human nervous system.4 For example, it is validated that the roles of H2S in cardiovascular protection, vasodilation, cell growth, and neuromodulation are very important.5 As a consequence, aberrant levels of H2S can lead to gastric mucosal injury, liver cirrhosis, Alzheimer's disease and other diseases.6
H2S and HClO have interplaying roles in many important physiological processes and are important mediators in brain function.7 It is found that HClO causes extensive oxidative stress and oxidative damage in human neurodegenerative diseases.8In vivo, as a reducing agent, H2S serves as a specific HClO inhibitor for oxidative stress, cytotoxicity, protein oxidation and lipid peroxidation.7a,8,9 Therefore, the respective and continuous detection of H2S and HClO can help understand the interplay and cross-talk of these two species in cells.
Fluorescence techniques offer an attractive tool to monitor biological species with high spatial and temporal resolution in a noninvasive manner in vivo.10 In the past decade, many efforts have been focused on the development of fluorescent probes to individually detect HClO or H2S.11 To date, to the best of our knowledge, there are only two fluorescent probes reported to simultaneously detect HClO and H2S,11e,12 both of which work in a turn-on manner. It is known that the ratiometric mode in fluorescence sensing is more desirable than the turn-on type due to the little interference from the background, minimized influences from instrumental factors, the concentration of the probe and other environmental factors.13 Therefore, it is of great importance and a big challenge to develop a single-molecule fluorescent probe that can simultaneously detect HClO and H2S in a ratiometric manner.
Herein, a novel dual-ratiometric fluorescent probe, Han-HClO-H2S, was designed and synthesized for the discriminative detection of HClO and H2S respectively and continuously with good linearity (Scheme 1). Probe Han-HClO-H2S was obtained in five steps, as illustrated in Scheme S1, ESI.† As shown in Scheme 1, probe Han-HClO-H2S holds two reaction sites: (1) the phenothiazine moiety is sensitive to HClO; (2) the azide group serves as a recognition group for H2S. Probe Han-HClO-H2S was expected to fluoresce in the red spectral region. We hypothesized that the presence of HClO would oxidize the phenothiazine moiety in probe Han-HClO-H2S to largely decrease its electron-donating ability and thereby result in a fluorescence change from red to green, and ratiometric detection could be realized. With regard to H2S, the azide group is expected to be reduced to form a blue fluorescent coumarin, while retaining the red-emitting phenothiazine coumarin, which can also afford a ratiometric fluorescence change to determine H2S. If probe Han-HClO-H2S is treated with H2S and HClO continuously, we expected that both recognition sites would be triggered to offer a mixed green-blue fluorescence. All in all, probe Han-HClO-H2S would sense H2S or HClO and both through distinct fluorescence signal combinations.
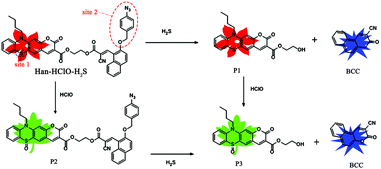 | ||
| Scheme 1 The proposed sensing mechanisms of probe Han-HClO-H2S for HClO, H2S and HClO/H2S with different fluorescence readouts. | ||
Using a single molecule to sense two analytes respectively and simultaneously in a ratiometric manner, two specific reaction sites are at least needed. First, phenothiazine-coumarin was used as the fluorophore because of its red emission, fairly high fluorescence quantum yield, large Stokes shift and good photo-stability.14 The sulfur atom (site 1) of phenothiazine-coumarin can be specifically oxidized by HClO to generate a green fluorescent coumarin. Second, we armed a precursor of blue-emitting coumarin with a H2S-specific azide group (site 2). Once the azide group was reduced by H2S, a blue-emitting coumarin BCC would be formed (Scheme 1). Finally, we conjugated these two above-mentioned moieties through an ester bond to obtain probe Han-HClO-H2S, which would exhibit three kinds of ratiometric fluorescence changes for H2S or HClO and both: red to red-blue for H2S, red to green for HClO and red to green-blue for H2S + HClO.
