Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reaction-based indicator displacement assay (RIA) for the development of a triggered release system capable of biofilm inhibition†
Bethany L.
Patenall‡
 a,
George T.
Williams‡
a,
George T.
Williams‡
 a,
Lauren
Gwynne
a,
Lauren
Gwynne
 a,
Liam J.
Stephens
a,
Liam J.
Stephens
 a,
Emma V.
Lampard
a,
Emma V.
Lampard
 a,
Hollie J.
Hathaway
a,
Hollie J.
Hathaway
 b,
Naing T.
Thet
a,
Amber E.
Young
b,
Naing T.
Thet
a,
Amber E.
Young
 c,
Mark J.
Sutton
c,
Mark J.
Sutton
 d,
Robert D.
Short
d,
Robert D.
Short
 b,
Steven D.
Bull
b,
Steven D.
Bull
 a,
Tony D.
James
a,
Tony D.
James
 *a,
Adam C.
Sedgwick
*a,
Adam C.
Sedgwick
 *e and
A. Toby A.
Jenkins
*e and
A. Toby A.
Jenkins
 *a
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; A.T.A.Jenkins@bath.ac.uk
bDepartment of Chemistry, Lancaster University, UK
cThe Scar Free Foundation Centre for Children's Burns Research, The Bristol Royal Hospital for Children, Bristol, UK
dPublic Health England, Porton Down, Salisbury, Wiltshire SP4 0JG, UK
eDepartment of Chemistry, University of Texas at Austin, 105 East 24th Street A5300, Austin, Texas 78712-1224, USA. E-mail: a.c.sedgwick@utexas.edu
First published on 2nd December 2019
Abstract
Here, a reaction-based indicator displacement hydrogel assay (RIA) was developed for the detection of hydrogen peroxide (H2O2) via the oxidative release of the optical reporter Alizarin Red S (ARS). In the presence of H2O2, the RIA system displayed potent biofilm inhibition for Methicillin-resistant Staphylococcus aureus (MRSA), as shown through an in vitro assay quantifying antimicrobial efficacy. This work demonstrated the potential of H2O2-responsive hydrogels containing a covalently bound diol-based drug for controlled drug release.
Dye displacement assays exploit the chemoselective reactivity of certain chemical moieties and the reversible binding of dye molecules to a specific receptor.1 Such chemistry has begun to find widespread use with marked enhancement over traditional sensing assays.1–8 More complex systems containing multiple dyes also offer new paradigms for microarray development.9 Not surprisingly, dye displacement assays have been elegantly employed by a number of research groups. These constructs often rely on boronic acid systems as the receptor (host) subunit with a 1,2- and 1,3-diol guest.10
Previously our group has developed boronate-based hydrogel systems as dye displacement assays (borogel) for monosaccharide detection.11,12 As shown in Scheme 1, the commercially available 1,2-diol dye Alizarin Red S (ARS) was shown to successfully bind to the boronate hydrogel and result in a colour change from red to orange. Upon the addition of a monosaccharide, the competitive displacement of ARS was observed with concomitant observation of an increase in absorption at 513 nm in solution (ARS wavelength).
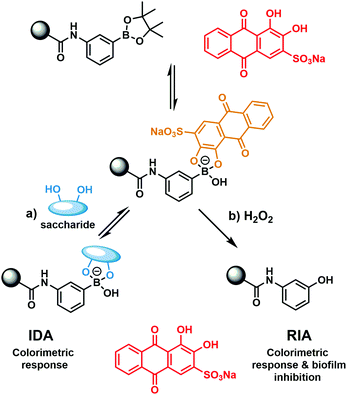 | ||
| Scheme 1 (a) Previously developed hydrogel bound dye displacement assay (IDA) for the detection of monosaccharides.11,12 (b) Present work – the development of a hydrogel bound reaction-based indicator displacement (RIA) assay for the detection of H2O2 and for the inhibition of MRSA biofilm formation. | ||
Aryl boronic acids/boronate esters are well known to undergo hydrogen peroxide (H2O2)-mediated oxidation to form the corresponding phenol.13 This unique synthetic transformation has been exploited in organic synthesis and fluorescence sensing.13 We thus envisaged that modification of the previously developed ARS hydrogel bound indicator displacement assay (IDA) would yield a multimodal detection platform for the detection of H2O2 with attendant antimicrobial activity (Scheme 1).14–16 Here, we report the construction of a covalently incorporated ARS polyacrylamide hydrogel that undergoes oxidative activation in the presence of H2O2 to release ARS and afford a reaction-based indicator displacement assay (RIA).15In vitro antibacterial assays with Methicillin-resistant Staphylococcus aureus (MRSA) indicated significant activity against biofilm formation for the combination of ARS and H2O2.17,18
In brief, phenylboronic acid (PBA) and benzoxaborole (BOB) acrylamide monomers were synthesised as previously reported.11,12 Polyacrylamide hydrogels were synthesised using water (60% w/w), acrylamide (38% w/w), methylene bisacrylamide (1% w/w), and BOB (1% w/w) or PBA (1% w/w). For qualitative purposes, hydrogel slabs containing BOB and PBA were immersed in 2.5 × 10−4 M ARS (PBS solution). Covalent incorporation was qualitatively measured via the observed colour change from red to orange, as measured against a blank hydrogel (Fig. S1 and S2, ESI†). For quantitative purposes, hydrogel cylinders (0.1 g) were immersed in a 2.5 × 10−4 M ARS solution (1 mL) and the UV-Vis absorption at 513 nm was measured over time. As shown in Fig. S3 and S4 (ESI†), a decrease in absorbance at 513 nm was observed, which corresponded to ARS uptake into the gel. After approximately 5 h, both PBA and BOB gels were saturated with ARS, which was indicated by no further decrease in absorption at 513 nm. Each gel was then placed into a solution of PBS (1 mL) to wash out any unbound ARS, which was shown by an increase in absorption at 513 nm (Fig. S5 and S6, ESI†). No further increase in absorption was observed after 3 h, which indicated the full release of any unbound ARS from each gel.
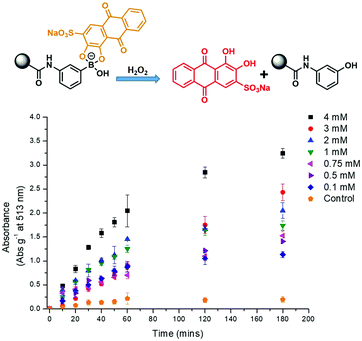
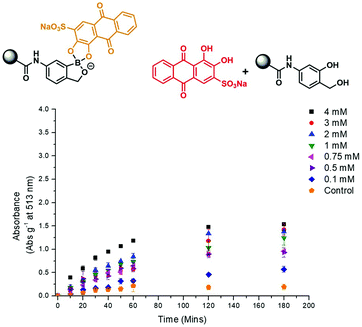
The prepared gels were then used to evaluate the response towards H2O2. Each gel (PBA and BOB) was placed into a solution of PBS (1 mL) and then exposed to various concentrations of H2O2 (0–4 mM). As shown in Fig. 1 and 2, increasing the concentration of H2O2 led to an increased release of ARS from the borogels, as seen in the higher absorption at 513 nm. Interestingly, the greatest sensitivity and ARS release was observed for the PBA-based gels, indicative of a greater reactivity towards H2O2 over the BOB-based gels (see Fig. S7 and S8, ESI†). This change in sensitivity is rationalised as the BOB moiety displays an enhanced binding affinity towards 1,2-diols due to an adjacent alkyl alcohol coordinating to the boron atom.12,19 Therefore, we believe the adjacent methyl alcohol retards oxidation of ARS bound-boronic acid by H2O2.
Recent efforts by Lee and co-workers have demonstrated that alizarin (10 μg mL−1) is an effective inhibitor of biofilm formation for three Staphylococcus aureus (S. aureus) strains and one Staphylococcus epidermidis strain.20,21 Biofilms are complex bacterial communities that can facilitate antibiotic resistance and impair wound healing.22 Hence, the development of new systems that can effectively treat or inhibit biofilm formation are highly desirable.
H2O2 is a commonly used disinfectant and antiseptic in wound care. Therefore, we explored the potential of this system in the development of a H2O2-responsive hydrogel for the triggered release of ARS for biofilm inhibition against the three key stages of bacterial growth: lag, exponential and stationary. Due to the PBA-based gel displaying the greatest sensitivity towards H2O2 over BOB-based gels (see Fig. S7 and S8, ESI†), only PBA gels were evaluated for biofilm inhibition. Control studies showed that the minimum inhibitory concentration (MIC) of H2O2 was 3.5–7 mM for Staphylococcus aureus (S. aureus) H560 and MRSA252, 0.8–1.6 mM for Pseudomonas aeruginosa PAO1 (P. aeruginosa PAO1) and 3–6 mM for Escherichia coli NCTC 10418 (E. coli NCTC 10418). Unfortunately, due to poor solubility, no MIC was determined for ARS against all the bacterial strains used in this study (see Fig. S9–S11, ESI†).23–25
ARS was able to inhibit biofilm formation for S. aureus MRSA252 and S. aureus H560 at 100 μM when added during the lag phase of growth (0 h), similar to other reports in the literature for Alizarin.21 However, ARS was unsuccessful in the inhibition of P. aeruginosa PAO1 and E. coli NCTC 10418 biofilms at concentrations below 100 μM (see Fig. S14–S16, ESI†). Additionally, H2O2 inhibited biofilm formation, albeit at much higher concentrations, prevents growth at 5 mM for S. aureus MRSA252 and S. aureus H560, 10 mM for E. coli NCTC 10418, and 100 mM for P. aeruginosa PAO1 (see Fig. S17–S19, ESI†). H2O2 (2 mM) with ARS (50 μM) acted synergistically, effecting biofilm inhibition of S. aureus MRSA252 when added during the lag phase (Fig. S20, ESI†). Unfortunately, this combination was unable to inhibit biofilm formation at all other growth phases for each bacterial strain (Fig. S21 and S22, ESI†).
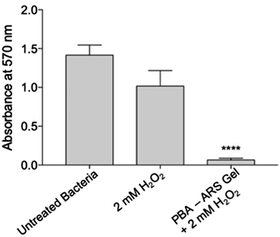
We next turned our attention towards the ability of the PBA-ARS hydrogel system to inhibit MRSA biofilm formation. Initial control experiments were carried out. PBA-ARS gel incubated in PBS solution for 3 h was shown to result in no biofilm inhibition, which indicates the requirement of H2O2 for ARS release and no off-target gel toxicity (Fig. S23, ESI†). Control “blank” acrylamide gels were subsequently tested to evaluate the requirement of the boronic acid units for the H2O2-mediated release of ARS. Following the usual ARS-loading protocol (see ESI†), acrylamide gels loaded with ARS (ARS uptake through passive diffusion – see Fig. S2, ESI†) were treated with H2O2. No biofilm inhibition was observed, which illustrated the requirements of the boronic acid units for H2O2-mediated release of ARS from the hydrogel (Fig. S24, ESI†). To capitalize upon the H2O2-mediated release of ARS from the boronic acid containing polyacrylamide hydrogel, PBA-based gels (0.1 g comprising of 2.5 × 10−4 M ARS) were incubated with H2O2 (2 mM) for 3 h to achieve maxiumum ARS release (cf.Fig. 1). The resultant ARS release was then applied to S. aureus MRSA252 at the lag phase. Remarkably, this resulted in complete biofilm inhibition (Fig. 3) thus demonstrating the potential of the PBA-based gels as a “smart” wound dressing.
In summary, we have developed a multimodal reaction-based indicator displacement (RIA) hydrogel assay for the detection of H2O2 with concomitant release of ARS for antimicrobial application. The greatest reactivity towards H2O2 was observed for the PBA-based gel compared to the BOB-based gel, attributed to attenuated reactivity of the cyclic BOB-boronate ester. In addition, the antimicrobial efficacy of each assay component was evaluated with the aim of developing a “triggered release” antimicrobial hydrogel. ARS was discovered to be a potent biofilm inhibitor in combination with hydrogen peroxide against S. aureus MRSA252, with the ARS loaded PBA-based gel successfully inhibiting biofilm formation. These results lead us to suggest that PBA-based gels, in combination with an early bacterial detection system for a MRSA biomarker, might find use as a “smart” wound dressing capable of preventing MRSA biofilm formation.26
The authors would like to thank the EPSRC for grant EP/R003556/1. BLP would also like thank James Tudor and Mr and Mrs Watson for additional funding. GTW would like to thank the EPSRC and Public Health England. ACS, ATAJ and NTT wish to thank the EPSRC for funding on Smartwound plasma – EP/R003939/1. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award. We would like to thank James T. Brewster II at Harvard college for helpful discussions and suggestions to improve the manuscript.
Conflicts of interest
No conflicts of interest.Notes and references
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118–3127 CrossRef CAS.
- A. M. Piatek, Y. J. Bomble, S. L. Wiskur and E. V. Anslyn, J. Am. Chem. Soc., 2004, 126, 6072–6077 CrossRef CAS PubMed.
- D. Leung, J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2008, 130, 12318–12327 CrossRef CAS PubMed.
- B. T. Nguyen, S. L. Wiskur and E. V. Anslyn, Org. Lett., 2004, 6, 2499–2501 CrossRef CAS PubMed.
- V. Janowski and K. Severin, Chem. Commun., 2011, 47, 8521–8523 RSC.
- J. H. Zhang, S. Umemoto and K. Nakatani, J. Am. Chem. Soc., 2010, 132, 3660–3661 CrossRef CAS PubMed.
- T. Minami, Y. L. Liu, A. Akdeniz, P. Koutnik, N. A. Esipenko, R. Nishiyabu, Y. Kubo and P. Anzenbacher, J. Am. Chem. Soc., 2014, 136, 11396–11401 CrossRef CAS PubMed.
- S. Comby, S. A. Tuck, L. K. Truman, O. Kotova and T. Gunnlaugsson, Inorg. Chem., 2012, 51, 10158–10168 CrossRef CAS PubMed.
- Y. Sasaki, Z. J. Zhang and T. Minami, Front. Chem., 2019, 7, 150, DOI:10.3389/fchem.2019.00049.
- X. Wu, Z. Li, X. X. Chen, J. S. Fossey, T. D. James and Y. B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC.
- W. M. J. Ma, M. P. P. Morais, F. D'Hooge, J. M. H. van den Elsen, J. P. L. Cox, T. D. James and J. S. Fossey, Chem. Commun., 2009, 532–534 RSC.
- E. V. Lampard, A. C. Sedgwick, T. Sombuttan, G. T. Williams, B. Wannalerse, A. T. A. Jenkins, S. D. Bull and T. D. James, ChemistryOpen, 2018, 7, 266–268 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- A. Romieu, Org. Biomol. Chem., 2015, 13, 1294–1306 RSC.
- X. L. Sun, M. L. Odyniec, A. C. Sedgwick, K. Lacina, S. Y. Xu, T. T. Qiang, S. D. Bull, F. Marken and T. D. James, Org. Chem. Front., 2017, 4, 1058–1062 RSC.
- X. L. Sun, K. Lacina, E. C. Ramsamy, S. E. Flower, J. S. Fossey, X. H. Qian, E. V. Anslyn, S. D. Bull and T. D. James, Chem. Sci., 2015, 6, 2963–2967 RSC.
- C. H. Ren, L. P. Chu, F. Huang, L. J. Yang, H. R. Fan, J. F. Liu and C. H. Yang, RSC Adv., 2017, 7, 1313–1317 RSC.
- F. Liu, L. B. Bai, H. L. Zhang, H. Z. Song, L. D. Hu, Y. G. Wu and X. W. Ba, ACS Appl. Mater. Interfaces, 2017, 9, 31626–31633 CrossRef CAS PubMed.
- M. Dowlut and D. G. Hall, J. Am. Chem. Soc., 2006, 128, 4226–4227 CrossRef CAS PubMed.
- J. H. Lee, Y. G. Kim, S. Y. Ryu and J. Lee, Sci. Rep., 2016, 6, 19267, DOI:10.1038/srep19267.
- R. K. Manoharan, J. H. Lee, Y. G. Kim and J. Lee, Front. Cell. Infect. Microbiol., 2017, 7, 447, DOI:10.3389/fcimb.2017.00447.
- C. W. Hall and T. F. Mah, FEMS Microbiol. Rev., 2017, 41, 276–301 CrossRef CAS PubMed.
- G. Zhu, Q. Wang, S. Lu and Y. Niu, Med. Princ. Pract., 2017, 26, 301–308 CrossRef PubMed.
- G. McDonnell and A. D. Russell, Clin. Microbiol. Rev., 2001, 14, 227–228 Search PubMed.
- Y. Yamada, T. Mokudai, K. Nakamura, E. Hayashi, Y. Kawana, T. Kanno, K. Sasaki and Y. Niwano, J. Toxicol. Sci., 2012, 37, 1091 CrossRef CAS.
- N. T. Thet, D. R. Alves, J. E. Bean, S. Booth, J. Nzakizwanayo, A. E. R. Young, B. V. Jones and A. T. A. Jenkins, ACS Appl. Mater. Interfaces, 2016, 8, 14909–14919 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc07759f |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2019 |