Potassium phosphotungstate spheres as an anode material for a solar rechargeable battery†
NanFu
Yan
 *a,
WenHua
Zhang
b,
HongMin
Cui
a,
XueJiao
Feng
c,
YueWei
Liu
a and
JinSong
Shi
a
*a,
WenHua
Zhang
b,
HongMin
Cui
a,
XueJiao
Feng
c,
YueWei
Liu
a and
JinSong
Shi
a
aInstitute of Applied Chemistry, Jiangxi Academy of Sciences, Nanchang 330029, China. E-mail: nfyan@mail.nankai.edu.cn
bNanchang Institute of Technology, Nanchang, 330099, China
cInstitute of Science and Technology Strategy, Jiangxi Academy of Sciences, Nanchang 330029, China
First published on 27th November 2017
Abstract
Potassium phosphotungstate spheres, prepared by a simple coprecipitation method, are introduced for the first time as an anode material for a solar rechargeable battery. The microstructure of the as-prepared potassium phosphotungstate spheres is characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The results show that the obtained potassium phosphotungstate particles were monodisperse and uniform spheres and present good photo-generated electron storage capacity with suitable redox potentials. Correspondingly, the as-fabricated battery using potassium phosphotungstate as the anode material exhibits satisfactory solar energy conversion and storage as well as rechargeable capability. Therefore, the potassium phosphotungstate spheres can be used as potential anode material for applications in the solar rechargeable battery.
Exploring renewable and clean energy sources is the practical solution to solving the problem of global warming and the energy crisis.1,2 Solar energy has been considered as the best substitute for conventional fossil sources, due to its advantage of being abundant, environmentally benign, and inexpensive.3 However, the bottleneck in efficiently using solar energy is the intermittent nature of solar irradiation, which poses significant impacts to the reliability and stability of the electric grid. Therefore, developing affordable solar energy storage devices with the bi-function of converting solar energy to electricity and storing the solar-generated electricity is a feasible way to solve the bottleneck problem of how to efficiently use solar energy.
A solar rechargeable battery is a type of solar energy conversion and storage system. The battery can convert the solar energy into chemical/electrical energy, and the chemical energy can be stored directly inside the batteries.4–9 In general, a solar rechargeable battery consists of a dye-sensitized TiO2 electrode, membrane, cathode, and anode. In recent years, various materials have been developed as anode materials to store photo-generated electrons, such as WO3 and conducting polymer.10–12 In order to efficiently store photo-generated electrons, the anode material should possess the bi-function of receiving photo-generated electrons and storing energy. The selection of anode materials is highly important to improve the performance of the solar rechargeable battery. Polyoxometalates (POMs) have numerous applications in catalysis, photochromism, electrochromism, and photoluminescence, and promising multifunctional materials have been created for future use by utilizing these phenomena.13,14 Recently, polyoxometalates have received extensive interest because they are attractive active species used in electrochemical rechargeable batteries, due to their versatile, tunable properties and multielectron redox reactions.15–17 In particular, the unique electrical properties of polyoxometalates are caused by the reversible intercalation of ions (e.g., H+, Li+).13,14 Also, the conduction band of the phosphotungstate is lower compared to TiO2,18,19 which makes it suitable for photo-generated electron storage. With this in mind, we focused on phosphotungstate as an anode material for a solar rechargeable battery to store the photo-generated electrons.
In this work, potassium phosphotungstate (K3PW12O40) spheres are prepared by a simple coprecipitation method and are introduced for the first time as an anode material for a solar rechargeable battery. Our research shows that the as-fabricated solar rechargeable battery using potassium phosphotungstate spheres as an anode material for photo-generated electron storage is an effective, workable strategy for solar energy conversion and storage.
Fig. 1 displays typical SEM and TEM images of the as-prepared K3PW12O40. As shown in Fig. 1(a), the K3PW12O40 particles are uniform spheres with a diameter of approximately 300 nm. The TEM image (Fig. 1(b)) shows that the discrete and uniform spherical morphology of K3PW12O40 is well preserved after calcination. Fig. 1(b) shows K3PW12O40 spheres with diameters of 300 ± 50 nm, which agrees well with the aforementioned SEM image.
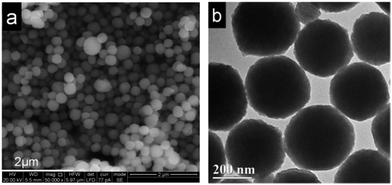 | ||
| Fig. 1 (a) SEM image of the as-prepared K3PW12O40 and (b) the TEM image of the as-prepared K3PW12O40. | ||
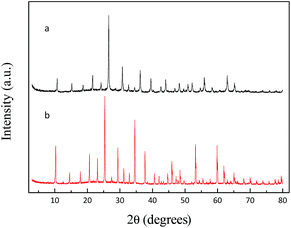
The structure of as-prepared K3PW12O40 and H3PW12O40 was studied by means of XRD. As displayed in Fig. 2(b), it is clear that H3PW12O40 shows characteristic diffraction peaks at 10.2°, 21.3°, 25.3°, 29.4°, 34.6° 53.2°, and 59.8°. After the co-precipitation process, it is shown that the XRD pattern of the as-prepared K3PW12O40 spheres has obvious diffraction peaks at 10.7°, 21.6°, 26.5°, 30.7°, 36.2° 55.9°, and 62.9°, which is similar to the results reported by Haber and Chen et al.20,21 It is clear that the diffraction peak with the maximum intensity shifted from 25.3° to 26.5° after the exchange of the H+ by K+. The diffraction patterns of K3PW12O40 reveal that no traces of pure H3PW12O40 were detected, which proved the presence of only one phase with good crystallinity.
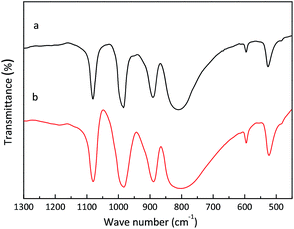
The structure of the K3PW12O40 sample was further characterized by FT-IR spectra. As shown in Fig. 3, the absorption peaks of H3PW12O40 appear at 1080.1, 982.7, 889.0, 802.0, 595.2, and 523.5 cm−1, which correspond to the peaks of absorption frequencies of the typical Keggin anion.22 After the simple co-precipitation process, the peaks of the as-prepared K3PW12O40 appear at 1080.8, 983.9, 890.6, 810.6, 595.6, and 526.1 cm−1, which were similar to the peaks of H3PW12O40. It is indicated that the as-prepared K3PW12O40 maintains a relatively complete Keggin structure with H3PW12O40. It is clear that the peak of νas(W–Oc–W) shifted from 802.0 to 810.6 cm−1. It has been reported that the H+ of the H3PW12O40 was linked to the bridging oxygen (W–Oc–W), and thus, the main reason for the peak shift is probably the exchange of the H+ by K+.20,23 It is evident that as-prepared K3PW12O40 spheres maintain the integrity of the Keggin structure after the co-precipitation process.
To verify the feasibility of using potassium phosphotungstate spheres as an anode, cyclic voltammograms (CVs) of the cathode/anode were tested. The CV of the K3PW12O40 is shown in Fig. 4, and it is clear that there are two pairs of peaks appearing at −0.08/−0.28 V and −0.76/−0.87 V. The reduction potential (−0.28 V vs. Ag/Ag+) of the K3PW12O40 is more positive than the potential of the conduction band edge of TiO2 (−0.68 V vs. Ag/Ag+). It has previously been demonstrated that the photo-generated electrons can transport from the TiO2 to the K3PW12O40 anode through the external circuit under illumination.10,12 However, the reduction potential of the second redox peak of the K3PW12O40 (−0.76 V) is lower than the potential of the conduction band edge of TiO2. This indicates that the K3PW12O40 can be only partially reduced by the photo-generated electrons. In the typical CVs of LiI, two pairs of redox peaks appear at 0.17/−0.005 V and −0.29/−0.56 V, which correspond to a two-step reversible reaction.8,24,25 The redox potential of the I3−/I− couple is lower than that of dye N719 (0.4 V; the CV is shown in Fig. S1, ESI†), which indicates that excited state dye N719 can oxidize I− to a potential as high as 0.40 V under illumination.23,24 Basically, the K3PW12O40 used as the anode material would be feasible for photo-generated electron storage on the basis of the energy level or potential.
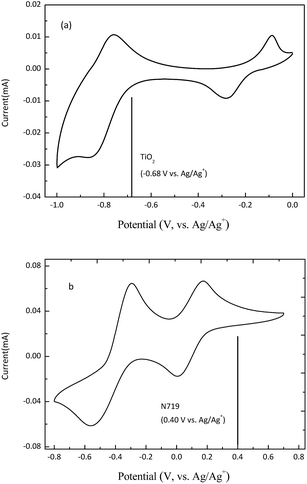 | ||
| Fig. 4 Cyclic voltammograms of (a) the as-prepared K3PW12O40 and (b) LiI (0.01 M LiI in propylene carbonate with 0.1 M LiClO4). | ||
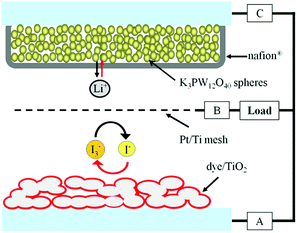
In order to demonstrate the electrochemical performance of K3PW12O40, the battery was fabricated with a dye-sensitized TiO2 electrode, LiI as the cathode, and K3PW12O40 as the anode. The configuration of the battery based on K3PW12O40 as the anode is shown in Fig. 5. The working mechanism of the battery has been previously reported.10,12
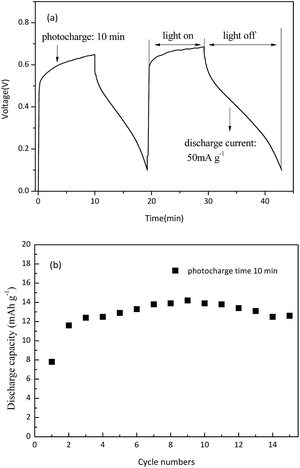
The fabricated battery is photo-charged for 10 min with an illumination intensity of 100 mW cm−2, and then discharged in the dark. As shown in Fig. 6(a), after under-illumination for 10 min (the J–V performance of the N719-based DSSCs is shown in Fig. S2 in the ESI†), the battery can be galvanostatically discharged for 9 min at a current density of 50 mA g−1 in the dark, which indicates that the battery can successfully convert chemical energy into electrical energy. After photo-charging, the voltage of the battery reaches 0.65 V, indicating that the redox couples (I− and K3PW12O40) converted into the redox couples (I3− and LixK3PW12O40) in the cathode and anode, respectively. This indicates that the solar energy can be converted into chemical energy. It is clear that the average working voltage of the battery is approximately 0.35 V in the discharge process, which corresponds to the average potential difference between the redox couples I−/I3− and K3PW12O40/LixK3PW12O40. For the first cycle, the discharge capacity of the K3PW12O40 anode is 7.8 mA h g−1, with the cut-off voltage at 0.1 V. Obviously, a battery based on K3PW12O40 spheres can repeat the photo-charge and electrochemical discharge processes. After 15 cycles, the anode retains a discharge capacity of 12.6 mA h g−1, demonstrating the good stability of the K3PW12O40 anode for storing photo-generated electrons. Based on the working mechanism analysis and the experimental results shown above, the K3PW12O40 spheres as an anode material were successfully applied to a solar rechargeable battery.
Conclusions
In summary, K3PW12O40 spheres were successfully prepared by a simple coprecipitation method. It was demonstrated that the K3PW12O40 spheres show suitable redox potentials and good electrochemical activity. The as-fabricated battery with K3PW12O40 spheres as the anode shows excellent solar energy conversion, storage capability, and cycle stability. Therefore, the K3PW12O40 spheres as an anode material were successfully applied to a solar rechargeable battery. The facile synthesis technique and the good electrochemical performance make K3PW12O40 spheres a promising candidate as an anode material for solar rechargeable batteries.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from the NSFC (21603093) and Natural Science Foundation of Jiangxi (20161BBE50095) are greatly appreciated.Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- M. D. Bhatt and J. S. Lee, J. Mater. Chem. A, 2015, 3, 10632–10659 CAS.
- M. A. Modestino, S. M. H. Hashemi and S. Haussener, Energy Environ. Sci., 2016, 9, 1533–1551 CAS.
- H. Nagai and H. Segawa, Chem. Commun., 2004, 974–975 RSC.
- W. Li, H. C. Fu, L. S. Li, M. Cabán-Aceved, J. H. He and S. Jin, Angew. Chem., 2016, 128, 1–6 CrossRef.
- O. Nguyena, E. Courtina, F. Sauvageb, N. Krins, C. Sancheza and C. Laberty-Robert, J. Mater. Chem. A, 2017, 5, 5927–5933 Search PubMed.
- B. Lei, G. R. Li, P. Chen and X. P. Gao, Nano Energy, 2017, 38, 257–262 CrossRef CAS.
- N. F. Yan, G. R. Li and X. P. Gao, J. Mater. Chem. A, 2013, 1, 7012–7015 CAS.
- D. Schmidt, M. D. Hager and U. S. Schubert, Adv. Energy Mater., 2016, 6, 1500369 CrossRef.
- N. F. Yan, G. R. Li, G. L. Pan and X. P. Gao, J. Electrochem. Soc., 2012, 159, A1770–A1774 CrossRef CAS.
- W. J. Zhao, X. F. Wang, E. Q. Zhang, Y. J. Wei, Y. Sanehir and G. Chen, J. Power Sources, 2017, 350, 28–34 CrossRef CAS.
- P. Liu, H. X. Yang, X. P. Ai, G. R. Li, G. L. Pan and X. P. Gao, Electrochem. Commun., 2012, 16, 69–72 CrossRef CAS.
- T. Yamase, Chem. Rev., 1998, 98, 307–325 CrossRef CAS PubMed.
- M. Sadakane and E. Steckhan, Chem. Rev., 1998, 98, 219–237 CrossRef CAS PubMed.
- D. Harry Pratt III, S. Nicholas, X. k. Fang and T. M. Anderson, J. Power Sources, 2013, 236, 259–264 CrossRef.
- H. X. Yang, T. Song, L. Liu, A. Devadoss, F. Xia, H. k. Han, H. J. Park, W. G. Sigmund, K. J. Kwon and U. Y. Paik, J. Phys. Chem. C, 2013, 117, 17376–17381 CAS.
- J. J. Chen, M. D. Symes, S. C. Fan, M. S. Zheng, H. N. Miras, Q. F. Dong and L. Cronin, Adv. Mater., 2015, 27, 4649–4654 CrossRef CAS PubMed.
- D. Friesen, J. Headley and C. Langford, Environ. Sci. Technol., 1999, 33, 3193–3198 CrossRef CAS.
- J. J. Chen and M. A. Barteau, Ind. Eng. Chem. Res., 2016, 55, 9857–9864 CrossRef CAS.
- C. C. Chen, Q. Wang, P. X. Lei, W. J. Song, W. H. Ma and J. C. Zhao, Environ. Sci. Technol., 2006, 40, 3965–3970 CrossRef CAS PubMed.
- J. Haber, K. Pamin, L. Matachowski, B. Napruszewska and J. Połtowicz, J. Catal., 2002, 207, 296–306 CrossRef CAS.
- N. Essayem, A. Holmqvist, P. Y. Gayraud, J. C. Vedrine and Y. Ben Taarit, J. Catal., 2001, 197, 273–280 CrossRef CAS.
- K. Y. Lee, N. Mizuno, T. Okuhara and M. Misono, Bull. Chem. Soc. Jpn., 1989, 62, 1731–1739 CrossRef CAS.
- Y. L. Wang, Q. L. Sun, Q. Q. Zhao, J. S. Cao and S. H. Ye, Energy Environ. Sci., 2011, 4, 3947 CAS.
- P. Liu, Y. L. Cao, G. R. Li, X. P. Gao, X. P. Ai and H. X. Yang, ChemSusChem, 2013, 6, 802–806 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00510e |
| This journal is © The Royal Society of Chemistry 2018 |