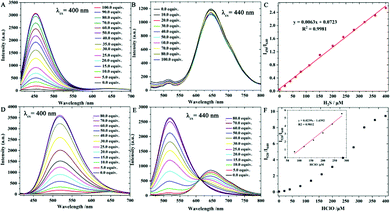
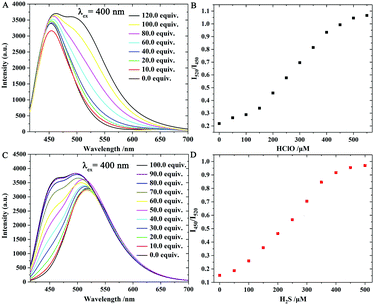
With probe Han-HClO-H2S in hand, we first investigated its optical properties toward H2S and HClO in PBS buffer (10.0 mM, pH = 7.4) containing 50% acetonitrile, respectively. Probe Han-HClO-H2S exhibited a main visible absorption band centred at 450 nm (Fig. S1, ESI†). The addition of H2S to the solution of probe Han-HClO-H2S caused negligible absorption changes. In contrast, the addition of HClO to the solution of probe Han-HClO-H2S blue-shifted the 450 nm band to 380 nm. Next, we turned our attention to the fluorescence behaviours of probe Han-HClO-H2S in response to H2S and HClO (Fig. 1). Probe Han-HClO-H2S exhibited a red fluorescence with λmax at 640 nm being excited at 440 nm. After the addition of H2S to the solution of probe Han-HClO-H2S, besides the stable red fluorescence, we observed a strong blue fluorescence with λmax at 450 nm (λex = 400 nm). Qualitatively, the fluorescence changes of probe Han-HClO-H2S toward H2S and HClO were in accordance with our expectation. To our delight, the red fluorescence signals exhibited an unchanged intensity before and after the addition of H2S and could serve as a built-in reference for the ratiometric detection. It was seen that the fluorescence intensity ratio at 450 nm and 640 nm (I450/I640) was proportional to the added concentration of H2S (0.0–400.0 μM) (Fig. 1C), and the limit of detection was 26 nM based on signal to noise = 3. Under excitation at 440 nm, the fluorescence of probe Han-HClO-H2S displayed a green fluorescence with λmax at 520 nm in response to HClO. The fluorescence intensity ratio at 520 nm and 640 nm (I520/I640) showed a good linear relationship with the concentration of HClO in the range of 50.0–300.0 μM with a 17 nM limit of detection (Fig. 1F). These optical results indicated that probe Han-HClO-H2S could sensitively and quantitatively respond to H2S and HClO with different fluorescence signal patterns in ratiometric manners, respectively.
Next, the fluorescence responses of probe Han-HClO-H2S toward both H2S and HClO were investigated (Fig. 2). First, probe Han-HClO-H2S was treated with 100.0 equiv. of H2S and then incubated with different concentrations of HClO. The addition of HClO induced a fluorescence enhancement at 520 nm, while the emission intensity at 450 nm remained unchanged, and the ratio (I520/I450) versus the concentration of HClO (100.0–450.0 μM) exhibited a good linear relationship. Meanwhile, we observed a fluorescence change from red to red-blue and to green-blue during the successive addition of H2S and HClO. In a reverse addition order (HClO first, followed by H2S), the further addition of H2S in the presence of HClO triggered an obvious fluorescence enhancement at 450 nm, and we were very pleased that the ratio of I450/I520 presented a linear-relationship with the concentration of H2S (50.0–400.0 μM). The solution of probe Han-HClO-H2S underwent a fluorescence color change from red to green and to green-blue. Therefore, probe Han-HClO-H2S could continuously and quantitatively detect H2S and HClO in dual-ratiometric manners, and the detection was not affected by the existence of each other.
The above optical studies were in good agreement with our proposed sensing mechanism. In order to obtain solid support for the sensing mechanism, compounds 3 and 5, the derivatives of the two main segments in probe Han-HClO-H2S, were prepared and their absorption and emission spectra in the absence/presence of H2S and HClO were determined, respectively (Fig. S2 and S3, ESI†). Compound 3 absorbed at 330 nm and was nearly non-fluorescent. In the presence of H2S, compound 3 displayed an emission at 450 nm with negligible absorption change. Compound 5 had an intense absorption band at 440 nm and fluoresced at 640 nm. When treated with HClO, compound 5 exhibited an absorption at 385 nm and strong fluorescence with a maximum at 520 nm. These results matched the optical behaviors of probe Han-HClO-H2S in response to H2S or HClO and both very well. Moreover, mass spectral analysis was carried out to elucidate the mechanism (Figs. S21–23, ESI†). As shown in Fig. S21 (ESI†), the mass spectrum of the reaction mixture of probe Han-HClO-H2S with HClO had a peak at 802.1869, which was assigned to the corresponding product P2 (Scheme 1). In the spectrum of probe Han-HClO-H2S with H2S, the expected mass peaks (434.1011 and 248.0914) for the proposed reaction products P1 and BCC were found (Fig. S22, ESI†). What is more, the mixture of probe Han-HClO-H2S with continuous addition of H2S and HClO gave two mass peaks at m/z 450.0944 and 248.0914, corresponding to P3 and BCC regardless of the addition order (Fig. S23, ESI†). The mass spectral analysis and the optical studies forcefully supported our proposed reaction mechanisms (Scheme 1).
