Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using a BiVO4/FeVO4 heterojunction composite†
Muhammad Munir Sajidac,
Sadaf Bashir Khan c,
Naveed Akthar Shadab,
Nasir Amin
c,
Naveed Akthar Shadab,
Nasir Amin a and
Zhengjun Zhang
a and
Zhengjun Zhang *d
*d
aDepartment of Physics, Government College University, Allama Iqbal Road, Faisalabad, 38000, Pakistan
bNational Institute for Biotechnology and Genetic Engineering (NIBGE), P. O. Box. 577, Jhang Road, Faisalabad, Pakistan
cThe State Key Laboratory for New Ceramics & Fine Processing, School of Materials Science & Engineering, Tsinghua University, Beijing, China 100084
dAdvanced Key Laboratory for New Ceramics, School of Materials Science & Engineering, Tsinghua University, Beijing, China 100084. E-mail: zjzhang@tsinghua.edu.cn
First published on 27th June 2018
Abstract
A BiVO4/FeVO4 nanocomposite photocatalyst was successfully synthesized via a hydrothermal method. The prepared heterojunction photocatalyst was characterized physically and chemically using XRD, SEM, EDX, XPS, BET, FT-IR, Raman, UV-vis DRS, EPR and photoluminescence techniques. BiVO4/FeVO4 was explored for its photocatalytic activity by the decomposition of crystal violet (CV) organic dye under visible radiation. This experiment showed that BiVO4/FeVO4 at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 completely degrades CV within 60 min. In addition, BiVO4/FeVO4 was investigated for the electrochemical detection of the useful analyte ascorbic acid using electrochemical impedance spectroscopy (EIS) and cyclic voltammetry techniques. This work reveals the potential of the BiVO4/FeVO4 nanocomposite for applications in environmental disciplines as well as in biosensing.
1 completely degrades CV within 60 min. In addition, BiVO4/FeVO4 was investigated for the electrochemical detection of the useful analyte ascorbic acid using electrochemical impedance spectroscopy (EIS) and cyclic voltammetry techniques. This work reveals the potential of the BiVO4/FeVO4 nanocomposite for applications in environmental disciplines as well as in biosensing.
Introduction
The large-scale disposal of dyes from fabrics, plastics, cosmetics, paper, leather, food, municipal waste and other industries into the water poses a significant threat to the environment. Approximately 1–20% of the dye produced globally is lost during the dyeing process and is expelled in waste water.1–5 These dyes are major causes of water pollution, so it is urgent that we develop either facile methods or eco-friendly techniques to treat polluted water and make it usable for human beings and other living organisms. A lot of work has been conducted in this area, involving approaches including filtration, electrochemical methods, precipitation, coagulation and adsorption, which are all common techniques for water remediation.6 Among these processes, heterogeneous photocatalysis is an advanced oxidant process (AOP) that has emerged in recent years. AOPs are used extensively as a facile and cost-effective way to mineralize pollutant water without generating secondary harmful pollutants, by using light in the presence of a catalyst.7–9 The effectiveness of an AOP is proportional to its ability to generate hydroxyl radicals. Photocatalysis is based on the principle in which the absorption of sunlight energy produces electrons and holes to activate oxidation–reduction responses on the surface of a semiconductor to degrade particular compounds.Metallic oxide (MO) semiconductors are generally considered as photocatalysts, but because of their wide band gaps, the light absorption ability of binary MOs is limited, affecting their photocatalytic efficiency. Therefore, it is necessary to fabricate MOs with smaller band gaps for effective employment of solar energy. To this end, ternary metal oxides (TMOs), which have valence bands made from orbitals of more than one element, come forward. TMOs have narrow band gaps with high visible light illumination absorption ability. Among these TMOs, metal vanadate is of great importance in several areas including photocatalysis, catalysis and batteries.10–13 One of the various metal vanadates that a lot of effort has been devoted to synthesizing and characterizing is bismuth vanadate (BiVO4), owing to its good optical, conductivity and ferroelasticity properties. It is used in yellow pigments, O2 evaluation, degradation of pollutants, electrodes for batteries and gas sensors.14–17
Bismuth vanadate was first reported as a photocatalyst in 1998 for water splitting by Kudo and Ueda, with the monoclinic-scheelite (m-BiVO4) phase showing the highest efficiency. In the next year, Kudo's group described the increased visible radiation photocatalytic attributes of BiVO4 for O2 evolution in an aqueous silver nitrate solvent. In another exciting investigation reported by Kudo et al., monoclinic and tetragonal BiVO4, which both have a scheelite structure, and the same composition, components and energy structure, showed extensively divergent photocatalytic efficiencies for O2 evolution in an AgNO3 mixture under ultraviolet and visible light, owing to the distortion of the Bi–O polyhedron due to the 6S2 lone pairs of Bi3+.18–20
Fabricating heterojunction photocatalysts based on various semiconductor materials represents an economical process to aid the separation of photo-excited charges (electron–hole pairs (ehps)) and increase the photocatalytic properties.21 Heterojunction photocatalysts are manufactured in several different ways, including sol–gel synthesis, ball milling,22 hydrothermal synthesis23 and co-precipitation,24 which lead to a heterojunction photocatalyst with an improved photocatalytic response as compared to that of its single ingredients. From a literature survey, it was found that heterojunction photocatalysts such as InVO4/BiVO4, CuO/BiVO4, C3N4/BiVO4, BiVO4/Bi2S3 and CaFe2O4/Ag3VO4 exhibit higher photocatalytic efficiency compared to single semiconductors.25–29 When a large band gap semiconductor is combined with a short band gap semiconductor, the electron–hole separation is prolonged and a superior photocatalytic response is achieved.22
To the best of our knowledge, there are few reports in the literature regarding the use of BiVO4/FeVO4 nanostructured heterojunction composites for the photocatalytic degradation of crystal violet dye, as well as the electrochemical detection of ascorbic acid. In this study, a BiVO4/FeVO4 heterojunction composite was prepared via a hydrothermal method. Both BiVO4 and FeVO4 are earth-abundant minerals with narrow band gaps and show innate visible light absorption at around 600 nm at about 2.05 eV. Therefore, combining BiVO4 and FeVO4 can increase the extent of electron and hole separation and enhance the photocatalytic process. The as-synthesized BiVO4/FeVO4 nanocomposite was investigated using XRD, SEM, EDS, XPS, BET, UV-vis and photoluminescence techniques for physical and chemical behavior studies. The photocatalytic response of the as-synthesized nanocomposite was evaluated by investigating the degradation of crystal violet (CV) dye under visible light. The electrochemical properties of the BiVO4/FeVO4 nanocomposite were also investigated for the detection of the important analyte ascorbic acid. This work exposes the potential of the BiVO4/FeVO4 nanocomposite for applications in environmental science as well as biosensor domains.