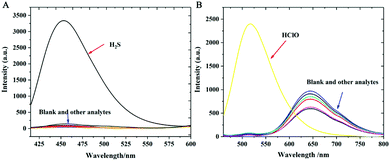
To verify its selectivity, we studied the optical responses of probe Han-HClO-H2S toward various biologically relevant species including NO, ONOO−, ˙OH, 1O2, O2−, ROO−, t-BuO˙, Cys, Hcy, GSH, Na2S2O3, Na2S2O5, NaCl, NaNO2, SCN−, Na2SO3, KI, Na2CO3, NaF, ZnCl2, Na2SO4, NaHCO3 and H2S2. Only H2S induced a remarkable fluorescence enhancement at 450 nm (Fig. 3A), whereas the fluorescence aroused by other interfering analytes was negligible even by extending the incubation time up to 100 min. Likewise, only HClO resulted in a strong fluorescence enhancement at 520 nm and a fluorescence decline at 640 nm (Fig. 3B). As expected, negligible fluorescence changes could be observed for other test species. As a result, it could be concluded that Han-HClO-H2S displayed good selectivity to H2S and HClO.
In addition, the response kinetic profiles of probe Han-HClO-H2S toward H2S and HClO were investigated (Fig. S5, ESI†). The addition of HClO to the solution of probe Han-HClO-H2S induced a dramatic enhancement in the fluorescence ratio (I520/I640) within seconds. In the presence of H2S, the fluorescence intensity ratio of I450/I640 gradually increased and reached a plateau within about 70 min. As a control, the probe itself showed no fluorescence changes during the same time period.
In order to investigate whether Han-HClO-H2S has the ability to sense H2S and HClO under physiological conditions, we then investigated the effect of pH on its fluorescence performance. The fluorescence of free probe Han-HClO-H2S remained unchanged within a wide pH range (Fig. S6, ESI†). In the presence of HClO or H2S, the probe showed a striking enhancement in fluorescence ratio (I520/I640) or (I450/I640) within pH from 5.0 to 9.0, indicating that this probe was stable and could be used in physiological environments.
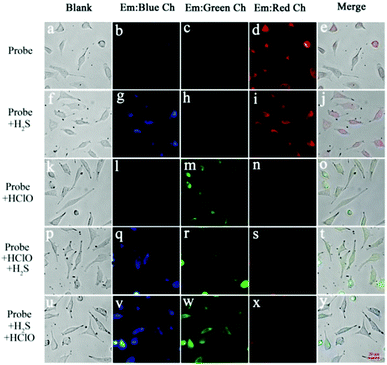
The attractive properties of probe Han-HClO-H2S in solution encouraged us to explore its capability to image the intracellular H2S and HClO in cells. MTT assay on MCF-7 cells with probe Han-HClO-H2S indicated that it had a low cytotoxicity (Fig. S7, ESI†). Three channels (blue, green and red) were used to detect H2S and HClO in living cells. Cells treated with probe Han-HClO-H2S gave off strong fluorescence in the red channel (Fig. 4d). When cells were pretreated with probe Han-HClO-H2S and then incubated with H2S, strong fluorescence signals were observed from both the blue and red channels (Fig. 4g and i). The incubation of probe Han-HClO-H2S with HClO induced a striking fluorescence in the green channel (Fig. 4m). We then further tested the fluorescence behavior of probe Han-HClO-H2S with the continuous addition of H2S and HClO in different orders in living cells. Probe-loaded cells were incubated with HClO for 20 min, washed with PBS buffer, and further treated with H2S for another 1 h. As expected, intense fluorescence signals appeared from both the blue and green channels (Fig. 4q and r). When we changed the addition order of the two analytes, similar results could be seen (Fig. 4v and w). The above results demonstrated that probe Han-HClO-H2S could monitor intracellular H2S or HClO and both through three different channels without interference from each other.
In summary, a fluorescent probe was developed to individually and continuously detect HClO or H2S and both for the first time in a ratiometric mode with high selectivity and sensitivity. In solution, this probe can qualitatively and quantitatively detect both H2S and HClO regardless of the addition order. Moreover, this probe was successfully applied to sense H2S or HClO and both in living cells with distinct fluorescence colors. The rational design strategy in this work holds potential to inspire the development of more powerful dual-response fluorescent probes for H2S/HClO and other biological molecules.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) P. Jia, Z. Zhuang, C. Liu, Z. Wang, Q. Duan, Z. Li, H. Zhu, B. Du, B. Zhu, W. Sheng and B. Kang, Anal. Chim. Acta, 2019, 1052, 131–136 CrossRef CAS PubMed; (b) Z. M. Prokopowicz, F. Arce, R. Biedron, C. L. L. Chiang, M. Ciszek, D. R. Katz, M. Nowakowska, S. Zapotoczny, J. Marcinkiewicz and B. M. Chain, J. Immunol., 2010, 184, 824–835 CrossRef CAS PubMed.
- (a) T. Henrique de Araujo, S. S. Okada, E. E. B. Ghosn, N. N. Taniwaki, M. R. Rodrigues, S. Rogerio de Almeida, R. A. Mortara, M. Russo, A. Campa and R. C. Albuquerque, Cell. Immunol., 2013, 281, 27–30 CrossRef CAS PubMed; (b) D. I. Pattison, C. L. Hawkins and M. J. Davies, Chem. Res. Toxicol., 2003, 16, 439–449 Search PubMed.
- (a) C. L. Hawkins, D. I. Pattison and M. J. Davies, Amino Acids, 2003, 25, 259–274 CrossRef CAS PubMed; (b) Y. W. Yap, M. Whiteman and N. S. Cheung, Cell. Signalling, 2007, 19, 219–228 CrossRef CAS PubMed; (c) G. Li, Q. Lin, L. Sun, C. Feng, P. Zhang, B. Yu, Y. Chen, Y. Wen, H. Wang and L. Ji, Biomaterials, 2015, 53, 285–295 CrossRef CAS PubMed; (d) D. I. Pattison, C. L. Hawkins and M. J. Davies, Chem. Res. Toxicol., 2003, 16, 439–449 Search PubMed.
- (a) H. Kimura, Y. Nagai, K. Umemura and Y. Kimura, Antioxid. Redox Signaling, 2005, 7, 795–803 Search PubMed; (b) B. L. Predmore, D. J. Lefer and G. Gojon, Antioxid. Redox Signaling, 2012, 17, 119–140 Search PubMed.
- (a) Y. Cai, L. Li, Z. Wang, J. Z. Sun, A. Qin and B. Z. Tang, Chem. Commun., 2014, 50, 8892–8895 RSC; (b) G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu and S. Zhang, Science, 2008, 322, 587–590 CrossRef CAS PubMed; (c) G.-J. Mao, T.-T. Wei, X.-X. Wang, S.-y. Huan, D.-Q. Lu, J. Zhang, X.-B. Zhang, W. Tan, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2013, 85, 7875–7881 CrossRef CAS PubMed.
- (a) S. Fiorucci, E. Antonelli, E. Distrutti, G. Rizzo, A. Mencarelli, S. Orlandi, R. Zanardo, B. Renga, M. Di Sante and A. Morelli, Gastroenterology, 2005, 129, 1210–1224 CrossRef CAS PubMed; (b) S. Fiorucci, E. Antonelli, A. Mencarelli, S. Orlandi, B. Renga, G. Rizzo, E. Distrutti, V. Shah and A. Morelli, Hepatology, 2005, 42, 539–548 CrossRef CAS PubMed; (c) K. Qu, S. Lee, J. Bian, C.-M. Low and P.-H. Wong, Neurochem. Int., 2008, 52, 155–165 CrossRef CAS PubMed; (d) K. Eto, T. Asada, K. Arima, T. Makifuchi and H. Kimura, Biochem. Biophys. Res. Commun., 2002, 293, 1485–1488 CrossRef CAS PubMed.
- (a) Y. Kimura, Y.-I. Goto and H. Kimura, Antioxid. Redox Signaling, 2010, 12, 1–13 CrossRef CAS PubMed; (b) L. A. Sena and N. S. Chandel, Mol. Cell, 2012, 48, 158–167 CrossRef CAS PubMed.
- J. K. Andersen, Nat. Med., 2004, 10, S18 CrossRef PubMed.