Experimental methods
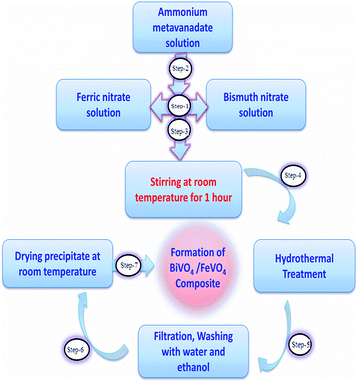
All of the materials were of analytical grade and used without any purification. The heterojunction composite BiVO4/FeVO4 was fabricated via a hydrothermal method. Firstly, 1 mmol Bi(NO3)3·5H2O and 1 mmol Fe(NO3)3·9H2O were dissolved in 60 mL distilled water, then 2 mmol of NH4VO3 was poured into the solution and vigorous stirring was carried out for 1 h at ambient temperature. The pH was adjusted to around 8 using NaOH, and the solution was transferred into a 100 mL Teflon-lined stainless autoclave, which was heated at 180 °C for 24 h. After cooling the autoclave, the precipitate was centrifuged and washed many times with ethanol and distilled water. It was then dried at 80 °C.30,31 To check the effects of various concentrations on the crystallinity, morphology, photocatalytic and electrochemical sensing properties, BiVO4/FeVO4 heterojunctions were prepared in different molar ratios while keeping all other parameters the same, resulting in gradual color changes from blackish to yellowish as the BiVO4 concentration increased. The flow chart of the preparation method is shown in Fig. 1.Characterization of BiVO4/FeVO4
A Rigaku 2500 X-ray diffractometer was used to analyze the crystallinity, phase and particle size of the synthesized nanoparticles. The morphological features and topography were characterized using field emission scanning electron microscopy, on a JEOL-7001F working at an operating voltage of 20 kV. The elemental analysis of the nanoparticles was examined using energy-dispersive X-ray spectroscopy (EDX), with an FESEM microscope attached to an XPS spectrometer (PHI 5000 Versa Probe). The absorbance spectra of organic CV dye were measured using a PerkinElmer λ-35 UV-vis spectrophotometer. The porosity and Brunauer–Emmett–Teller (BET) surface areas of the products were evaluated by a multi-point BET method using adsorption data. FT-IR measurements were obtained on a Nicolet Magna-550 spectrometer. Photoluminescence (PL) and Raman analysis were conducted on Horiba Scientific Fluoromax-4 spectrofluorometer. Electronic Paramagnetic Resonance (EPR) analysis was performed on an ESR-JES-FA 2010 spectrometer.Photocatalytic activity
To check the photocatalytic responses of powdered BiVO4/FeVO4, samples were illuminated using a UV-visible light xenon lamp with an accumulative intensity of 300 W for degrading CV dye, which was 25 cm away from the lamp. For studying the kinetics, dye solutions with fixed concentrations (150 mL) at neutral pH were prepared and 0.1 g of the prepared BiVO4/FeVO4 photocatalyst was mixed into each separate solution. The pH was maintained by adding HNO3 and NaOH, and then each prepared solution was stirred for 15 min in the dark and finally centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. To avoid any thermal degradation and to keep the temperature at 0 °C, nitrogen cooling was used throughout the experiment.32 The degradation phenomenon of the dye was analyzed using a PerkinElmer λ-35 UV-vis spectrometer in the spectral range of 400–800 nm.
000 rpm for 5 min. To avoid any thermal degradation and to keep the temperature at 0 °C, nitrogen cooling was used throughout the experiment.32 The degradation phenomenon of the dye was analyzed using a PerkinElmer λ-35 UV-vis spectrometer in the spectral range of 400–800 nm.
Sensor measurements
The electrochemical evaluation was carried using a model Chi760D electrochemical work station, and a three-electrode scheme. A glassy carbon electrode (GCE) was applied in this work for electrode adjustment. BiVO4/FeVO4 (0.5 mg) was first dispersed in 1 mL distilled water. Then, 10 μL of nanomaterial was put on the GC electrodes and dehydrated in the oven at 80 °C for 2 min, after which 2–3 μL of 5% Nafion was drop-casted onto the electrodes and dried out. Each solution for sensor measurements comprised distilled water, and the required concentrations were made up using suitable dilutions from the respective stock solutions. The BiVO4/FeVO4 modified glassy electrode was applied as the working electrode, a carbon glassy rod as the counter electrode and Ag/AgCl electrode as the reference electrode. Before the electrochemical measurements, each solution was degassed using nitrogen gas for 4–5 min. Electrochemical impedance spectroscopy (EIS) was carried out at an amplitude of 0.01 V in the frequency range of 0.1–4000 Hz. Cyclic voltammetry measurements were recorded in the potential range of −1.0 V to +1.0 V at a scan rate of 0.05 V s−1 in a mixed solution of 0.1 M H3PO4, Li2SO4, NaSO4 and NaOH with ascorbic acid at 0.05 mM concentration. All measurements were executed at room temperature.Results and discussion
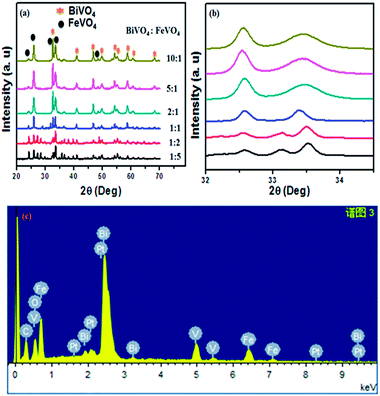
Fig. 2a shows the XRD diffraction pattern of the as-prepared BiVO4/FeVO4 nanophotocatalysts at different concentration ratios for phase structures. The XRD diffraction peaks of BiVO4/FeVO4 are in agreement with pure BiVO4 (JCPD card 85-1730)33 and FeVO4 (JCPD card 24-0541). The patterns of the BiVO4/FeVO4 heterojunction photocatalyst compounds display typical diffraction peaks from both BiVO4 and FeVO4 distinct phases, verifying that the BiVO4/FeVO4 nanocomposites at different molar ratios were prepared successfully by the autoclave hydrothermal process, as shown in Fig. 2a. The intense and edged peaks of BiVO4 suggest a larger crystallite size, while the small peaks of FeVO4 indicate a small particle size. There is a shift in the peaks (Fig. 2b) first towards the left when the ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then towards the right as the ratio increases from 5
1, and then towards the right as the ratio increases from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The color also changes gradually into yellowish as the BiVO4 concentration is increased. It can be clearly observed that the broadening of BiVO4 peaks takes place and the intensity of the FeVO4 peaks declines. Here, the optimum conditions were revealed for making BiVO4/FeVO4 (2
1. The color also changes gradually into yellowish as the BiVO4 concentration is increased. It can be clearly observed that the broadening of BiVO4 peaks takes place and the intensity of the FeVO4 peaks declines. Here, the optimum conditions were revealed for making BiVO4/FeVO4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at which high intensity peaks are found, as indicated in Fig. 2a. From the EDS analysis shown in Fig. 2c, it is observed that the elements vanadium, iron, bismuth and oxygen are homogeneously spread throughout the composite, and the presence of carbon and platinum peaks are due to carbon tape and platinum coating. Aside from this, no impurities were detected within the detection limit.
1) at which high intensity peaks are found, as indicated in Fig. 2a. From the EDS analysis shown in Fig. 2c, it is observed that the elements vanadium, iron, bismuth and oxygen are homogeneously spread throughout the composite, and the presence of carbon and platinum peaks are due to carbon tape and platinum coating. Aside from this, no impurities were detected within the detection limit.
The surface topography and morphological analysis of the BiVO4/FeVO4 nanocomposites were characterized using field emission scanning electron microscopy, on a JEOL-7001F. SEM images of the composites at different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are illustrated in Fig. 3a–f. It was noticed that at higher concentrations of FeVO4, small sized nanocomposites developed, as shown in Fig. 3a and b. When the BiVO4/FeVO4 ratio was 1
1) are illustrated in Fig. 3a–f. It was noticed that at higher concentrations of FeVO4, small sized nanocomposites developed, as shown in Fig. 3a and b. When the BiVO4/FeVO4 ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, rod and particle shaped nanostructures formed, while at a ratio of 1
5, rod and particle shaped nanostructures formed, while at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, polyhedron and particle shaped nanostructures formed. In the opposite case, upon increasing the molar ratio of BiVO4 in the BiVO4/FeVO4 nanocomposite, large crystallite sizes were found, as shown in Fig. 3d–f. In Fig. 3d the BiVO4/FeVO4 nanocomposite at a molar ratio of 2
2, polyhedron and particle shaped nanostructures formed. In the opposite case, upon increasing the molar ratio of BiVO4 in the BiVO4/FeVO4 nanocomposite, large crystallite sizes were found, as shown in Fig. 3d–f. In Fig. 3d the BiVO4/FeVO4 nanocomposite at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 comprises rods and plates, and some plates are linked with each other to form chains that look like flower type structures. At a molar ratio of 5
1 comprises rods and plates, and some plates are linked with each other to form chains that look like flower type structures. At a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the SEM image captured shows rod and plate-like structures, in which there are more agglomerations of plates than at the previous concentration ratio. The SEM image in Fig. 3f of the BiVO4/FeVO4 nanocomposite at a molar ratio 10
1, the SEM image captured shows rod and plate-like structures, in which there are more agglomerations of plates than at the previous concentration ratio. The SEM image in Fig. 3f of the BiVO4/FeVO4 nanocomposite at a molar ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shows flowers and just a few plate structures are found.
1 shows flowers and just a few plate structures are found.
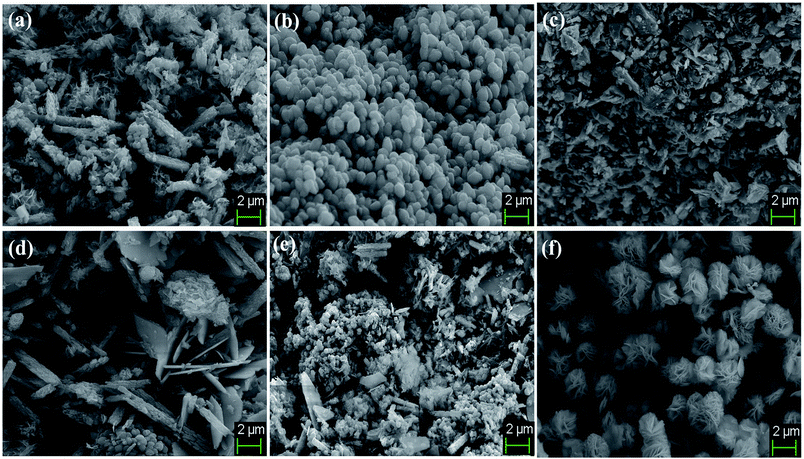 | ||
Fig. 3 SEM images of BiVO4/FeVO4 composite heterojunction photocatalysts at different molar ratios of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (b) 1 5, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (c) 1 2, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (d) 2 1, (d) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (e) 5 1, (e) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (f) 10 1 and (f) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The SEM morphologies of pure BiVO4 and FeVO4 are given in Fig. S1 (ESI).† The formation and conversion from particles to nanorods and flower shape morphologies are totally dependent on the experimental conditions, reaction mechanisms and material compositions. In our experimental results, rod and particle shaped nanostructure formation occurs when the FeVO4 concentration is higher than that of BiVO4. Contrarily, as the BiVO4 concentration increases, plate-like crystallites begin to appear, ultimately forming flower shaped nanostructures. These morphological changes take place due to differences in structure, atomic radii, crystal orientation and reactions occurring during the hydrothermal synthesis. Thus, our experimental results validate that nanorod and particle conversion into nanoplate flower-like morphologies takes place as the ratio of BiVO4 and FeVO4 is changed during the synthesis of the BiVO4/FeVO4 nanocomposites, as shown in the SEM image (Fig. 3).
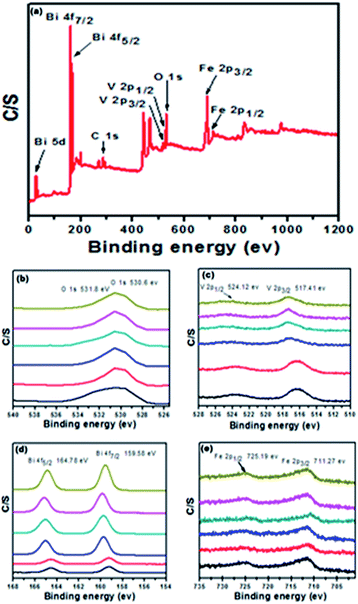
X-ray photoelectron spectroscopy (XPS) is a surface analysis method for investigating the composition and chemical states of the constituents. In order to validate further, elemental analysis was carried out by performing XPS characterization. Fig. 4a shows the typical X-ray photoelectron spectrum of the BiVO4/FeVO4 nanocomposite, which is composed of O, Bi, V, and Fe. In the XPS analysis of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiVO4/FeVO4 nanocomposite, two peaks of oxygen at 1s are located at 530.6 and 531.8 eV34,35 in Fig. 4b, showing the two different peaks of O2 in the experiment. Two peaks of V 2p3/2 at 517.41 eV and V 2p1/2 at 524.12 eV were noticed, as shown in Fig. 4c. Fig. 4d highlights the XPS peaks of Bi 4f7/2 and Bi 4f5/2, which are found at 159.44 eV and 164.68 eV.36 In Fig. 4e, the Fe 2p spectrum comprises two leading peaks (2p1/2 and 2p3/2) with many sub-peaks, with the main peaks positioned at 711.27 and 725.19 eV.37 A peak shift was found similar to that observed in the XRD analysis, which may be attributed to morphology effects or the increasing concentration of BiVO4. From the above XRD and XPS detailed spectra, the formation of the BiVO4/FeVO4 heterojunction photocatalysts can be confirmed.
1 BiVO4/FeVO4 nanocomposite, two peaks of oxygen at 1s are located at 530.6 and 531.8 eV34,35 in Fig. 4b, showing the two different peaks of O2 in the experiment. Two peaks of V 2p3/2 at 517.41 eV and V 2p1/2 at 524.12 eV were noticed, as shown in Fig. 4c. Fig. 4d highlights the XPS peaks of Bi 4f7/2 and Bi 4f5/2, which are found at 159.44 eV and 164.68 eV.36 In Fig. 4e, the Fe 2p spectrum comprises two leading peaks (2p1/2 and 2p3/2) with many sub-peaks, with the main peaks positioned at 711.27 and 725.19 eV.37 A peak shift was found similar to that observed in the XRD analysis, which may be attributed to morphology effects or the increasing concentration of BiVO4. From the above XRD and XPS detailed spectra, the formation of the BiVO4/FeVO4 heterojunction photocatalysts can be confirmed.
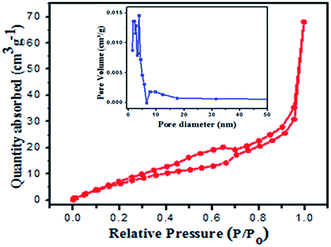
The N2 sorption isotherms of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nanocomposite photocatalyst BiVO4/FeVO4 were produced and examined. The Brunauer–Emmett–Teller surface area was found to be 70.147 cm2 g−1. The pore size was estimated from the desorption isotherms, using the Barret–Joyner–Halender (BJH) method.38,39 The evaluated pore volume and average pore diameter were found to be 0.124 cm3 g−1 and 3.798 nm, respectively, as shown in Fig. 5. The adsorption curve is a type III curve presenting the hysteresis of H2 and H3 types, which associates slit shape capillaries with large and narrow short plate-like aggregates of particles, resulting in a lamellar pore structure and slit shaped pores.40 The lamellar wedge shaped pore construction clearly indicates the presence of mesopores within the structure.
1 nanocomposite photocatalyst BiVO4/FeVO4 were produced and examined. The Brunauer–Emmett–Teller surface area was found to be 70.147 cm2 g−1. The pore size was estimated from the desorption isotherms, using the Barret–Joyner–Halender (BJH) method.38,39 The evaluated pore volume and average pore diameter were found to be 0.124 cm3 g−1 and 3.798 nm, respectively, as shown in Fig. 5. The adsorption curve is a type III curve presenting the hysteresis of H2 and H3 types, which associates slit shape capillaries with large and narrow short plate-like aggregates of particles, resulting in a lamellar pore structure and slit shaped pores.40 The lamellar wedge shaped pore construction clearly indicates the presence of mesopores within the structure.
The Brunauer–Emmett–Teller surface areas of the BiVO4/FeVO4 composites at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were 136.188, 6.019, 7.097, 9.325 and 14.679 cm2 g−1, the pore volumes were 0.213, 0.010, 0.012, 0.013 and 0.025 cm3 g−1, and the pore diameters were 1.682, 1.932, 1.682, 1.937 and 2.181 nm, respectively, as shown in Fig. S2 (ESI).†
1 were 136.188, 6.019, 7.097, 9.325 and 14.679 cm2 g−1, the pore volumes were 0.213, 0.010, 0.012, 0.013 and 0.025 cm3 g−1, and the pore diameters were 1.682, 1.932, 1.682, 1.937 and 2.181 nm, respectively, as shown in Fig. S2 (ESI).†
Fig. 6a shows the FT-IR spectra of the BiVO4/FeVO4 heterogeneous composites prepared under different molar ratios, where the absorption from 3000 to 3600 cm−1 is due to the stretching vibration of OH of absorbed water. The absorption at 1631 cm−1 is due to absorbed water molecule bending vibration. The band at 512 cm−1 is due to V–O–V deformation caused by the V–O stretching mode. The peaks at 741, 837, 915 and 1052 cm−1 correspond to V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and V–O–V joined vibrations and the stretching of the short vanadyl bond, while the Bi–O bending and asymmetric vibrations appear at 474 cm−1 and 1362 cm−1, respectively.41–45
O and V–O–V joined vibrations and the stretching of the short vanadyl bond, while the Bi–O bending and asymmetric vibrations appear at 474 cm−1 and 1362 cm−1, respectively.41–45
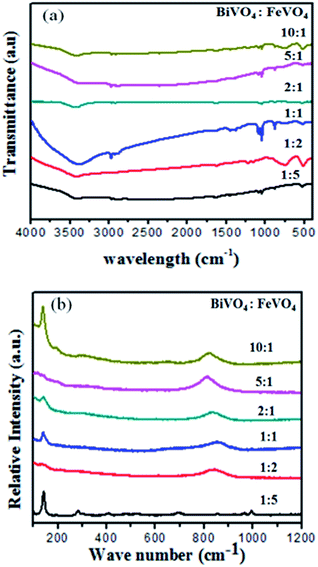 | ||
| Fig. 6 (a) FT-IR spectra and (b) Raman spectra of the BiVO4/FeVO4 heterogeneous composites prepared with different molar ratios. | ||
Raman analysis is a useful tool for providing structural information and is also a sensitive method for investigating crystallization, local structure and the electronic dimensions of materials. In Fig. 6b, Raman bands at around 198, 333, 367, 640 and 836 cm−1 corresponding to BiVO4 were observed for all samples, while bands at around 139, 300, 354, 655, 962 and 996 cm−1 represent FeVO4 in the samples.46–48 A shift in the peaks was observed as the molar ratio changed. At higher concentrations of BiVO4, the peaks were broader and covered the FeVO4 peaks, as observed in the XRD and XPS analysis, which also confirms and supports our previous results.
Photocatalytic activity of crystal violet solution
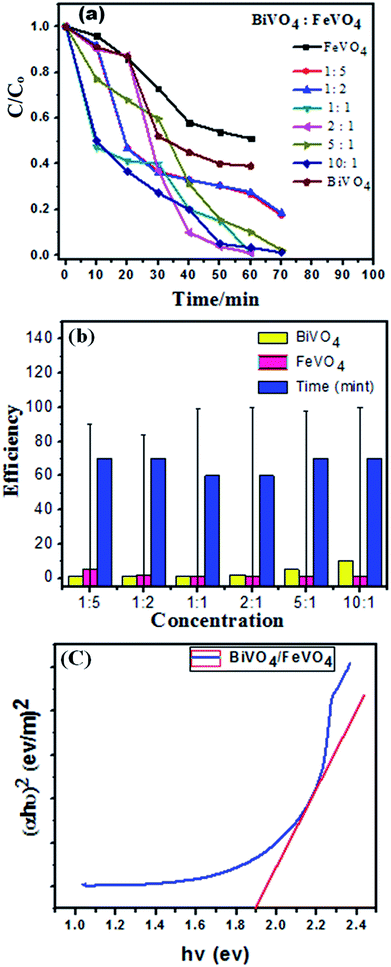
To determine the photocatalytic activity of FeVO4, BiVO4 and the BiVO4/FeVO4 nanophotocatalysts, the degradation rate of CV dye was investigated in water under visible radiation from 400 < λ < 800 nm. The chemical formula of CV is C25H30ClN3, and its chemical structure with its adsorption spectrum is shown in Fig. S3 (ESI).† The photocatalytic response of the BiVO4/FeVO4 photocatalysts is shown in Fig. S4 (ESI).† It is noticed that the photocatalytic action of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiVO4/FeVO4 is significantly higher compared to the other samples. It degraded 99.1% of CV dye within 60 min under visible radiation illumination. It is clear that in both cases of either increasing the FeVO4 or BiVO4 molar ratio, the photocatalytic activity is reduced. However, in the case of a higher concentration of FeVO4, the photocatalytic activity first increases and then reduces.
1 BiVO4/FeVO4 is significantly higher compared to the other samples. It degraded 99.1% of CV dye within 60 min under visible radiation illumination. It is clear that in both cases of either increasing the FeVO4 or BiVO4 molar ratio, the photocatalytic activity is reduced. However, in the case of a higher concentration of FeVO4, the photocatalytic activity first increases and then reduces.
When the molar ratio of BiVO4/FeVO4 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the photocatalytic degradation efficiency of CV is 71% in 60 min. Perhaps the most appropriate BiVO4/FeVO4 molar ratio to form a nanocomposite heterojunction photocatalyst is 2
5, the photocatalytic degradation efficiency of CV is 71% in 60 min. Perhaps the most appropriate BiVO4/FeVO4 molar ratio to form a nanocomposite heterojunction photocatalyst is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The 2
1. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiVO4/FeVO4 heterojunction photocatalyst facilitates effective electron–hole separation, reduces the recombination rate of charges and enhances the absorption of visible light. According to the above results and discussion, a higher concentration of BiVO4 or FeVO4 leads to reduced photocatalytic activity, which may be attributed to some particles of BiVO4 or FeVO4 that cannot be effectively incorporated into the composite photocatalyst. In this study, the 2
1 BiVO4/FeVO4 heterojunction photocatalyst facilitates effective electron–hole separation, reduces the recombination rate of charges and enhances the absorption of visible light. According to the above results and discussion, a higher concentration of BiVO4 or FeVO4 leads to reduced photocatalytic activity, which may be attributed to some particles of BiVO4 or FeVO4 that cannot be effectively incorporated into the composite photocatalyst. In this study, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiVO4/FeVO4 composite exhibited the highest degradation efficiency and was therefore determined to have the optimal ratio.
1 BiVO4/FeVO4 composite exhibited the highest degradation efficiency and was therefore determined to have the optimal ratio.
The degradation efficiency is determined by following the equation:
| Degradation efficiency% = (Co − Ct)/Co | (1) |
| (αhν) = A(hν − Eg)n | (2) |
The energy band gap (Eg) of the BiVO4/FeVO4 compound was calculated by interpolating the linear portion of the plot of (αhν)2 versus hν to the energy axis, as shown in Fig. 7c. The Eg value came out to be 1.9 eV for BiVO4/FeVO4, which is closely matched to the described values. The photocatalytic mechanism for the degradation of CV dye solution by BiVO4/FeVO4 under visible light is illustrated in Fig. 8 and can be represented by the following equations:
| BiVO4/FeVO4 + hν → e− + h+ + BiVO4/FeVO4 | (3) |
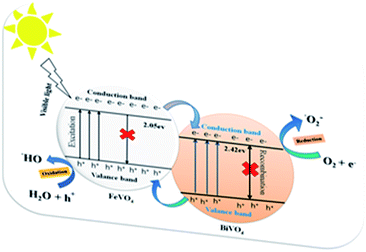 | ||
| Fig. 8 Reaction mechanism of CV photodegradation over the BiVO4/FeVO4 composite under visible light. | ||
Oxidation occurs at the FeVO4 surface:
| h+ + H2O → ˙OH + H+ | (4) |
| 2h+ + 2H2O → 2H+ + H2O2 | (5) |
Reduction reaction occurs at BiVO4:
| e− + O2 → ˙O2− | (6) |
When visible light falls on the solution containing CV dye and the BiVO4/FeVO4 photocatalyst, the electrons move fast from FeVO4 to BiVO4, while the holes of BiVO4 move to the valence band (VB) of FeVO4. An oxidation reaction occurs at the VB of FeVO4, where positive holes react with water to form hydroxyl radicals (˙OH), while a reduction reaction occurs at the conduction band (CB) of BiVO4, where negative electrons (e−) produce superoxide radicals (˙O2−) by reacting with dissolved oxygen. The superoxide radicals and hydroxide radicals from both ends oxidize the toxic dye C25H30ClN3 molecules and decompose them into harmless or non-toxic molecules with CO2, H2O and NO3 byproducts, as eqn (7) indicates below.
| C25H30ClN3 + (˙OH, ˙O2−) → CO2 + H2O + NO3 + other intermediates | (7) |
During the photocatalysis process, various primary active species including photogenerated holes, singlet oxygen atoms, hydroxyl radicals and superoxide radicals could be created during the UV-visible degradation process.50–52 According to previous studies, in the presence of N2 and radical scavengers, ˙OH and ˙O2− are the two main active species in the entire process.5,45,50,52–56 From previous results, the dominant active oxygen species generated in direct oxidation and photocatalytic reactions are 1O2 and ˙OH radicals.32,50,57,58 On the basis of the above studies, the probability of forming ˙OH should be much lower than that for ˙O2−; however, the hydroxyl radical is an extremely strong oxidizing agent, which takes the degradation process to either partial or complete mineralization of various organic contaminations. The photocatalytic degradation of CV dye by pure FeVO4, pure BiVO4 and the BiVO4/FeVO4 heterojunction nanophotocatalysts at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was performed and absorbance spectra are provided in the ESI (Fig. S4†).
1 was performed and absorbance spectra are provided in the ESI (Fig. S4†).
On the basis of the aforementioned studies,5,32,52,56,58 once the electrons enter the conduction band of BiVO4, it induces the production of oxygen, which causes decomposition of CV dye. Hydroxyl radicals are also produced by the reaction of ˙O2− radicals with H+ ions and h+ holes with OH− ions or H2O. No electron paramagnetic resonance (EPR) signal was noticed when the reaction was executed in the dark. However, the signals with intensities corresponding to the characteristic peaks of DMPO-˙OH and DMPO-˙O2− adducts were observed during the reaction process in the EPR experiment. In Fig. 9, not only are the six characteristic peaks of the DMPO-˙O2− observed, but the four characteristic peaks of the DMPO-˙OH radical (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; quartet pattern) are also observed by irradiation of the BiVO4/FeVO4 heterojunction nanocomposite solution under visible light. The decay of CV dye by the generated oxidant species is represented by eqn (8) and (9).
1; quartet pattern) are also observed by irradiation of the BiVO4/FeVO4 heterojunction nanocomposite solution under visible light. The decay of CV dye by the generated oxidant species is represented by eqn (8) and (9).
| CV + ˙O2− → decomposed compounds | (8) |
| CV + ˙OH → decomposed compounds | (9) |
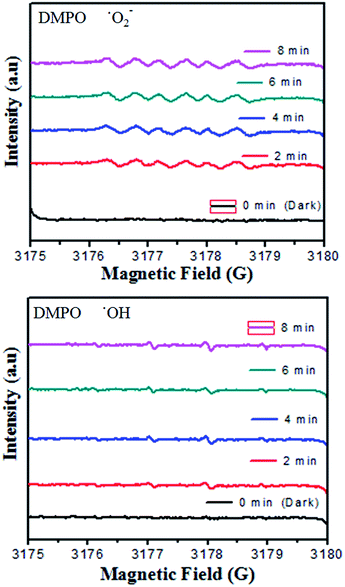 | ||
| Fig. 9 DMPO spin trapping EPR spectra for DMPO-˙O2− and DMPO-˙OH under visible light irradiation with the BiVO4/FeVO4 photocatalyst. | ||
The effects of catalyst dose, dye concentration, stability and recyclability factors were also investigated and the results are provided in the ESI (Fig. S5†). In the absorption spectra of CV solution using BiVO4/FeVO4 at different doses of 0, 2.5 and 5 mg/10 mL, it is observed that the rate of degradation decreases as the dose of the BiVO4/FeVO4 decreases by 5 mg/10 mL to 0 mg/10 mL in CV solutions.
This implies that the amount of BiVO4/FeVO4 catalyst has a significant effect on the photocatalytic reaction for the decomposition of CV organic dye. Dye concentration effect was evaluated by taking different initial concentrations of CV dye (10–50 mg L−1) and the results are shown in Fig. S5 (ESI).† The color removal efficiency decreases as the CV concentration is increased; this is due to the fact that the increasing concentration of CV prevents light penetration into the solution. Secondly, the number of CV molecules absorbed on the catalyst surface is increased, while the numbers of OH and O radicals remain the same under specific conditions. Furthermore, the stability and recyclability of the BiVO4/FeVO4 heterogeneous photocatalyst were also investigated, as shown in Fig. S5 (ESI).† The photocatalytic activity was evaluated three times with the BiVO4/FeVO4 photocatalyst and the sample showed superb stability as well as recyclability.
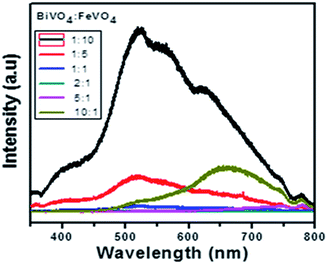
The photoluminescence spectra exhibit the recombination rate and electron–hole separation within the photocatalysts. A higher PL intensity reveals a higher degree of electron–hole recombination, which reduces the photocatalytic degradation efficiency.59 A lower PL peak intensity indicates reduced recombination, which results in efficient charge transfer over the catalyst surface in a semiconductor.60,61 So, the low recombination allows superior dye degradation, which is consistent with the photocatalytic results shown in Fig. 10.
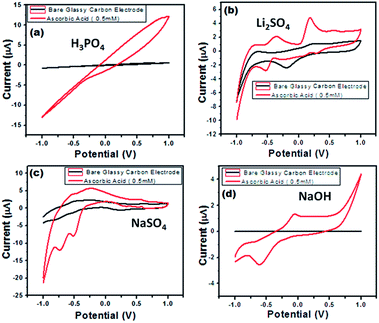
Electrochemical impedance spectroscopy is a versatile tool for measuring the conductivity, surface analysis and electron transfer.62 It was noticed that in all electrolytes, the impedance spectrum is either a semicircle or nearly a circle at higher ac frequency and a line at low modulation ac frequency, as shown in Fig. 11. Moreover, in the modified BiVO4/FeVO4 nanocomposite GCE, the diameter of the Nyquist circle decreases as compared to that of the bare GCE, indicating that the BiVO4/FeVO4 nanocomposite exhibits much higher electron transfer on the surface and superior electrochemical activity, and as a result, the resistance decreases and improves the electron transfer process. Cyclic voltammetry was used to assess the detection of ascorbic acid analyte by the BiVO4/FeVO4 nanocomposite material. Cyclic voltammetry showed the response of the bare electrode and glassy carbon modified electrode both in the presence and absence of 0.5 mM ascorbic acid at a scan rate of 50 mV s−1 at −1 to 1 V s−1 as shown in Fig. 12a. The bare electrode and modified glassy carbon electrode in H3PO4 solution did not show any oxidation peak both in the absence and presence of ascorbic acid. However, an enhanced current was observed, which shows that the semiconductor material has the potential to change the current intensity.
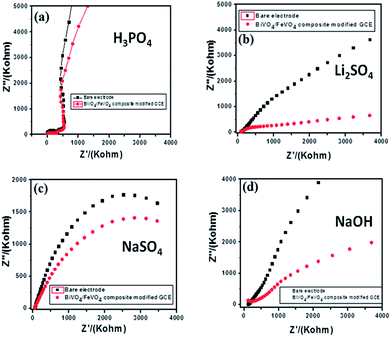 | ||
| Fig. 11 Electrochemical impedance spectroscopy of bare and modified BiVO4/FeVO4 nanocomposite GCEs in different electrolytes: (a) 0.1 M H3PO4, (b) 0.1 M Li2SO4, (c) 0.1 M NaSO4 and (d) 0.1 M NaOH. | ||
In LiSO4 solution (Fig. 12b) the bare GCE gives one oxidation peak and one reduction peak located at 0.20 V and −0.20 V, respectively. Meanwhile, the BiVO4/FeVO4 nanocomposite GCE response shows two anodic peaks (cvp1 = −0.36 V, cvp2 = −0.17 V) and two cathodic peaks (cvp1′ = 0.52 V, cvp2′ = 0.19 V) as well as an increase in the current intensity. In the case of NaSO4 solution (Fig. 12c), the bare electrode did not show any oxidation and reduction but the modified BiVO4/FeVO4 nanocomposite GCE showed two anodic peaks (cvp1 = −0.49 V, cvp2 = −0.71 V). In the case of the basic solution (NaOH), the bare GCE did not give any response, as shown in Fig. 12d. However, the modified BiVO4/FeVO4 nanocomposite GCE showed a change in current intensity, as well as one reduction peak (cvp = −0.05 V) and one oxidation peak located at cvp = −0.59 V.
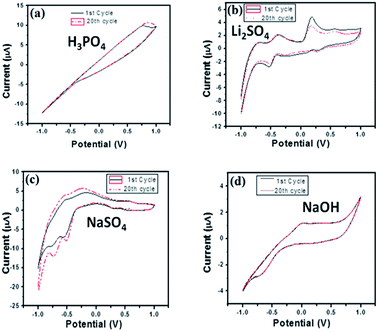
For long-term stability confirmation we stored the modified BiVO4/FeVO4 nanocomposite GCE for one month at room temperature and used it for sensing ascorbic acid. It was found that it detects ascorbic acid without any decrease in current. Furthermore, we checked its reproducibility after twenty measurements, and it was found to vary slightly relative to the standard deviation of the original values, as indicated in Fig. 13. Thus, the modified BiVO4/FeVO4 nanocomposite GCE exhibits excellent stability and reproducibility for the electrochemical determination of ascorbic acid.
Conclusions
BiVO4/FeVO4 nanocomposites were synthesized via an autoclave hydrothermal method using bismuth nitrate dehydrate (Bi(NO3)3·5H2O) and iron nitrate dehydrate (Fe(NO3)3·9H2O) as bismuth and ferric ion sources and ammonia metavanadate NH4VO3 as a vanadium ion source, at different BiVO4 and FeVO4 molar ratios. The prepared nanocomposites were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray photoelectron spectroscopy (XPS), the Brunauer–Emmett–Teller (BET) method, Fourier transform infrared (FT-IR) spectroscopy, Raman spectroscopy, photoluminescence (Pl), electron paramagnetic resonance, cyclic voltammetry and electrochemical impendence spectroscopy (EIS). The BiVO4/FeVO4 nanocomposites were investigated for their activity as nanophotocatalysts by the photocatalytic degradation of crystal violet (CV) dye under visible light irradiation. The as-synthesized heterojunction photocatalysts exhibited good photocatalytic activity, especially BiVO4/FeVO4 at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which showed superior degradation efficiency owing to the higher specific surface area of 70.147 cm2 g−1 and pore size of 3.798 nm. The material was also studied for the electrochemical detection of an important analyte, ascorbic acid, and it showed good results. This work reveals the potential of BiVO4/FeVO4 nanocomposites for applications in environmental science as well as in biosensor fields.
1, which showed superior degradation efficiency owing to the higher specific surface area of 70.147 cm2 g−1 and pore size of 3.798 nm. The material was also studied for the electrochemical detection of an important analyte, ascorbic acid, and it showed good results. This work reveals the potential of BiVO4/FeVO4 nanocomposites for applications in environmental science as well as in biosensor fields.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful to the financial support by the Basic Science Center Project of NSFC under grant No. 51788104, the National Natural Science Foundation of China (Grant No. 51572148 and 51531006), and the Tsinghua University Initiative Scientific Research Program. The present work was also supported by Ministry of Science and Technology (MoST), Govt. of Pakistan, under a program to initiate nano-biotechnology research at Government College University, Faisalabad, Pakistan. This work was also supported by Higher Education Commission (HEC) of Pakistan.Notes and references
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef.
- F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam and R. Naidu, Appl. Catal., A, 2009, 359, 25–40 CrossRef.
- C. L. Wong, Y. N. Tan and A. R. Mohamed, J. Environ. Manage., 2011, 92, 1669–1680 CrossRef PubMed.
- Y. Li, X. Yuan, Z. Wu, H. Wang, Z. Xiao, Y. Wu, X. Chen and G. Zeng, Chem. Eng. J., 2016, 303, 636–645 CrossRef.
- Y.-R. Jiang, S.-Y. Chou, J.-L. Chang, S.-T. Huang, H.-P. Lin and C.-C. Chen, RSC Adv., 2015, 5, 30851–30860 RSC.
- H. S. Peavy and G. Tchobanoglous, Environ. Eng., 1985, 628 Search PubMed.
- F. Sayılkan, S. Erdemoğlu, M. Asiltürk, M. Akarsu, Ş. Şener, H. Sayılkan, M. Erdemoğlu and E. Arpaç, Mater. Res. Bull., 2006, 41, 2276–2285 CrossRef.
- R. Andreozzi, V. Caprio, A. Insola and R. Marotta, Catal. Today, 1999, 53, 51–59 CrossRef.
- D. Wang, F. Jia, H. Wang, F. Chen, Y. Fang, W. Dong, G. Zeng, X. Li, Q. Yang and X. Yuan, J. Colloid Interface Sci., 2018, 519, 273–284 CrossRef PubMed.
- R. Konta, H. Kato, H. Kobayashi and A. Kudo, Phys. Chem. Chem. Phys., 2003, 5, 3061–3065 RSC.
- V. Conte and B. Floris, Dalton Trans., 2011, 40, 1419–1436 RSC.
- L. Mai, L. Xu, C. Han, X. Xu, Y. Luo, S. Zhao and Y. Zhao, Nano Lett., 2010, 10, 4750–4755 CrossRef PubMed.
- S. Tokunaga, H. Kato and A. Kudo, Chem. Mater., 2001, 13, 4624–4628 CrossRef.
- A. Iwase, H. Kato and A. Kudo, J. Sol. Energy Eng., 2010, 132, 021106 CrossRef.
- T. Masui, T. Honda and N. Imanaka, Dyes Pigm., 2013, 99, 636–641 CrossRef.
- X. Zhang, Z. Ai, F. Jia, L. Zhang, X. Fan and Z. Zou, Mater. Chem. Phys., 2007, 103, 162–167 CrossRef.
- Y. Zhao, Y. Xie, X. Zhu, S. Yan and S. Wang, Chem.–Eur. J., 2008, 14, 1601–1606 CrossRef PubMed.
- A. Kudo, K. Ueda, H. Kato and I. Mikami, Catal. Lett., 1998, 53, 229–230 CrossRef.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 RSC.
- M. Long, W. Cai, J. Cai, B. Zhou, X. Chai and Y. Wu, J. Phys. Chem. B, 2006, 110, 20211–20216 CrossRef PubMed.
- S. Shenawi-Khalil, V. Uvarov, S. Fronton, I. Popov and Y. Sasson, J. Phys. Chem. C, 2012, 116, 11004–11012 CrossRef.
- M. Wang, Q. Liu and D. Zhang, Adv. Mater. Res., 2010, 129, 784–788 Search PubMed.
- J. Li, W. Zhao, Y. Guo, Z. Wei, M. Han, H. He, S. Yang and C. Sun, Appl. Surf. Sci., 2015, 351, 270–279 CrossRef.
- M. Baojun, L. Keying, S. Weiguang and L. Wanyi, Appl. Surf. Sci., 2014, 317, 682–687 CrossRef.
- C. Shifu, Z. Wei, L. Wei, Z. Huaye, Y. Xiaoling and C. Yinghao, J. Hazard. Mater., 2009, 172, 1415–1423 CrossRef PubMed.
- L. Ma, S. Liang, X. L. Liu, D. J. Yang, L. Zhou and Q. Q. Wang, Adv. Funct. Mater., 2015, 25, 898–904 CrossRef.
- C. Li, S. Wang, T. Wang, Y. Wei, P. Zhang and J. Gong, Small, 2014, 10, 2783–2790 CrossRef PubMed.
- X. Gao, H. B. Wu, L. Zheng, Y. Zhong, Y. Hu and X. W. D. Lou, Angew. Chem., 2014, 126, 6027–6031 CrossRef.
- S. Chen, W. Zhao, W. Liu, H. Zhang, X. Yu and Y. Chen, J. Hazard. Mater., 2009, 172, 1415–1423 CrossRef PubMed.
- H. Ma, X. Yang, Z. Tao, J. Liang and J. Chen, CrystEngComm, 2011, 13, 897–901 RSC.
- C. Yu, S. Dong, J. Feng, J. Sun, L. Hu, Y. Li and J. Sun, Environ. Sci. Pollut. Res., 2014, 21, 2837–2845 CrossRef PubMed.
- S.-Y. Chou, W.-H. Chung, L.-W. Chen, Y.-M. Dai, W.-Y. Lin, J.-H. Lin and C.-C. Chen, RSC Adv., 2016, 6, 82743–82758 RSC.
- S. Gao, B. Gu, X. Jiao, Y. Sun, X. Zu, F. Yang, W. Zhu, C. Wang, Z. Feng and B. Ye, J. Am. Chem. Soc., 2017, 139, 3438–3445 CrossRef PubMed.
- S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, Appl. Catal., B, 2014, 144, 885–892 CrossRef.
- S. Wang, Y. Guan, L. Wang, W. Zhao, H. He, J. Xiao, S. Yang and C. Sun, Appl. Catal., B, 2015, 168, 448–457 CrossRef.
- S. Gu, W. Li, F. Wang, S. Wang, H. Zhou and H. Li, Appl. Catal., B, 2015, 170, 186–194 CrossRef.
- W. Yang, G. Tan, J. Huang, H. Ren, A. Xia and C. Zhao, Ceram. Int., 2015, 41, 1495–1503 CrossRef.
- D. Y. Chung, S. W. Jun, G. Yoon, H. Kim, J. M. Yoo, K.-S. Lee, T. Kim, H. Shin, A. K. Sinha and S. G. Kwon, J. Am. Chem. Soc., 2017, 139, 6669–6674 CrossRef PubMed.
- S. S. Imam, Z. U. Zango and H. Abdullahi, Am. Sci. Res. J. Eng., Technol., Sci., 2018, 41, 26–39 Search PubMed.
- S. B. Khan, M. Hou, S. Shuang and Z. Zhang, Appl. Surf. Sci., 2017, 400, 184–193 CrossRef.
- A. Š. Vuk, B. Orel and G. Dražič, J. Solid State Electrochem., 2001, 5, 437–449 CrossRef.
- M. Alagiri, S. Ponnusamy and C. Muthamizhchelvan, J. Mater. Sci.: Mater. Electron., 2012, 23, 728–732 CrossRef.
- M. Ghiyasiyan-Arani, M. Salavati-Niasari, M. Masjedi-Arani and F. Mazloom, J. Mater. Sci.: Mater. Electron., 2018, 29, 474–485 CrossRef.
- P. Pookmanee, S. Kojinok and S. Phanichphant, Trans. Mater. Res. Soc. Jpn., 2014, 39, 431–434 CrossRef.
- Y.-H. Lee, Y.-M. Dai, J.-Y. Fu and C.-C. Chen, Mol. Catal., 2017, 432, 196–209 CrossRef.
- T. Lehnen, M. Valldor, D. Nižňanský and S. Mathur, J. Mater. Chem. A, 2014, 2, 1862–1868 RSC.
- V. Merupo, S. Velumani, G. Oza, M. Makowska-Janusik and A. Kassiba, Mater. Sci. Semicond. Process., 2015, 31, 618–623 CrossRef.
- S. Nikam and S. Joshi, RSC Adv., 2016, 6, 107463–107474 RSC.
- P. Brack, J. S. Sagu, T. Peiris, A. McInnes, M. Senili, K. Wijayantha, F. Marken and E. Selli, Chem. Vap. Deposition, 2015, 21, 41–45 CrossRef.
- W. W. Lee, C.-S. Lu, C.-W. Chuang, Y.-J. Chen, J.-Y. Fu, C.-W. Siao and C.-C. Chen, RSC Adv., 2015, 5, 23450–23463 RSC.
- H.-P. Lin, C.-C. Chen, W. W. Lee, Y.-Y. Lai, J.-Y. Chen, Y.-Q. Chen and J.-Y. Fu, RSC Adv., 2016, 6, 2323–2336 RSC.
- C.-T. Yang, W. W. Lee, H.-P. Lin, Y.-M. Dai, H.-T. Chi and C.-C. Chen, RSC Adv., 2016, 6, 40664–40675 RSC.
- S.-Y. Chou, C.-C. Chen, Y.-M. Dai, J.-H. Lin and W. W. Lee, RSC Adv., 2016, 6, 33478–33491 RSC.
- F.-Y. Liu, Y.-R. Jiang, C.-C. Chen and W. W. Lee, Catal. Today, 2018, 300, 112–123 CrossRef.
- C.-W. Siao, H.-L. Chen, L.-W. Chen, J.-L. Chang, T.-W. Yeh and C.-C. Chen, J. Colloid Interface Sci., 2018, 596 Search PubMed.
- F.-Y. Liu, J.-H. Lin, Y.-M. Dai, L.-W. Chen, S.-T. Huang, T.-W. Yeh, J.-L. Chang and C.-C. Chen, Catal. Today, 2018, 613 Search PubMed.
- S.-T. Huang, Y.-R. Jiang, S.-Y. Chou, Y.-M. Dai and C.-C. Chen, J. Mol. Catal. A: Chem., 2014, 391, 105–120 CrossRef.
- C.-C. Chen, C.-T. Yang, W.-H. Chung, J.-L. Chang and W.-Y. Lin, J. Taiwan Inst. Chem. Eng., 2017, 78, 157–167 CrossRef.
- A. Arshad, J. Iqbal, M. Siddiq, Q. Mansoor, M. Ismail, F. Mehmood, M. Ajmal and Z. Abid, J. Appl. Phys., 2017, 121, 024901 CrossRef.
- C. Shifu, Z. Wei, L. Wei and Z. Sujuan, J. Sol-Gel Sci. Technol., 2009, 50, 387–396 CrossRef.
- S. Fatima, S. I. Ali, M. Z. Iqbal and S. Rizwan, RSC Adv., 2017, 7, 35928–35937 RSC.
- L. Pei, N. Lin, T. Wei, H. Liu and H. Yu, J. Mater. Chem. A, 2015, 3, 2690–2700 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03890b |
| This journal is © The Royal Society of Chemistry 2018 |