- M. Whiteman, N. S. Cheung, Y.-Z. Zhu, S. H. Chu, J. L. Siau, B. S. Wong, J. S. Armstrong and P. K. Moore, Biochem. Biophys. Res. Commun., 2005, 326, 794–798 CrossRef CAS PubMed.
- B. N. Giepmans, S. R. Adams, M. H. Ellisman and R. Y. Tsien, Science, 2006, 312, 217–224 CrossRef CAS PubMed.
- (a) M. Ren, K. Zhou, L. He and W. Lin, J. Mater. Chem. B, 2018, 6, 1716–1733 RSC; (b) X. Wu, Z. Li, L. Yang, J. Han and S. Han, Chem. Sci., 2013, 4, 460–467 RSC; (c) L. Yuan, L. Wang, B. K. Agrawalla, S.-J. Park, H. Zhu, B. Sivaraman, J. Peng, Q.-H. Xu and Y.-T. Chang, J. Am. Chem. Soc., 2015, 137, 5930–5938 CrossRef CAS PubMed; (d) B. Deng, M. Ren, J.-Y. Wang, K. Zhou and W. Lin, Sens. Actuators, B, 2017, 248, 50–56 CrossRef CAS; (e) M. Ren, Z. Li, B. Deng, L. Wang and W. Lin, Anal. Chem., 2019, 91, 2932–2938 CrossRef CAS PubMed; (f) B. Wang, P. Li, F. Yu, P. Song, X. Sun, S. Yang, Z. Lou and K. Han, Chem. Commun., 2013, 49, 1014–1016 RSC; (g) F. Liu, Y. Gao, J. Wang and S. Sun, Analyst, 2014, 139, 3324–3329 RSC; (h) C. Zhang, Q. Nie, I. Ismail, Z. Xi and L. Yi, Chem. Commun., 2018, 54, 3835–3838 RSC; (i) X.-L. Liu, X.-J. Du, C.-G. Dai and Q.-H. Song, J. Mater. Chem. B, 2014, 79, 9481–9489 CAS; (j) P. S. Zhang, X. Z. Nie, M. Gao, F. Zeng, A. J. Qin, S. Z. Wu and B. Z. Tang, Mater. Chem. Front., 2017, 1, 838–845 RSC; (k) Y. X. Hong, P. S. Zhang, H. Wang, M. L. Yu, Y. Gao and J. Chen, Sens. Actuators, B, 2018, 272, 340–347 CrossRef CAS; (l) Y. Huang, P. S. Zhang, M. Gao, F. Zeng, A. J. Qin, S. Z. Wu and B. Z. Tang, Chem. Commun., 2016, 52, 7288–7291 RSC; (m) J. Y. Ren, P. S. Zhang, H. Liu, C. H. Zhang, Y. Gao, J. X. Cui and J. Chen, Sens. Actuators, B, 2020, 304, 127299 CrossRef; (n) D. L. Shi, S. Q. Chen, B. Dong, Y. H. Zhang, C. Q. Sheng, T. D. James and Y. Guo, Chem. Sci., 2019, 10, 3715–3722 RSC; (o) Y. H. Zhang, L. Ma, C. C. Tang, S. N. Pan, D. L. Shi, S. J. Wang, M. Y. Li and Y. Guo, J. Mater. Chem. B, 2018, 6, 725–731 RSC; (p) Z. R. Lou, P. Li, Q. Pan and K. L. Han, Chem. Commun., 2013, 49, 1014–1016 RSC.
- X. Jiao, Y. Xiao, Y. Li, M. Liang, X. Xie, X. Wang and B. Tang, Anal. Chem., 2018, 90, 7510–7516 CrossRef CAS PubMed.
- H. Xiong, L. He, Y. Zhang, J. Wang, X. Song and Z. Yang, Chin. Chem. Lett., 2019, 30, 1075–1077 CrossRef CAS.
- (a) K. Kazlauskas, J. Mikulėnaitė, R. Karpicz, V. Gulbinas, J. Simokaitiene, A. Michaleviciute, J. Keruckas and J. Grazulevicius, Dyes Pigm., 2009, 83, 168–173 CrossRef CAS; (b) L. Găină, I. Torje, E. Gal, A. Lupan, C. Bischin, R. Silaghi-Dumitrescu, G. Damian, P. Lönnecke, C. Cristea and L. Silaghi-Dumitrescu, Dyes Pigm., 2014, 102, 315–325 CrossRef; (c) H. Xiao, K. Xin, H. Dou, G. Yin, Y. Quan and R. Wang, Chem. Commun., 2015, 51, 1442–1445 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc10028h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |