Hybridization chain reaction: a versatile molecular tool for biosensing, bioimaging, and biomedicine
Sai
Bi
a,
Shuzhen
Yue
a and
Shusheng
Zhang
*b
aCollaborative Innovation Center for Marine Biomass Fiber, Materials and Textiles of Shandong Province, College of Chemistry and Chemical Engineering, Shandong Sino-Japanese Center for Collaborative Research of Carbon Nanomaterials, Laboratory of Fiber Materials and Modern Textiles, the Growing Base for State Key Laboratory, Qingdao University, Qingdao 266071, P. R. China
bShandong Provincial Key Laboratory of Detection Technology for Tumor Makers, College of Chemistry and Chemical Engineering, Linyi University, Linyi 276005, P. R. China. E-mail: shushzhang@126.com
First published on 2nd June 2017
Abstract
Developing powerful, simple and low-cost DNA amplification techniques is of great significance to bioanalysis and biomedical research. Thus far, many signal amplification strategies have been developed, such as polymerase chain reaction (PCR), rolling circle amplification (RCA), and DNA strand displacement amplification (SDA). In particular, hybridization chain reaction (HCR), a type of toehold-mediated strand displacement (TMSD) reaction, has attracted great interest because of its enzyme-free nature, isothermal conditions, simple protocols, and excellent amplification efficiency. In a typical HCR, an analyte initiates the cross-opening of two DNA hairpins, yielding nicked double helices that are analogous to alternating copolymers. As an efficient amplification platform, HCR has been utilized for the sensitive detection of a wide variety of analytes, including nucleic acids, proteins, small molecules, and cells. In recent years, more complicated sets of monomers have been designed to develop nonlinear HCR, such as branched HCR and even dendritic systems, achieving quadratic and exponential growth mechanisms. In addition, HCR has attracted enormous attention in the fields of bioimaging and biomedicine, including applications in fluorescence in situ hybridization (FISH) imaging, live cell imaging, and targeted drug delivery. In this review, we introduce the fundamentals of HCR and examine the visualization and analysis techniques for HCR products in detail. The most recent HCR developments in biosensing, bioimaging, and biomedicine are subsequently discussed with selected examples. Finally, the review provides insight into the challenges and future perspectives of HCR.
1 Introduction
DNAs, as genetic information carriers and protein encoders, play central roles in life processes that translate the genetic code into proteins and further regulate diverse cellular functions.1,2 In addition to their biological functions, DNAs have been considered functional materials for the construction of DNA nanostructures3–6 and circuits.7 More recently, functional nucleic acid structure-based amplification techniques have emerged as attractive tools in the fields of biotechnology. These nucleic acid amplification techniques can be divided into two categories: thermocycling amplification techniques, which include polymerase chain reaction (PCR)8 and ligase chain reaction (LCR),9 and isothermal amplification techniques, including helicase-dependent amplification (HDA),10 rolling circle amplification (RCA),11 and strand displacement amplification (SDA), such as enzyme-mediated SDA (e.g., polymerase-mediated SDA)12 and enzyme-free SDA (e.g., toehold-mediated strand displacement [TMSD] amplification).13 Compared to thermocycling amplification methods, which often involve the use of thermal cyclers and suffer from externally driven melting and rehybridization, isothermal amplification protocols can be implemented under simple conditions (e.g., at a constant temperature).14 In particular, TMSD amplification is one of the most popular isothermal amplification strategies because of its advantages, which include being enzyme-free and having high amplification efficiency and controllable kinetics. In a typical TMSD, the reaction is initiated at the complementary single-stranded overhang region (termed as a toehold), and a long single-stranded DNA (ssDNA) displaces a short one and hybridizes with a third strand to form a double-helix structure with more complementary base pairs.15–17 The kinetics of the strand displacement process has been determined to be regulated by the number and species of bases in the toehold region.13 Theoretically, the rate of strand displacement can be accelerated 106-fold using TMSD.17Among TMSD systems, hybridization chain reaction (HCR) is a simple and efficient isothermal amplification process proposed by Dirks and Pierce in 2004.19 In a typical HCR, an initiator triggers a cascade of hybridization events between two species of DNA hairpins, leading to the formation of a nicked double helix with tens to hundreds of repeated units until the hairpins are exhausted. Given the advantages of its enzyme-free nature, efficient isothermal amplification, ultrahigh sensitivity and structural flexibility, HCR has emerged as a powerful molecular tool with versatile applications in biosensing, bioimaging, and biomedicine. Biosensing, signal transduction, or transforming input molecules into readable signal outputs, can readily be achieved via incorporation of HCR products with functional moieties such as fluorophores,25–28 nanoparticles (NPs),29 and electrochemical reagents.30,31 To date, HCR has been used for the sensitive detection of various targets, including nucleic acids (DNAs and RNAs),32 proteins,33,34 enzyme activities,35,36 small biomolecules,37–39 metal ions,40,41 and even tumour cells.42,43 In addition to target analyte detection, recognition molecules (e.g., antibodies [Abs] and aptamers) have been combined with HCR to develop novel methods for in situ or intracellular imaging to better understand the biological processes, and these methods also can be applied to disease diagnosis and therapy.20,21,45,128 More recently, HCR applications have expanded to include biomedicine; for example, HCR products have been used as drug delivery carriers with high payload capacities.23,24,46 Although the applications of HCR in biosensing have undergone tremendous development during the last few decades, the most significant HCR developments in bioimaging and biomedicine have occurred in recent years. Given its advantages (efficient isothermal amplification, ultrahigh sensitivity, structural versatility, and enzyme-free nature), HCR holds great promise as a powerful molecular biotechnology tool for versatile applications in nanomedicine and nanotechnology.
In this review, we first describe the fundamental principles of HCR and demonstrate visualization and analysis techniques of HCR products. Then, we discuss recent representative works using HCR, which are classified into three sections based on their application fields: biosensing, bioimaging, and biomedicine. Finally, the challenges and outlook of HCR development are considered.
2 Fundamentals of HCR
2.1 Basic features of HCR
As a target-triggered amplification system, HCR was first demonstrated and named by Dirks and Pierce in 2004.19 They introduced a novel strategy in which DNA can accomplish the roles of recognition and signal amplification simultaneously by binding to a substrate. In a typical HCR process, the recognition of a target initiates the cross-opening of two DNA hairpins to form DNA polymeric nanowires (Fig. 1). Briefly, two species of hairpins (H1 and H2) caught in a kinetic trap can coexist metastably in the absence of an initiator (I) (Fig. 1a). The introduction of I (a*–b*) opens H1 via a TMSD reaction in which I binds to the sticky end of H1 (domain a) and undergoes a branch migration (Fig. 1b). The newly exposed domain (c–b*) of H1 acts as another initiator to interact with the sticky end of H2 (domain c*), resulting in the release of sequence a*–b* of H2 that is identical to I and, thus, subsequently opens H1 (Fig. 1c). Finally, DNA polymeric nanowires can be produced by the cross-opening of two DNA hairpins until the supply of H1 or H2 is exhausted. Dirks and Pierce also showed that the molecular weight of the HCR product is inversely proportional to the amount of initiator. In addition, based on aptamer recognition, they demonstrated a versatile application of HCR in the detection of adenosine triphosphate (ATP). In 2007, the same group proposed an autonomous polymerization motor in which the target triggered a four-way branch migration to perform HCR, resulting in the formation of a long nicked double helix.47 Unlike the traditional analytical technique of PCR, HCR is an isothermal and non-enzymatic amplification strategy. Another significant difference between HCR and PCR is that HCR is a probe-amplification technique, whereas PCR is a target-amplification technique.32 Thus, HCR can effectively reduce false-positive results and cross-contamination from amplicons, which often occur in PCR.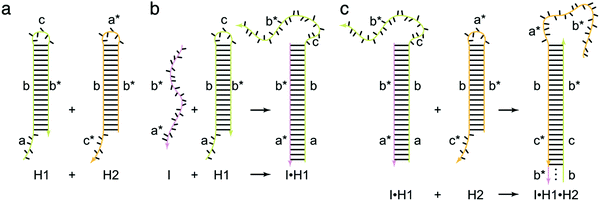 | ||
| Fig. 1 Fundamentals of HCR. The initiator (I) triggers a chain hybridization reaction between alternating H1 and H2 hairpins to form a nicked double helix. Reprinted with permission from ref. 19. Copyright (2004) National Academy of Sciences. | ||
The crucial elements of HCR are two auxiliary hairpins that contain three domains: toehold, stem, and loop. In the hairpin structure, the short loop is protected by the long stem to store potential energy.19 The kinetics of HCR can be precisely controlled by changing the length and binding strength of the toehold sequence. Thus, to programmably control the HCR process, the sequence and length of the toehold are crucial factors in the rational design of an HCR. Generally, HCR cannot be effectively initiated if the toehold domain is too short. It has been found that the reaction rate remains stable and almost unchanged when the toehold length is approximately 6–10 nucleotides.18 Recently, Yung and co-workers established a four-point set of guidelines for designing the lengths and GC contents of the hairpin toehold and stem domains to improve the kinetics of HCR.48 Up to now, a variety of novel strategies for activation and control of the HCR process have been reported such as photoactivation,49,50 pH control,51 and proximity-dependent initiation.52
2.2 Nonlinear HCR
In most cases, HCR is fundamentally a linear growth mechanism in response to an initiator. In recent years, more complicated sets of monomers have been designed to develop nonlinear HCR, such as branched HCR and even dendritic systems. Compared with linear HCR, nonlinear HCR can readily achieve quadratic or exponential growth kinetics, thereby facilitating construction of amplified biosensing platforms with ultrahigh sensitivity and excellent selectivity. For example, based on triggered HCR, LaBean's group proposed a dendritic DNA nanostructure for the first time.53 Although this system could be used to detect an arbitrary target, many problems remained such as the thermal process (from 25 °C to 30 °C), leakage of hairpins, and need for a relatively long stem sequence (15 base pairs), which could lead to linear growth rather than dendritic growth.Alternatively, Hsing's group developed a target DNA-triggered nonlinear HCR protocol using two double-stranded DNAs (dsDNAs) as substrates (substrate-A and substrate-B) and two ssDNAs as assistants (assistant-A and assistant-B) (Fig. 2A).27 The A and B substrates were designed with toehold and bulge loop domains. A trigger DNA initiated successive TMSD reactions, resulting in the self-assembly of double-stranded substrates into dendritic DNA structures via exponential growth. Moreover, the same group successfully applied this strategy to immobilization-free and enzyme-free electrochemical detection of DNA, which offered a limit of detection as low as 100 fM and excellent specificity for detecting single-nucleotide polymorphisms.54 Although the above target-triggered nonlinear HCR strategies could achieve exponential amplification for nucleic acid detection and efficiently controlled system leakage, the introduction of double-stranded substrates required purification steps to remove the unreacted ssDNAs. A hairpin-based hyperbranched HCR (HB-HCR) strategy was also proposed (Fig. 2B).28 This system consisted of two super-hairpins (SH1 and SH2), two hairpins (H1 and H2), and two assistant ssDNAs (AS1 and AS2). In the super-hairpin structures, two base pairs were pre-programmed in the bulged loop, which divided the stem into two domains and effectively avoided the spontaneous initiation of HB-HCR in the absence of an initiator. The introduction of a target DNA triggered a cascade of TMSD events, resulting in the self-assembly of hyperbranched and nicked double-stranded DNA structures. This HB-HCR demonstrated exponential growth kinetics and allowed reliable detection of target DNA at concentrations as low as 10−13 M. More recently, through the integration of catalytic hairpin assembly (CHA) with HCR, a triggered and catalytic system for the self-assembly of hyperbranched DNA structures was reported by the same group.55 In addition, HCR has been applied to build a variety of complex DNA nanostructures, such as ladder-like and ring-like DNA nanostructures56 and DNA hydrogels.57
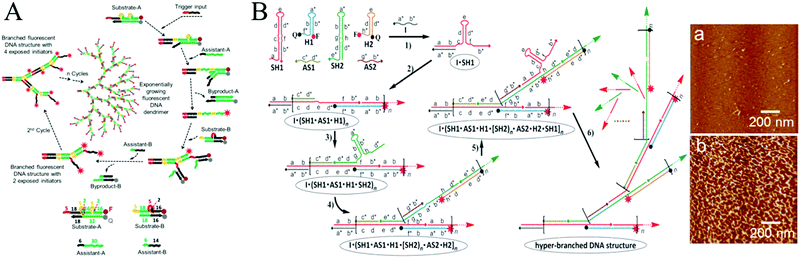 | ||
| Fig. 2 Nonlinear HCR systems. (A) Self-assembly of DNA dendrimers via triggered chain-branching growth. Reprinted with permission from ref. 27. Copyright (2014) American Chemical Society. (B) HB-HCR. Inset: Atomic force microscopy (AFM) images of assembled products in (a) the absence and (b) presence of initiator DNA (I). Adapted with permission from ref. 28. Copyright (2015) John Wiley and Sons. | ||
2.3 Visualization and analysis techniques of HCR products
Since numerous signal readout techniques are available, the HCR process and its products can be flexibly monitored and detected. Gel electrophoresis is one of the most common ways to verify the formation of HCR products, but it is rarely used for quantitative determination.19,21 AFM has been applied to observe the morphology of HCR products, such as nanowires (Fig. 5A and 13A)23,58,59 and dendritic or hyperbranched structures (Fig. 2B).27,28,55 The length and height of assembled HCR products can also be obtained directly from AFM.Optical detection methods provide effective tools for the quantitative determination of analytes. Optical readout techniques mainly include fluorescence, chemiluminescence, bioluminescence, colorimetry, surface plasmon resonance (SPR), and surface-enhanced Raman scattering (SERS). HCR-based fluorescence methods can be classified into four types: (1) fluorophores that are chemically conjugated to the hairpins;25–28,60 (2) a label-free fluorescent strategy in which the fluorescent dyes (e.g., SYBR Green I) intercalate into the grooves of dsDNA;61 (3) a fluorescence resonance energy transfer (FRET) strategy in which a fluorescence donor and an acceptor are labelled at the appropriate positions of hairpins;62 and (4) NPs used as fluorescent signal reporters, such as quantum dots (QDs),63,84 silver nanoclusters (AgNCs),64 and copper NPs (CuNPs).43 For chemiluminescence, a signal is usually generated by immobilizing horseradish peroxidase (HRP) molecules on the HCR products; HRP catalyses the oxidation of luminol by H2O2.35 DNAzyme-based chemiluminescence strategies, such as the hemin/G-quadruplex HRP-mimicking DNAzyme59 and manganese porphyrin (MnTMPyP)-dsDNA mimicking peroxidase,34 have also attracted increasing attention in recent years. For bioluminescence, HCR can be used for the homogeneous detection of various targets.65 Additionally, colorimetric assays can be mainly divided into two major classes. The first is gold nanoparticle (AuNP)-based assays. In this case, colorimetric and spectroscopic analysis of HCR products can be realized based on the different aggregative behaviours of AuNPs induced by the analytes.29,66 The other class of HCR-amplified colorimetric methods is based on HRP42 or hemin/G-quadruplex HRP-mimicking DNAzyme59 catalysed colorimetric systems, such as the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS2−) or 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2. In addition, SPR67 and SERS68 have also emerged as powerful optical techniques for quantitative analysis of HCR products.
In addition to optical techniques, electrochemical methods have been used extensively to study HCR. HCR-based electrochemical strategies can be broadly classified into four varieties: (1) hybridization with hairpins that are chemically modified with electroactive indicators;31 (2) employment of HRP molecules69 or DNAzymes70 (e.g., hemin/G-quadruplex HRP-mimicking DNAzyme) immobilized on HCR products to catalyse the H2O2-mediated oxidization of electrochemical substrate; (3) label-free electrochemical strategies in which electrochemical indicators (e.g., methylene blue [MB],30,33 doxorubicin [DOX],71 or hexaammineruthenium(III) chloride ([Ru(NH3)6]3+, RuHex))72 are intercalated into the double-stranded HCR products; and (4) incorporation of NPs, especially silver NPs (AgNPs), into the HCR products via labelling at DNA hairpins73 or electrostatic adsorption.74 Moreover, HCR-based photoelectrochemical (PEC) detection methods have been identified as powerful tools for biological analysis.75,76 Additionally, the HCR process can be monitored by quartz crystal microbalances (QCMs), which have been demonstrated to be a simple and label-free method for sensitive and real-time detection of nucleic acids.77
3 Applications of HCR in biosensing assays
HCR exploits DNAs as amplification transducers that can amplify an output signal over hundreds of times. Thus, combined with its advantages of isothermal and enzyme-free conditions, HCR has become an ideal analytical method for ultrasensitive and selective detection of various targets, offering new perspectives for point-of-care (POC) diagnostics. Herein, we classify the HCR amplification biosensing methods into five categories based on the type of analyte: nucleic acids, protein molecules, enzyme activities, tumour cells, and small molecules.3.1 Detection of nucleic acids
Nucleic acids, including DNAs and RNAs, are fascinating biopolymers that have been considered as specific markers for the early diagnosis of diseases, especially cancers. Therefore, sensitive and specific detection of nucleic acids is of great significance for biological and biomedical studies and clinical diagnosis. To achieve this goal, a variety of HCR-based strategies have been developed for nucleic acid detection, and they can be artificially divided into two classes based on whether they occur in solution or on a solid support.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (target
1 (target![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) probe) binding model,78,79 the sensitivity of this method was improved by more than 2 orders of magnitude. Luminescent NPs have also attracted considerable interest as fluorescent reporters for label-free biosensors. By combining highly fluorescent AgNCs as reporters with HCR amplification, a fluorescent biosensing platform was established for the detection of let-7a microRNA with a detection limit of 0.78 nM and the identification of single-nucleotide polymorphisms in the let-7 microRNA family.64
probe) binding model,78,79 the sensitivity of this method was improved by more than 2 orders of magnitude. Luminescent NPs have also attracted considerable interest as fluorescent reporters for label-free biosensors. By combining highly fluorescent AgNCs as reporters with HCR amplification, a fluorescent biosensing platform was established for the detection of let-7a microRNA with a detection limit of 0.78 nM and the identification of single-nucleotide polymorphisms in the let-7 microRNA family.64
 | ||
| Fig. 3 HCR-based homogeneous fluorescent assays for nucleic acid detection. (A) Pyrene-excimer probes based on HCR for DNA detection. Reprinted with permission from ref. 25. Copyright (2011) John Wiley and Sons. (B) GO-assisted HCR for microRNA detection. Reprinted with permission from ref. 26. Copyright (2012) American Chemical Society. | ||
Colorimetric assays have attracted substantial interest for nucleic acid detection because of their advantages of low cost, easy operation, and practicality.80 AuNPs can be considered one of the most competitive materials in colorimetric biosensing.81–83 There are two major mechanisms of HCR-based colorimetric assays using AuNPs. The first is the use of nucleotide-modified AuNPs as signal reporters. Based on this mechanism, an HCR-amplified colorimetric approach has been proposed (Fig. 4A).66 In this method, AuNPs were modified with capture DNA probes. The target oncomiR specifically hybridized with the capture DNA, forming a RNA/DNA heteroduplex that exposed a sticky RNA end to bind with a specific initiator to initiate HCR on the AuNPs. The resulting long double helix on the surface of the AuNPs protected the AuNPs from aggregation induced by Mg2+ ions, resulting in a red colour. Conversely, since no HCR product was assembled on the AuNPs in the absence of a target, an immediate colour transition (within seconds) or black precipitate (after an hour) can be observed upon Mg2+ treatment, which results from aggregation of the AuNPs. This visual strategy permitted the sensitive and selective analysis of three circulating oncomiRs separately and simultaneously with a detection limit of 20 fM. The other, more notable HCR-based colorimetric method mechanism is based on the different binding capacities of ssDNA and dsDNA toward unmodified AuNPs, which facilitate label-free analysis. Since no covalent functionalization of AuNPs is required, this type of strategy shows notable benefits for a visual analysis of nucleic acids (Fig. 4B).29 Target DNA concentrations as low as 100 pM can be identified by the naked eye, and 50 pM concentrations can be detected by ultraviolet (UV)-vis measurement.
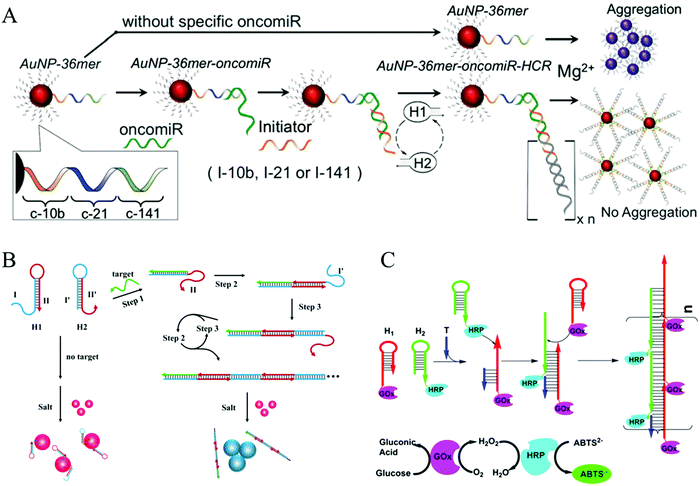 | ||
| Fig. 4 HCR-based colorimetric assays for nucleic acid detection. (A) HCR-amplified colorimetric approach using nucleotide-modified AuNPs as reporters. Reprinted with permission from ref. 66. Copyright (2016) Royal Society of Chemistry. (B) HCR-amplified colorimetric method based on different binding capacities of ssDNA and dsDNA towards unmodified AuNPs. Reprinted with permission from ref. 29. Copyright (2013) American Chemical Society. (C) HCR-triggered enzyme cascade amplification for colorimetric detection of target DNA (T). Reprinted with permission from ref. 85. Copyright (2016) American Chemical Society. | ||
More recently, Yang's group developed an ultrasensitive colorimetric platform based on HCR-triggered enzyme cascade amplification (Fig. 4C).85 The two hairpins used in this strategy were modified with glucose oxidase (GOx) and HRP. Then addition of the nucleic acid target (T) initiated HCR, resulting in the formation of GOx/HRP enzyme pairs. By combining HCR amplification with enhanced catalytic performance of the GOx/HRP enzyme pairs for the oxidation of ABTS2− by H2O2, a low detection limit of 5.2 fM was achieved for the nucleic acid target.
Catalytic units are often used as amplifying labels in biosensing strategies. Catalytic nucleic acids (DNAzymes or ribozymes), which are isolated through systematic evolution of ligands by exponential enrichment (SELEX), can be considered to be ideal candidates for developing bioanalytical platforms because of their good structural flexibility and high catalytic activity.90 Specifically, DNAzymes have been coupled with HCR to act as effective catalysts for amplified fluorescence, chemiluminescence, colorimetric, and electrochemical detection of nucleic acids. Willner's group combined a DNAzyme with HCR for DNA detection for the first time (Fig. 5A).58 In this system, the two hairpins included Mg2+-dependent DNAzyme subunits that were partially “caged” by the stem regions. In the presence of target DNA, HCR was activated to generate polymeric DNA nanowires consisting of catalytically active Mg2+-dependent DNAzyme units. The activated DNAzyme nanostructures catalytically cleaved the substrates that were labelled with fluorophore/quencher (F/Q) pairs at their 3′- and 5′-ends, respectively, resulting in the generation of fluorescence and allowing sensitive detection of DNA with a detection limit of 10 fM. In a related system, an HCR-based colorimetric and chemiluminescent biosensing platform was demonstrated by the same group using hemin/G-quadruplex HRP-mimicking DNAzymes as amplifying labels (Fig. 5B).59 The hairpin structures contained three-fourths and one-fourth of the HRP-mimicking DNAzyme sequence, which were both in inactive configurations in the absence of a target. The target DNA triggered an autonomous cross-opening of the hairpins, resulting in formation of DNA nanowires consisting of numerous hemin/G-quadruplex HRP-mimicking DNAzymes that could catalyse the H2O2-mediated oxidation of ABTS2− or luminol to generate a colorimetric or chemiluminescent signal for target DNA detection. G-Quadruplexes that kinetically conjugated on DNA nanowires with precise positioning were studied by Wang et al., who determined that they provided a solid support for label-free detection of various targets via signal amplification from HCR and hemin/G-quadruplex HRP-mimicking DNAzyme.91
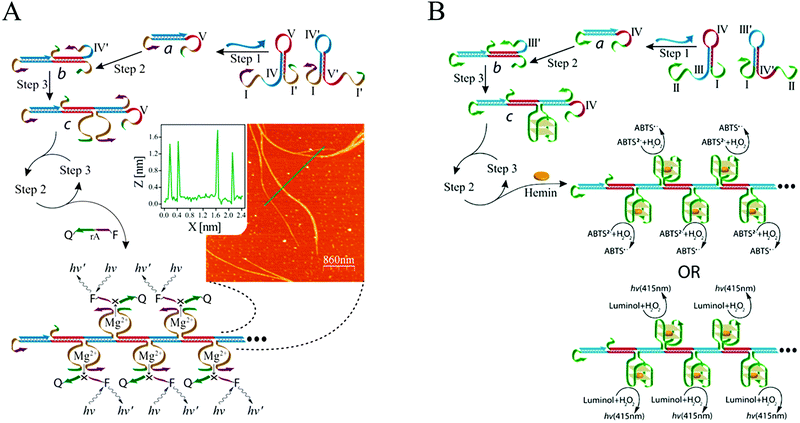 | ||
| Fig. 5 (A) Mg2+-dependent DNAzyme-based and (B) hemin/G-quadruplex DNAzyme-based HCR for nucleic acid detection. Inset of (A): AFM image and cross-sectional analysis of the resulting DNAzyme nanowires via HCR. Adapted with permission from ref. 58 and 59. Copyright (2011) and (2012) American Chemical Society. | ||
As a convenient, sensitive, and cost-effective detection technique, HCR-based electrochemical sensors have attracted substantial attention. Yang and co-workers introduced an enzyme-free and conjugation-free electrochemical genosensor (Fig. 6A).86 In this method, the capture probes were immobilized on the surface of an electrode via Au–S bonds. Upon the introduction of target DNA, sandwiched hybridization between the target and initiator strands initiated HCR, resulting in the formation of linear, electronegative DNA concatemers. Then, the electrochemical reporter [Ru(NH3)6]3+ interacted with the DNAs through electrostatic adsorption. This HCR-based electrochemical biosensor could accurately detect the breast cancer type 1 (BRCA1) gene with ultrahigh sensitivity (as low as 1 aM) and specifically discriminate single-base mismatched sequences.
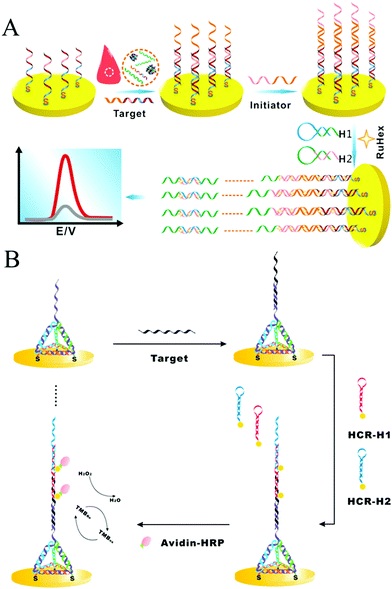 | ||
| Fig. 6 HCR-based electrochemical strategies for nucleic acid detection. (A) Enzyme-free and conjugation-free electrochemical genosensor for detection of the BRCA1 gene. Reprinted with permission from ref. 86. Copyright (2016) Elsevier. (B) Incorporation of tetrahedral DNA nanostructured probes with HCR amplification for microRNA detection. Reprinted with permission from ref. 69. Copyright (2014) American Chemical Society. | ||
Controlling the density and orientation of molecular probes on an electrode surface is the main challenge associated with electrochemical biosensors. To address this issue, a probe-conjugated three-dimensional (3D) tetrahedral DNA nanostructure-immobilized electrode was developed to increase the accessibility and reactivity of probes and avoid the introduction of spacer molecules (Fig. 6B).69 Target DNA was hybridized with the tetrahedral probe on the electrode surface, which then triggered the HCR to assemble HRP molecules and produce an electrocatalytic signal for quantitative analysis of the target DNA. Based on the synergetic effect of the tetrahedral probe and HCR, the detection limits of DNA and microRNA were as low as 100 aM and 10 aM, respectively. Similarly, another tetrahedral DNA nanostructure-based HCR-amplified electrochemical biosensor was constructed using AuNPs as initiator carriers, and its detection limit was as low as 2 aM microRNA.73
For optical solid-phase assays, microparticles or NPs, glass chips or microwell plates are usually employed as carriers to immobilize HCR products. For instance, Zhang and co-workers reported an amplification strategy for ultrasensitive and selective detection of DNA on magnetic particles that combined HCR with enzyme enhancement.87 In this method, one biotin-modified hairpin (Bio-H1) was first immobilized on streptavidin (SA)-coated magnetic beads. Upon addition of target DNA, the Bio-H1 was opened, and a sticky end was exposed to specifically hybridize to the toehold of the other kind of biotin-modified hairpin (Bio-H2), resulting in the initiation of HCR. Then, avidin-conjugated HRP molecules were attached to the resulting HCR products, and the fluorescence signal of bi-p,p′-4-hydroxyphenylacetic acid that was generated via HRP catalysis of hydroxyphenylacetic acid (HPA) was measured. Ultrasensitive detection of the target DNA was achieved, with a detection limit as low as 8.1 × 10−16 M.
Although this method achieved ultrahigh sensitivity, it suffered from the disadvantages of expensive labelling costs (e.g., the modification of biotin and avidin) and sophisticated processes for signal molecule immobilization (e.g., the biotin–avidin interaction). As an alternative, a label-free electrochemiluminescence (ECL) protocol was developed for DNA detection by intercalating Ru(phen)32+ into the dsDNA grooves of the HCR products and achieved an ultrasensitive analysis of femtomolar DNA.88 In addition, a label-free fluorescence strategy was proposed for a high-throughput detection of RNA based on branched HCR polymerization with binding to SYBR Green I dye.61 In recent years, SPR has emerged as a powerful optical tool for detecting a wide range of biomolecules.89 Accordingly, HCR-based SPR amplification strategy has been developed for label-free detection of DNAs and has achieved high sensitivity and selectivity.67 A comparison of different HCR strategies for detecting nucleic acids is listed in Table 1. Although all the strategies are based on HCR, they demonstrate different detection limits. Generally, sensitivity of HCR-based sensors is related to not only HCR amplification and signal readout methods but also the protocols that integrate with other isothermal amplification techniques and detection models (e.g., “signal-on” or “signal-off”). In addition, reaction conditions, especially stability of the designed hairpins, are of great significance to determine a detection limit. For instance, unstable hairpins partially open in the absence of a target would potentially reduce signal gain.
| Target | Method | Signal transduction | Detection limit | Ref. |
|---|---|---|---|---|
| Solution-based detection | ||||
| DNA | Pyrene molecules modified hairpins for HCR | Fluorescence | 256 fM | 25 |
| microRNA | GO-Assisted HCR | Fluorescence | 1 pM | 26 |
| microRNA | HCR modulated DNA-hosted AgNCs as signal reporters | Fluorescence | 0.78 nM | 64 |
| DNA | Mg2+-Dependent DNAzyme-based HCR assay | Fluorescence | 10 fM | 58 |
| DNA | Nonlinear HCR using dsDNAs as substrates | Fluorescence | 0.15 nM | 27 |
| DNA | Hairpin-based HB-HCR | Fluorescence | 100 fM | 28 |
| microRNA | Self-assembly of hyperbranched DNA structures through integrating CHA with HCR | Chemiluminescence | 0.72 pM | 55 |
| oncomiRs | HCR-amplified assay using nucleotide-modified AuNPs as signal reporters | Colorimetry | 20 fmol | 66 |
| DNA | Unmodified AuNPs as signal reporters that have different binding capacities toward hairpin monomers and double-stranded HCR products | Colorimetry | 50 pM (UV-vis detection) | 29 |
| 100 pM (naked eye detection) | ||||
| DNA | HCR-triggered enzyme cascade amplification | Colorimetry | 5.2 fM | 85 |
| DNA | Assembly of HCR products containing hemin/G-quadruplex HRP-mimicking DNAzymes as amplifying labels | Colorimetry and chemiluminescence | 100 fM | 59 |
| DNA | Target-triggered nonlinear HCR | Electrochemistry | 100 fM | 54 |
| DNA | Self-assembly of dendritic DNA nanostructure | Polyacrylamide gel | 10 nM | 53 |
| Solid-phase detection | ||||
| DNA | HCR-based enzyme-free and conjugation-free genosensor using Ru(NH3)6 as electrochemical reporter | Electrochemistry | 1 aM | 86 |
| DNA | Probe-conjugated 3D tetrahedral DNA nanostructure modified electrode for HCR | Electrochemistry | 100 aM (DNA) | 69 |
| microRNA | 10 aM (microRNA) | |||
| microRNA | Tetrahedral DNA nanostructure modified electrode using AuNPs as initiator carriers for HCR | Electrochemistry | 2 aM | 73 |
| microRNA | Tetrahedral DNA nanostructure modified electrode using metal-catalyst-free click chemical signal amplification via HCR | PEC | 27 aM | 75 |
| DNA | HCR-based label-free strategy using cyclometalated iridium(III) complex as signal indicator | PEC | 9.0 fM | 76 |
| DNA | HCR with enzyme-amplification using magnetic particles as carriers | Fluorescence | 0.81 fM | 87 |
| RNA | Branched HCR-based label-free detection platform on 96-well plate | Fluorescence | 1 pM | 61 |
| DNA | Label-free and real-time QCM with dissipation monitoring (QCM-D) biosensing platform | QCM | 0.1 nM | 77 |
| DNA | HCR-based label-free biosensor using Ru(phen)32+ as ECL reporter | ECL | 15 fM | 88 |
| DNA | HCR-based label-free SPR assay | SPR | 0.3 fM (DNA) | 67 |
3.2 Detection of proteins
Proteins are essential for the maintenance of life, and protein dysfunction has been demonstrated to cause many diseases.92 In addition, many proteins exist in quite low abundance in real samples. Therefore, development of signal amplification methods for highly sensitive and specific detection of proteins is very significant for bioanalysis and biomedicine. HCR, as a fascinating enzyme-free isothermal amplification strategy, has been widely exploited for the ultrasensitive detection of proteins. Using recognition mechanisms, these methods can be divided into two types: Ab-based and aptamer-based strategies.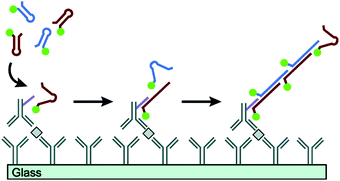 | ||
| Fig. 7 Ab-Based immuno-HCR for protein detection. Reprinted with permission from ref. 60. Copyright (2011) American Chemical Society. | ||
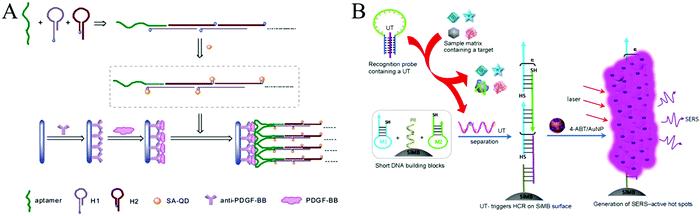 | ||
| Fig. 8 HCR-based aptasensors for protein detection. (A) Fluorescence detection of PDGF-BB through the coupling of aptamer recognition with HCR. Reprinted with permission from ref. 63. Copyright (2012) Royal Society of Chemistry. (B) Construction of HCR-based universal SERS aptasensors for the detection of multiple analytes. Reprinted with permission from ref. 68. Copyright (2014) American Chemical Society. | ||
In addition to heterogeneous assays, a homogeneous aptasensing strategy was developed for protein analysis by combining target-triggered HCR with GO-based selective fluorescence quenching.98 Based on the different adsorption capacity of GO for ssDNAs and double-stranded HCR polymer products, a strong fluorescence signal could be detected when the target protein was present in a sample, resulting in the highly sensitive and selective detection of PDGF-BB with a detection limit of 1.25 pM. A comparison of different HCR strategies for detecting proteins is provided in Table 2.
| Target | Method | Signal transduction | Detection limit | Ref. |
|---|---|---|---|---|
| Cytokines | Immuno-HCR through linking an oligonucleotide initiator to Ab2 | Fluorescence | 0.27–2.2 pM | 60 |
| Human IgG | Immuno-HCR using AuNPs as DNA initiator carriers for signal amplification | Electrochemistry | 0.1 fg mL−1 | 31 |
| PDGF-BB | HCR-based aptasensor | Fluorescence | 50 pM | 63 |
| Thrombin | An universal aptasensor using DNA triple-helix probe | SERS | 18 pM (thrombin) | 68 |
| Adenosine | 1.5 nM (adenosine) | |||
| CEM cells | 10 cells per mL (CEM cell) | |||
| PDGF-BB | Homogeneous aptasensing strategy combining HCR with GO-based selective fluorescence quenching | Fluorescence | 1.25 pM | 98 |
| T4 PNK | HCR-based FCBA | Fluorescence | 4 × 10−6 U mL−1 | 106 |
| DNA MTase | HCR-based branched RCA | Chemiluminescence | 0.52 U mL−1 | 35 |
| Uracil-DNA glycosylase (UDG) | Nuclease-responsive HCR strategy using TWJ with specific nuclease sites | Fluorescence | 4.3 × 10−5 U mL−1 (UDG) | 36 |
| CpG methyltransferase M.SssI | 0.019 U mL−1 (M.SssI) |
3.3 Detection of enzyme activities
Enzymes are unique proteins that can catalyse biochemical reactions with high rates and selectivity and play a vital role in almost all life processes.99,100 Additionally, some enzymes (e.g., telomerase,101,102 DNA methyltransferase [MTase],103 and polynucleotide kinase [PNK]104,105) have been found to be biomarkers of diseases. Therefore, developing assays for detecting enzyme activity is important for biomedical and clinical research. To date, various HCR-based biosensing platforms have been reported for highly sensitive and selective analysis of enzyme activity. Zhang and co-workers proposed an HCR-based flow cytometric bead assay (FCBA) for ultrasensitive detection of T4 PNK activity.106 In the absence of T4 PNK, the 5′-OH terminus of the blocking DNA cannot be phosphorylated. Thus, the blocking DNA will not be digested by λ exonuclease, resulting in the prevention of HCR. In contrast, when T4 PNK was present in a sample, HCR was triggered on the surface of the magnetic beads. The fluorophores that accumulated on the magnetic beads could be directly analysed using a flow cytometer, and excellent analytical performance for determination of T4 PNK activity was achieved, with a detection limit of 4 × 10−6 U mL−1. Detecting DNA MTase activity and its inhibitors has also been achieved by HCR.35 Moreover, a universal nuclease-responsive HCR strategy has been developed.36 Through the rational design of a three-way junction (TWJ) with specific nuclease sites, this method can be readily used to detect a wide range of DNA-modified enzymes, which can be further expanded to the construction of advanced target-responsive DNA devices for diverse applications. A comparison of different HCR strategies for determining enzyme activities is shown in Table 2.3.4 Detection of tumour cells
Expression levels of some biomarkers, such as receptors, proteins or specific enzymes, on tumour cell surfaces or within tumour cells differ significantly from their normal values, offering opportunity for an early clinical diagnosis and treatment of cancers.92 To date, many studies have reported the detection of cancer cells through recognition of biomarkers on the cytomembrane.107,108 Unlike detection of other tumour markers (e.g., nucleic acids and proteins) within the cells, which is often associated with tedious treatment steps, such as cell lysis and target extraction, the direct detection of whole cancer cells is relatively convenient and efficient. Since the cell surface is complex and contains many different molecules, especially proteins,109 developing enzyme-free amplification strategies for cancer cell detection is highly desirable. For this purpose, a multibranched HCR (mHCR) was developed for detecting cancer cells through aptamer recognition (Fig. 9A).42 This mHCR was obtained by joining typical HCR to produce long products with multiple reporters for signal amplification and multiple branched arms for multivalent binding on a DNA tetrahedron nanostructured gold electrode. Because of the synergetic effect of mHCR amplification and multivalent binding, this biosensing platform achieved ultrahigh sensitivity with a detection limit as low as four MCF-7 cells.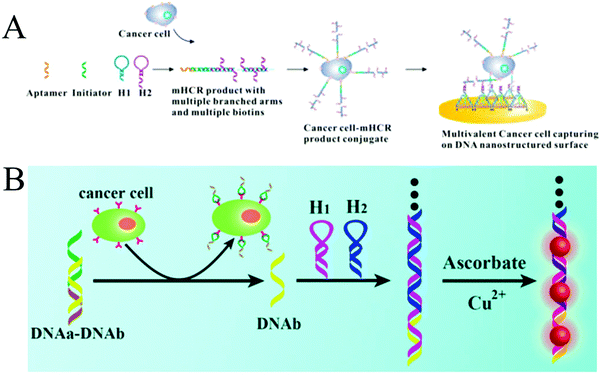 | ||
| Fig. 9 HCR-based strategies for tumour cell detection. (A) mHCR for electrochemical detection of tumour cells. Reprinted with permission from ref. 42. Copyright (2014) American Chemical Society. (B) Label-free detection of tumour cells through metallization of CuNPs on HCR products. Reprinted with permission from ref. 43. Copyright (2015) Royal Society of Chemistry. | ||
A label-free strategy was also developed through the metallization of NPs on HCR products (Fig. 9B).43 In this method, a partially complementary duplex was first formed through hybridization of aptamer sequence (DNAa) with a messenger DNA (DNAb). In the presence of target cells, the aptamers specifically bound to the targets, releasing DNAb to trigger HCR. The resultant long duplex DNA oligomers were employed as templates for the in situ synthesis of CuNPs with strong fluorescence, which were further used as a readout signal to achieve label-free quantitative determination of tumour cells with a detection limit of 50 cells.
3.5 Detection of small molecules
Currently, a number of functional nucleic acids, such as aptamers and DNAzymes, have been employed to expand HCR-based isothermal amplified techniques for detecting small molecules, including small biomolecules and metal ions. For small biomolecule detection, most strategies have relied on aptamer recognition, in which target molecules trigger the HCR upon binding to aptamers. Based on this mechanism, a variety of biomolecules, such as ATP, adenosine and glutathione (GSH), have been sensitively and selectively detected by combining HCR with SA assembly as a molar mass amplifier,110 exonuclease III (Exo III)-assisted DNA recycling,39 and specific thymine–Hg2+–thymine (T–Hg2+–T) coordination,38 respectively.As another kind of small molecules, metal ions have also been determined to exert serious effects on human health and environmental quality.111 Thus, construction of facile, sensitive, and reliable methods for detecting metal ions has attracted substantial interest. Traditional techniques for metal ion analysis include inductively coupled plasma mass spectrometry (ICP-MS)112 and atomic absorption spectroscopy.113 However, these methods usually require sophisticated instruments, complicated procedures, and relatively large amounts of sample. Recently, nucleic acid-based biosensors have been developed for metal ion detection with improved sensitivity. Generally, the interactions between metal ions and nucleic acids can be divided into two types: the specific response of DNAzymes or ribozymes to metal ions (e.g., Pb2+, Mg2+, and Cu2+)90 and the specific bridges between metal ions and nucleosides or ligandosides that form stable metal ion-coordination base pairs (e.g., T–Hg2+–T114 and C–Ag+–C115). Based on these mechanisms, HCR amplification strategies have been developed in which initiator DNA is generated in response to target metal ions, which, in turn, initiate HCR, and pM-level sensitivity has been achieved.41,116
4 Applications of HCR in bioimaging and biomedicine
Comprehensively understanding biological pathways is critical for the development of biological systems and their applications in clinical research. In recent years, imaging analysis has emerged as one of the most promising techniques for a complete understanding of biological processes and effective diagnosis and treatment of diseases. Some isothermal amplification techniques, such as RCA,117,118 SDA,119 and loop-mediated isothermal amplification (LAMP),120,121 have been used for bioimaging analysis. However, these methods need enzymes and often require complicated operations. More recently, HCR-based isothermal amplification techniques have been extended to image various targets, such as extracellular matrix,44 tumour cells,21,22 and RNAs,20,45,62,122,123 demonstrating these techniques' advantages of signal amplification and simplicity and their enzyme-free nature. In this section, we focus on the development of HCR-based bioimaging methods, including fluorescence in situ hybridization (FISH) imaging and live cell imaging (cell surface and intracellular imaging). In addition, the applications of HCR in biomedicine are discussed.4.1 FISH imaging based on HCR
In early studies, FISH offered an effective tool for mapping RNAs within cells. In a typical FISH method, fluorophore-labelled oligodeoxynucleotides are employed as probes, which enter the fixed cells or tissues by means of permeation and hybridize with target RNAs to generate a response signal. Through FISH, images of intracellular RNA expression and distribution can be obtained to elucidate relevant pathological processes and verify their functions in clinical diagnosis. However, since only one signal probe hybridizes to the target RNA, traditional FISH methods suffer from low signal intensity. Therefore, to improve the signal level, it is highly desirable to develop a method for targeting the same analyte with multiple probes.130 In recent years, HCR-based FISH (HCR-FISH) techniques have been developed to generate sufficient signal-to-background ratios, where a polymerization reaction between two fluorophore-labeled hairpins is initiated upon recognizing a single given target, thus achieving amplified fluorescence activation imaging. It has been reported that the mean signal obtained by HCR-FISH is ∼200-fold higher than using direct-labeled probes without HCR amplification.122Using HCR-FISH, Pierce's group conducted a series of innovative studies.20,122,124–128 They first proposed an HCR-FISH method to map five target mRNAs simultaneously in fixed whole-mount and sectioned zebrafish embryos (Fig. 10A).20 This strategy mainly involved two stages: the detection stage and the amplification stage. In the detection stage, target mRNAs hybridized in situ to the complementary RNA probes, and then, the samples were washed to remove the unreacted probes. In the amplification stage, orthogonal initiators triggered the in situ HCR to form distinct fluorophore-tethered DNA polymers, and the optical signals generated by all the target mRNAs were imaged simultaneously. As shown by the results in Fig. 10A, this HCR-FISH method exhibited deep sample penetration, high signal-to-background ratios, and sharp signal localization. The same group subsequently introduced the next generation of in situ HCR for imaging RNA expression.122 Unlike first-generation HCR-FISH, in this system, DNA probes and amplifiers were employed instead of RNA probes and amplifiers to improve reagent durability and decrease reagent costs. In addition, permissive in situ amplification conditions were established, and the DNA hairpins were engineered to increase the signal gain per amplifier. Recently, HCR-FISH has also been applied for detecting environmental microorganisms.129
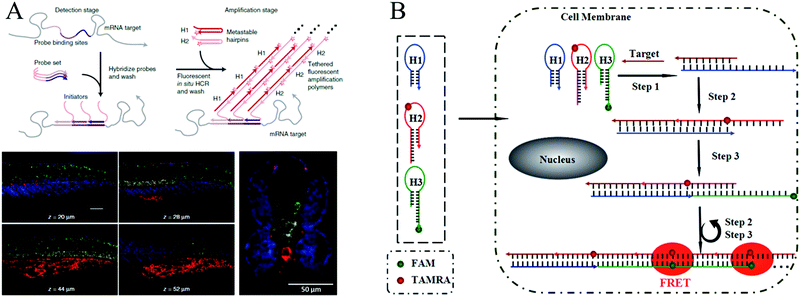 | ||
| Fig. 10 (A) HCR-FISH used for simultaneous mapping of multiple target mRNAs in fixed whole-mount and cross-sectioned zebrafish embryos. Adapted with permission from ref. 20. Copyright (2010) Nature Publishing Group. (B) Wash-free HCR-FISH used for in situ detection of target mRNA in cancer cells. Reprinted with permission from ref. 62. Copyright (2016) Royal Society of Chemistry. | ||
The above mentioned HCR-FISH methods required repeated washing steps to remove unbound probes and hairpins and were, thus, time consuming and required complicated procedures. Very recently, Wang introduced a wash-free HCR-FISH technique using FRET, which successfully eliminated the need for multiple washing steps and prevented false-positive signals caused by probe accumulation or degradation in quencher/dye systems (Fig. 10B).62 This system contained three DNA hairpins: H1, H2, and H3. H1 was partially complementary to the target mRNA, whereas H2 and H3 were modified with a fluorescence acceptor (carboxytetramethylrhodamine [TAMRA]) and donor (fluorescein amidite [FAM]), respectively, at the appropriate positions. As a result, H1 could be opened by the target mRNA, which further initiated HCR via the cross-opening of H2 and H3, facilitating the generation of amplified FRET signals. Therefore, washing steps were not needed in this FRET HCR-FISH strategy, which significantly simplified the operations and reduced experimental time.
In particular, a proximity-dependent initiation of HCR (proxHCR) was proposed by Söderberg et al.52 When hairpin-conjugated Abs bound in close proximity, an initiator strand was revealed to activate the HCR, which resulted in generation of fluorescence signals. This proxHCR protocol was successfully applied to the sensitive detection of protein interactions and post-translational modifications of native proteins in fixed cells and tissues and is promising as a robust alternative to proximity ligation assays.
4.2 Live cell imaging based on HCR
The above mentioned HCR-based imaging methods mainly focused on fixed cells or tissue sections. To obtain detailed spatial-temporal information on dynamics related to cellular functions and diseases, live cell imaging methods are required.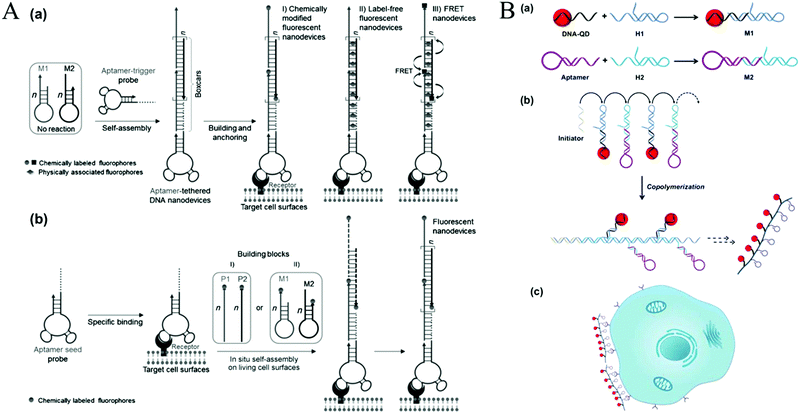 | ||
| Fig. 11 HCR-based cell surface imaging. (A) Construction of fluorescent DNA aptNDs on target cell surfaces using HCR via an anchoring model (a) and an in situ self-assembly model (b). Reprinted with permission from ref. 21. Copyright (2013) John Wiley and Sons. (B) Fabrication of linear QAPs for molecular imaging of cancer cells. Reprinted with permission from ref. 22. Copyright (2016) American Chemical Society. | ||
Recently, HCR-based imaging strategies have been proposed to investigate the dynamic expression and distribution of intracellular nucleic acids, enzyme activities, and small molecules in live cells with the assistance of nanomaterials (e.g., AuNPs,45 GO,123,131 mesoporous silica nanospheres [MSNs],132 and ZnO NPs133). In general, hairpins were assembled on the nanomaterials through electrostatic interaction, π-stacking, or hydrogen bonding. After transporting the conjugates into cells, HCR was initiated upon the recognition of targets, resulting in generation of long double helices with strong fluorescence. Jiang et al. developed an electrostatic DNA nanoassembly for ultrasensitive imaging of mRNA in live cells based on HCR amplification (Fig. 12A).45 In this strategy, the fluorescence donor and acceptor-labelled DNA hairpins were electrostatically assembled on cationic peptide-coated AuNPs, which were efficiently delivered into live cells through an endocytosis-independent mechanism. Because of efficient quenching of the fluorescence signals of the fluorophores by AuNPs, the DNA hairpins that assembled on the AuNPs produced very weak fluorescence signals in the absence of target mRNA. In contrast, presence of target mRNA triggered the HCR, resulting in dissociation of the hairpins from the particles and the recovery of FRET signals, indicating target mRNA expression. Similarly, via electrostatic interaction between DNA probes and positively charged group-modified MSNs, a GSH-initiated HCR was proposed for sensitive monitoring of GSH expression in single cancer cells.132 In addition, using GO as a probe carrier and fluorescence quencher, Tang's group proposed an HCR-amplified fluorescence imaging of microRNA in live cells (Fig. 12B).123 This method also achieved simultaneous imaging of two types of microRNAs in live cells, which would efficiently avoid false positive results. Moreover, a similar work has been reported by the same group using GO for fluorescence imaging of intracellular telomerase activity based on HCR amplification.131
4.3 Applications of HCR in biomedicine
In recent decades, cancer has emerged as one of the most devastating diseases and results in high death tolls worldwide. Currently, surgical intervention, radiotherapy, and chemotherapy are the main cancer treatment methods. However, these methods often kill normal cells or tissues, resulting in a requirement for insufficient doses of anticancer reagents and eventual treatment failure or even patient death.134 Therefore, developing efficient approaches for targeted therapy is essential. Ligand-targeted therapeutics (LTTs), such as radioimmunotherapy, has been considered to be a successful means to improve selectivity of anticancer drugs with cancer cells.134 In addition to Ab recognition, aptamer-based targeted drug delivery techniques have been developed.135–137 Compared to immunotherapeutics, aptamers have significant advantages in terms of their size and preparation. In addition, aptamers can transport themselves or anticancer reagents across a cell membrane via the endocytosis pathway.138 Thus, aptamers are ideal recognition molecules for targeted drug delivery.In recent years, aptamer-tethered DNA assemblies based on HCR have been devised that allow construction of enzyme-free platforms for targeted drug delivery with easy procedures and high drug payload capacities. In aptamer-HCR strategies, aptamers have been used as recognition elements to specifically bind to target cells, whereas HCR products with abundant spatially addressable sites have been employed as drug carriers that could be readily internalized by target cells for drug delivery and disease treatment. Chronic accumulation of nanomaterials is avoided because of the intrinsic biodegradability of nucleic acids. Moreover, assembly of aptamer-HCR-based drug carriers is implemented under enzyme-free and isothermal conditions, making this technique suitable for clinical applications. Based on this mechanism, Tan's group reported the first aptamer-HCR example of targeted anticancer drug delivery through construction of aptamer-tethered DNA nanotrains (aptNTrs) and demonstrated that these structures constitute a promising platform for a precise treatment of cancer (Fig. 13A).23 Recently, an activatable theranostic approach has been developed through a structure-switching aptamer-triggered HCR on cell surfaces (Fig. 13B).46 DNA polymers assembled on the target cells accumulated high-load prodrugs, which were then taken up and transported into cells for selective cancer therapy.
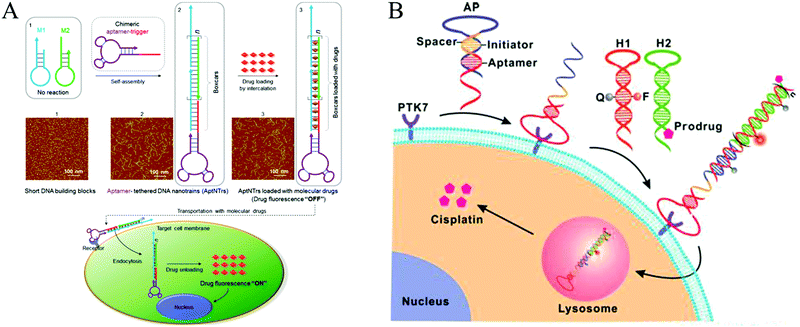 | ||
| Fig. 13 Applications of HCR in biomedicine. (A) Construction of aptNTrs for targeted anticancer drug delivery. Reprinted with permission from ref. 23. Copyright (2013) National Academy of Sciences. (B) Activatable theranostic approach based on a structure-switching aptamer-triggered HCR. Reprinted with permission from ref. 46. Copyright (2015) American Chemical Society. | ||
Structures with multiple binding sites have higher affinity and specificity compared to their monovalent counterparts, which can improve the cellular uptake ability of nanocarriers.139 Thus, self-assembly of multivalent DNA nanostructures as drug carriers is desirable for efficient drug delivery. Because of multivalent effects, the multiple binding approach has achieved enhanced binding affinity via HCR, facilitating cellular internalization with no loss of selectivity.24
5 Conclusions and perspectives
In this review, we have summarized development of HCR-based isothermal amplification strategies by highlighting several examples. Based on the unique properties of its enzyme-free and isothermal nature, controllable synthetic routes, and good biocompatibility, HCR has been demonstrated to be a powerful amplification tool in the fields of biosensing, bioimaging, and biomedicine.Although HCR-based methods have undergone substantial development, biosensing applications still face many challenges. (1) In an HCR, design of the hairpins is a crucial factor. Indeed, a small variation in the hairpins may lead to failure of the HCR process. Substantial effort has been expended to construct creative molecular design systems. For example, based on simulation and experimental validation, a set of four-point design guidelines have been proposed which can be used to design the lengths and GC contents of toehold and stem domains of a hairpin.48 Although substantial progress has been achieved, systematic guidelines for designing hairpin sequences remain lacking. (2) Generally, HCR can be combined with various signal readout techniques, such as fluorescence, chemiluminescence, colorimetry, and electrochemistry, to achieve sensitive detection of analytes. However, strategies based on HCR amplification alone often cannot provide sufficient sensitivity for biosensing. To further improve sensitivity, various enzyme-assisted HCR strategies have been proposed with detection limits at the sub-attomole level, which is even lower than that achieved by PCR. Although these enzyme-assisted HCR methods have ultrahigh sensitivities, given the harsh procedures and reaction conditions of the enzymes required, employing enzymes in HCR is incompatible with the goal to improve the detection sensitivity and limits its applications in complex sample analyses. Therefore, an ideal HCR-based biosensing strategy should be completely enzyme-free. Alternatively, developing functional nucleic acids, especially DNAzymes, promotes the establishment of enzyme-free HCR paradigms. HCR-mediated synthesis of DNAzyme products, such as hemin/G-quadruplex wires, not only improves detection sensitivity but also overcomes the drawbacks of enzyme-assisted HCR strategies. (3) For heterogeneous HCR, controlling the density and orientation of DNA probes on the surface of solid substrates is an important issue that must be overcome because it could cause nonspecific adsorption and crowding effects, and limit reaction efficiencies. Although employment of a DNA tetrahedron as a scaffold on solid surfaces has solved this problem to a certain degree because of its structural stability and mechanical rigidity, programmable and controllable immobilization of molecular probes on solid supports remains challenging. (4) In recent years, branched or even dendritic HCR systems have been developed through triggered self-assembly of complicated DNA monomers, achieving quadratic or exponential growth mechanisms. However, these nonlinear HCR strategies are still limited. Therefore, integration of HCR with other isothermal mechanisms to construct more complicated DNA structures is highly desirable. (5) Development of aptamers has benefitted HCR amplification methods, which have been extended to detect a wide range of analytes ranging from nucleic acids to proteins, small molecules, and even whole cells. However, a limited number of aptamers have been selected, which is an obstacle to developing aptamer-HCR strategies. (6) More importantly, currently, most HCR-based bioanalytical methods remain in the laboratory stage, and their practical applications are still challenging. In-depth studies on HCR should be implemented to establish a foundation for future clinical applications. Other fields, such as physical and engineering sciences, should focus on designing simple and sensitive “sample to answer” settings based on HCR to facilitate POC applications.
For bioimaging and biomedicine, HCR-based isothermal protocols provide exciting opportunities for bioimaging in fixed cells and tissues, or even live cells. Furthermore, nanometre-sized HCR products are being used as carriers for targeted drug delivery, highlighting their future utility in biomedicine. However, applications of HCR in bioimaging and biomedicine remain in their infancy, and several issues still need to be addressed. (1) Transportation of HCR products into cells is a crucial step for intracellular imaging or drug delivery. However, cellular uptake of HCR products carrying imaging reagents or drugs is not easy because of the charge and size of nucleic acids. Additionally, few target recognition probes (e.g., aptamers) can be internalized into live cells. Nevertheless, encouraged by ongoing advancements, we expect that a greater variety of transport modes for nucleic acid probes will be developed and that more aptamers will be selected in future HCR studies. (2) Because of the high molecular weight of HCR products, it is difficult to avoid its nonspecific binding to normal cells during cell targeting and drug delivery, which may lead to false signals in bioimaging and kill normal cells or tissues in biomedical applications. Another challenge for the use of HCR in biomedicine is lack of efficient and accurate drug release mechanisms, which would certainly reduce therapeutic effects. (3) The in situ imaging of intracellular non-nucleic acid bioanalytes (e.g., proteins and small molecules) is rarely reported. Moreover, developing enzyme-mediated HCR strategies to study nucleic acid–protein and protein–protein interactions in vivo should be considered. Employing intracellular enzymes or the transportation of enzymes into cells may resolve this issue.
Although many challenges lie ahead of us, we believe that HCR-based isothermal non-enzymatic platforms can be readily integrated with a wide variety of nanomaterials, synthetic and analytical approaches, and precision manufacturing technologies to stimulate development of DNA nanotechnology and further enhance the progress of these strategies in practical applications, such as clinical diagnostics and therapeutics, energy storage and transfer, and molecular computation.
Acknowledgements
The authors appreciate the financial support from the National Natural Science Foundation of China (21535002 and 21375056), the Special Funds of Taishan Scholar Program of Shandong Province (tsqn20161028), and the Shandong Provincial Natural Science Foundation (ZR2016QZ001).References
- P. Belmont, J.-F. Constant and M. Demeunynck, Chem. Soc. Rev., 2001, 30, 70–81 RSC.
- D. Schilter, Nat. Rev. Chem., 2017, 1, 0011 CrossRef.
- A. V. Pinheiro, D. Han, W. M. Shih and H. Yan, Nat. Nanotechnol., 2011, 6, 763–772 CrossRef CAS PubMed.
- F. Zhang, J. Nangreave, Y. Liu and H. Yan, J. Am. Chem. Soc., 2014, 136, 11198–11211 CrossRef CAS PubMed.
- T. Tørring, N. V. Voigt, J. Nangreave, H. Yan and K. V. Gothelf, Chem. Soc. Rev., 2011, 40, 5636–5646 RSC.
- H. Jabbari, M. Aminpour and C. Montemagno, ACS Comb. Sci., 2015, 17, 535–547 CrossRef CAS PubMed.
- C. Jung and A. D. Ellington, Acc. Chem. Res., 2014, 47, 1825–1835 CrossRef CAS PubMed.
- R. K. Saiki, D. H. Gelfand, S. Stoffel, S. J. Scharf, R. Higuchi, G. T. Horn, K. B. Mullis and H. A. Erlich, Science, 1988, 239, 487–491 CAS.
- F. Barany, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 189–193 CrossRef CAS.
- M. Vincent, Y. Xu and H. Kong, EMBO Rep., 2004, 5, 795–800 CrossRef CAS PubMed.
- M. M. Ali, F. Li, Z. Zhang, K. Zhang, D.-K. Kang, J. A. Ankrum, X. C. Le and W. Zhao, Chem. Soc. Rev., 2014, 43, 3324–3341 RSC.
- G. T. Walker, M. C. Little, J. G. Nadeau and D. D. Shank, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 392–396 CrossRef CAS.
- D. Y. Zhang and G. Seelig, Nat. Chem., 2011, 3, 103–113 CrossRef CAS PubMed.
- Y. Zhao, F. Chen, Q. Li, L. Wang and C. Fan, Chem. Rev., 2015, 115, 12491–12545 CrossRef CAS PubMed.
- B. Yurke, A. J. Turberfield, A. P. Mills Jr, F. C. Simmel and J. L. Neumann, Nature, 2000, 406, 605–608 CrossRef CAS PubMed.
- G. Seelig, D. Soloveichik, D. Y. Zhang and E. Winfree, Science, 2006, 314, 1585–1588 CrossRef CAS PubMed.
- A. J. Genot, D. Y. Zhang, J. Bath and A. J. Turberfield, J. Am. Chem. Soc., 2011, 133, 2177–2182 CrossRef CAS PubMed.
- D. Y. Zhang and E. Winfree, J. Am. Chem. Soc., 2009, 131, 17303–17314 CrossRef CAS PubMed.
- R. M. Dirks and N. A. Pierce, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15275–15278 CrossRef CAS PubMed.
- H. M. T. Choi, J. Y. Chang, L. A. Trinh, J. E. Padilla, S. E. Fraser and N. A. Pierce, Nat. Biotechnol., 2010, 28, 1208–1212 CrossRef CAS PubMed.
- G. Zhu, S. Zhang, E. Song, J. Zheng, R. Hu, X. Fang and W. Tan, Angew. Chem., Int. Ed., 2013, 52, 5490–5496 CrossRef CAS PubMed.
- Z. Li, X. He, X. Luo, L. Wang and N. Ma, Anal. Chem., 2016, 88, 9355–9358 CrossRef CAS PubMed.
- G. Zhu, J. Zheng, E. Song, M. Donovan, K. Zhang, C. Liu and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7998–8003 CrossRef CAS PubMed.
- W. Li, X. Yang, L. He, K. Wang, Q. Wang, J. Huang, J. Liu, B. Wu and C. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 25733–25740 CAS.
- J. Huang, Y. Wu, Y. Chen, Z. Zhu, X. Yang, C. J. Yang, K. Wang and W. Tan, Angew. Chem., Int. Ed., 2011, 50, 401–404 CrossRef CAS PubMed.
- L. Yang, C. Liu, W. Ren and Z. Li, ACS Appl. Mater. Interfaces, 2012, 4, 6450–6453 CAS.
- F. Xuan and I.-M. Hsing, J. Am. Chem. Soc., 2014, 136, 9810–9813 CrossRef CAS PubMed.
- S. Bi, M. Chen, X. Jia, Y. Dong and Z. Wang, Angew. Chem., Int. Ed., 2015, 54, 8144–8148 CrossRef CAS PubMed.
- P. Liu, X. Yang, S. Sun, Q. Wang, K. Wang, J. Huang, J. Liu and L. He, Anal. Chem., 2013, 85, 7689–7695 CrossRef CAS PubMed.
- T. Hou, W. Li, X. Liu and F. Li, Anal. Chem., 2015, 87, 11368–11374 CrossRef CAS PubMed.
- B. Zhang, B. Liu, D. Tang, R. Niessner, G. Chen and D. Knopp, Anal. Chem., 2012, 84, 5392–5399 CrossRef CAS PubMed.
- J. Ikbal, G. S. Lim and Z. Gao, TrAC, Trends Anal. Chem., 2015, 64, 86–99 CrossRef CAS.
- J. Zhou, M. Xu, D. Tang, Z. Gao, J. Tang and G. Chen, Chem. Commun., 2012, 48, 12207–12209 RSC.
- J. Xu, J. Wu, C. Zong, H. Ju and F. Yan, Anal. Chem., 2013, 85, 3374–3379 CrossRef CAS PubMed.
- S. Bi, T. Zhao, B. Luo and J.-J. Zhu, Chem. Commun., 2013, 49, 6906–6908 RSC.
- J. Zhu, L. Wang, X. Xu, H. Wei and W. Jiang, Anal. Chem., 2016, 88, 3817–3825 CrossRef CAS PubMed.
- J. Ge, Z.-M. Huang, Q. Xi, R.-Q. Yu, J.-H. Jiang and X. Chu, Chem. Commun., 2014, 50, 11879–11882 RSC.
- Y. Wang, L. Jiang, Q. Leng, Y. Wu, X. He and K. Wang, Biosens. Bioelectron., 2016, 77, 914–920 CrossRef CAS PubMed.
- J. Sun, W. Jiang, J. Zhu, W. Li and L. Wang, Biosens. Bioelectron., 2015, 70, 15–20 CrossRef CAS PubMed.
- J. Huang, X. Gao, J. Jia, J.-K. Kim and Z. Li, Anal. Chem., 2014, 86, 3209–3215 CrossRef CAS PubMed.
- S. Tang, P. Tong, M. Wang, J. Chen, G. Li and L. Zhang, Chem. Commun., 2015, 51, 15043–15046 RSC.
- G. Zhou, M. Lin, P. Song, X. Chen, J. Chao, L. Wang, Q. Huang, W. Huang, C. Fan and X. Zuo, Anal. Chem., 2014, 86, 7843–7848 CrossRef CAS PubMed.
- Y. Zhang, Z. Chen, Y. Tao, Z. Wang, J. Ren and X. Qu, Chem. Commun., 2015, 51, 11496–11499 RSC.
- N. Chen, S. Li, M. R. Battig and Y. Wang, Small, 2013, 9, 3944–3949 CrossRef CAS PubMed.
- Z. Wu, G.-Q. Liu, X.-L. Yang and J.-H. Jiang, J. Am. Chem. Soc., 2015, 137, 6829–6836 CrossRef CAS PubMed.
- Y.-M. Wang, Z. Wu, S.-J. Liu and X. Chu, Anal. Chem., 2015, 87, 6470–6474 CrossRef CAS PubMed.
- S. Venkataraman, R. M. Dirks, P. W. K. Rothemund, E. Winfree and N. A. Pierce, Nat. Nanotechnol., 2007, 2, 490–494 CrossRef PubMed.
- Y. S. Ang and L.-Y. L. Yung, Chem. Commun., 2016, 52, 4219–4222 RSC.
- F. Huang, M. You, D. Han, X. Xiong, H. Liang and W. Tan, J. Am. Chem. Soc., 2013, 135, 7967–7973 CrossRef CAS PubMed.
- F. Huang, X. Zhou, D. Yao, S. Xiao and H. Liang, Small, 2015, 11, 5800–5806 CrossRef CAS PubMed.
- A. Idili, A. Porchetta, A. Amodio, A. Vallée-Bélisle and F. Ricci, Nano Lett., 2015, 15, 5539–5544 CrossRef CAS PubMed.
- B. Koos, G. Cane, K. Grannas, L. Löf, L. Arngården, J. Heldin, C.-M. Clausson, A. Klaesson, M. K. Hirvonen, F. M. S. de Oliveira, V. O. Talibov, N. T. Pham, M. Auer, U. H. Danielson, J. Haybaeck, M. Kamali-Moghaddam and O. Söderberg, Nat. Commun., 2015, 6, 7294 CrossRef CAS PubMed.
- H. Chandran, A. Rangnekar, G. Shetty, E. A. Schultes, J. H. Reif and T. H. Labean, Biotechnol. J., 2013, 8, 221–227 CrossRef CAS PubMed.
- F. Xuan, T. W. Fan and I.-M. Hsing, ACS Nano, 2015, 9, 5027–5033 CrossRef CAS PubMed.
- S. Bi, S. Yue, Q. Wu and J. Ye, Chem. Commun., 2016, 52, 5455–5458 RSC.
- Z. Nie, P. Wang, C. Tian and C. Mao, Angew. Chem., Int. Ed., 2014, 53, 8402–8405 CrossRef CAS PubMed.
- J. Wang, J. Chao, H. Liu, S. Su, L. Wang, W. Huang, I. Willner and C. Fan, Angew. Chem., Int. Ed., 2017, 56, 2171–2175 CrossRef CAS PubMed.
- F. Wang, J. Elbaz, R. Orbach, N. Magen and I. Willner, J. Am. Chem. Soc., 2011, 133, 17149–17151 CrossRef CAS PubMed.
- S. Shimron, F. Wang, R. Orbach and I. Willner, Anal. Chem., 2012, 84, 1042–1048 CrossRef CAS PubMed.
- J. Choi, K. R. Love, Y. Gong, T. M. Gierahn and J. C. Love, Anal. Chem., 2011, 83, 6890–6895 CrossRef CAS PubMed.
- Y. Xu and Z. Zheng, Biosens. Bioelectron., 2016, 79, 593–599 CrossRef CAS PubMed.
- J. Huang, H. Wang, X. Yang, K. Quan, Y. Yang, L. Ying, N. Xie, M. Ou and K. Wang, Chem. Sci., 2016, 7, 3829–3835 RSC.
- W. Song, K. Zhu, Z. Cao, C. Lau and J. Lu, Analyst, 2012, 137, 1396–1401 RSC.
- X. Qiu, P. Wang and Z. Cao, Biosens. Bioelectron., 2014, 60, 351–357 CrossRef CAS PubMed.
- Q. Xu, G. Zhu and C.-Y. Zhang, Anal. Chem., 2013, 85, 6915–6921 CrossRef CAS PubMed.
- M. Rana, M. Balcioglu, M. Kovach, M. S. Hizir, N. M. Robertson, I. Khan and M. V. Yigit, Chem. Commun., 2016, 52, 3524–3527 RSC.
- X. Li, Y. Wang, L. Wang and Q. Wei, Chem. Commun., 2014, 50, 5049–5052 RSC.
- J. Zheng, Y. Hu, J. Bai, C. Ma, J. Li, Y. Li, M. Shi, W. Tan and R. Yang, Anal. Chem., 2014, 86, 2205–2212 CrossRef CAS PubMed.
- Z. Ge, M. Lin, P. Wang, H. Pei, J. Yan, J. Shi, Q. Huang, D. He, C. Fan and X. Zuo, Anal. Chem., 2014, 86, 2124–2130 CrossRef CAS PubMed.
- Y. Yu, Z. Chen, W. Jian, D. Sun, B. Zhang, X. Li and M. Yao, Biosens. Bioelectron., 2015, 64, 566–571 CrossRef CAS PubMed.
- C. Song, G. Xie, L. Wang, L. Liu, G. Tian and H. Xiang, Biosens. Bioelectron., 2014, 58, 68–74 CrossRef CAS PubMed.
- W.-J. Wang, J.-J. Li, K. Rui, P.-P. Gai, J.-R. Zhang and J.-J. Zhu, Anal. Chem., 2015, 87, 3019–3026 CrossRef CAS PubMed.
- P. Miao, Y. Tang and J. Yin, Chem. Commun., 2015, 51, 15629–15632 RSC.
- J. Zhuang, L. Fu, M. Xu, H. Yang, G. Chen and D. Tang, Anal. Chim. Acta, 2013, 783, 17–23 CrossRef CAS PubMed.
- C. Ye, M. Q. Wang, Z. F. Gao, Y. Zhang, J. L. Lei, H. Q. Luo and N. B. Li, Anal. Chem., 2016, 88, 11444–11449 CrossRef CAS PubMed.
- C. Li, H. Wang, J. Shen and B. Tang, Anal. Chem., 2015, 87, 4283–4291 CrossRef CAS PubMed.
- W. Tang, D. Wang, Y. Xu, N. Li and F. Liu, Chem. Commun., 2012, 48, 6678–6680 RSC.
- C.-H. Lu, H.-H. Yang, C.-L. Zhu, X. Chen and G.-N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS PubMed.
- S. He, B. Song, D. Li, C. Zhu, W. Qi, Y. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef CAS.
- Y. Song, W. Wei and X. Qu, Adv. Mater., 2011, 23, 4215–4236 CrossRef CAS PubMed.
- J. Zhang, L. Wang, D. Pan, S. Song, F. Y. C. Boey, H. Zhang and C. Fan, Small, 2008, 4, 1196–1200 CrossRef CAS PubMed.
- H. Li and L. J. Rothberg, J. Am. Chem. Soc., 2004, 126, 10958–10961 CrossRef CAS PubMed.
- H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS PubMed.
- Q. Guo, F. Bian, Y. Liu, X. Qu, X. Hu and Q. Sun, Chem. Commun., 2017, 53, 4954–4957 RSC.
- S. Lu, T. Hu, S. Wang, J. Sun and X. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 167–175 CAS.
- H. Yang, Y. Gao, S. Wang, Y. Qin, L. Xu, D. Jin, F. Yang and G.-J. Zhang, Biosens. Bioelectron., 2016, 80, 450–455 CrossRef CAS PubMed.
- S. Niu, Y. Jiang and S. Zhang, Chem. Commun., 2010, 46, 3089–3091 RSC.
- Y. Chen, J. Xu, J. Su, Y. Xiang, R. Yuan and Y. Chai, Anal. Chem., 2012, 84, 7750–7755 CrossRef CAS PubMed.
- J. Homola, Chem. Rev., 2008, 108, 462–493 CrossRef CAS PubMed.
- I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- J. Ren, J. Wang, L. Han, E. Wang and J. Wang, Chem. Commun., 2011, 47, 10563–10565 RSC.
- L. Wu and X. Qu, Chem. Soc. Rev., 2015, 44, 2963–2997 RSC.
- Q. Liu, C. Jin, Y. Wang, X. Fang, X. Zhang, Z. Chen and W. Tan, NPG Asia Mater., 2014, 6, e95 CrossRef CAS.
- J. Zhou and J. Rossi, Nat. Rev. Drug Discovery, 2016, 16, 181–202 CrossRef PubMed.
- L. Yang, X. Zhang, M. Ye, J. Jiang, R. Yang, T. Fu, Y. Chen, K. Wang, C. Liu and W. Tan, Nat. Rev. Drug Discovery, 2011, 63, 1361–1370 CAS.
- M.-H. Meng, T. Fu, B.-X. Zhang and W. Tan, Natl. Sci. Rev., 2015, 2, 71–84 CrossRef.
- M. Salunkhe, T. Wu and R. L. Letsinger, J. Am. Chem. Soc., 1992, 114, 8768–8772 CrossRef CAS.
- X. Wang, A. Jiang, T. Hou, H. Li and F. Li, Biosens. Bioelectron., 2015, 70, 324–329 CrossRef CAS PubMed.
- T. D. H. Bugg, Nat. Prod. Rep., 2001, 18, 465–493 RSC.
- A. Warshel, P. K. Sharma, M. Kato, Y. Xiang, H. Liu and M. H. M. Olsson, Chem. Rev., 2006, 106, 3210–3235 CrossRef CAS PubMed.
- X. Zhou and D. Xing, Chem. Soc. Rev., 2012, 41, 4643–4656 RSC.
- J. Nandakumar and T. R. Cech, Nat. Rev. Mol. Cell Biol., 2013, 14, 69–82 CrossRef CAS PubMed.
- N. B. Muren and J. K. Barton, J. Am. Chem. Soc., 2013, 135, 16632–16640 CrossRef CAS PubMed.
- C. J. Dobson and S. L. Allinson, Nucleic Acids Res., 2006, 34, 2230–2237 CrossRef CAS PubMed.
- Z. Tang, K. Wang, W. Tan, C. Ma, J. Li, L. Liu, Q. Guo and X. Meng, Nucleic Acids Res., 2005, 33, e97 CrossRef PubMed.
- Y. Zhang, C. Liu, S. Sun, Y. Tang and Z. Li, Chem. Commun., 2015, 51, 5832–5835 RSC.
- A. Bajaj, S. Rana, O. R. Miranda, J. C. Yawe, D. J. Jerry, U. H. F. Bunz and V. M. Rotello, Chem. Sci., 2010, 1, 134–138 RSC.
- M. M. Costa, A. de la Escosura-Muñiz, C. Nogués, L. Barrios, E. Ibáñez and A. Merkoçi, Nano Lett., 2012, 12, 4164–4171 CrossRef PubMed.
- W. Tan, M. J. Donovan and J. Jiang, Chem. Rev., 2013, 113, 2842–2862 CrossRef CAS PubMed.
- B. Yang, X.-B. Zhang, L.-P. Kang, G.-L. Shen, R.-Q. Yu and W. Tan, Anal. Chem., 2013, 85, 11518–11523 CrossRef CAS PubMed.
- G. Aragay, J. Pons and A. Merkoçi, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS PubMed.
- S. Caroli, G. Forte, A. L. Iamiceli and B. Galoppi, Talanta, 1999, 50, 327–336 CrossRef CAS PubMed.
- P. Pohl, TrAC, Trends Anal. Chem., 2009, 28, 117–128 CrossRef CAS.
- A. Ono and H. Togashi, Angew. Chem., Int. Ed., 2004, 43, 4300–4302 CrossRef CAS PubMed.
- A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto and Y. Tanaka, Chem. Commun., 2008, 4825–4827 RSC.
- J. Zhuang, L. Fu, M. Xu, Q. Zhou, G. Chen and D. Tang, Biosens. Bioelectron., 2013, 45, 52–57 CrossRef CAS PubMed.
- X.-B. Zhong, P. M. Lizardi, X.-H. Huang, P. L. Bray-Ward and D. C. Ward, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 3940–3945 CrossRef CAS PubMed.
- A. T. Christian, M. S. Pattee, C. M. Attix, B. E. Reed, K. J. Sorensen and J. D. Tucker, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 14238–14243 CrossRef CAS PubMed.
- G. J. Nuovo, Diagn. Mol. Pathol., 2000, 9, 195–202 CrossRef CAS PubMed.
- F. Maruyama, T. Kenzaka, N. Yamaguchi, K. Tani and M. Nasu, Appl. Environ. Microbiol., 2003, 69, 5023–5028 CrossRef CAS PubMed.
- S. Ikeda, K. Takabe, M. Inagaki, N. Funakoshi and K. Suzuki, Pathol. Int., 2007, 57, 594–599 CrossRef CAS PubMed.
- H. M. T. Choi, V. A. Beck and N. A. Pierce, ACS Nano, 2014, 8, 4284–4294 CrossRef CAS PubMed.
- L. Li, J. Feng, H. Liu, Q. Li, L. Tong and B. Tang, Chem. Sci., 2016, 7, 1940–1945 RSC.
- A. Z. Rosenthal, X. Zhang, K. S. Lucey, E. A. Ottesen, V. Trivedi, H. M. T. Choi, N. A. Pierce and J. R. Leadbetter, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 16163–16168 CrossRef CAS PubMed.
- H. M. T. Choi, C. R. Calvert, N. Husain, D. Huss, J. C. Barsi, B. E. Deverman, R. C. Hunter, M. Kato, S. M. Lee, A. C. T. Abelin, A. Z. Rosenthal, O. S. Akbari, Y. Li, B. A. Hay, P. W. Sternberg, P. H. Patterson, E. H. Davidson, S. K. Mazmanian, D. A. Prober, M. van de Rijn, J. R. Leadbetter, D. K. Newman, C. Readhead, M. E. Bronner, B. Wold, R. Lansford, T. Sauka-Spengler, S. E. Fraser and N. A. Pierce, Development, 2016, 143, 3632–3637 CrossRef CAS PubMed.
- S. Shah, E. Lubeck, M. Schwarzkopf, T.-F. He, A. Greenbaum, C. Ho Sohn, A. Lignell, H. M. T. Choi, V. Gradinaru and N. A. Pierce, Development, 2016, 143, 2862–2867 CrossRef CAS PubMed.
- D. Huss, H. M. T. Choi, C. Readhead, S. E. Fraser, N. A. Pierce and R. Lansford, Cold Spring Harbor Protocols, 2015, 3, 259–268 Search PubMed.
- Y. Tang, X.-L. Zhang, L.-J. Tang, R.-Q. Yu and J.-H. Jiang, Anal. Chem., 2017, 89, 3445–3451 CrossRef CAS PubMed.
- T. Yamaguchi, S. Kawakami, M. Hatamoto, H. Imachi, M. Takahashi, N. Araki, T. Yamaguchi and K. Kubota, Environ. Microbiol., 2015, 17, 2532–2541 CrossRef CAS PubMed.
- G. Bao, W. J. Rhee and A. Tsourkas, Annu. Rev. Biomed. Eng., 2009, 11, 25–47 CrossRef CAS PubMed.
- M. Hong, L. Xu, Q. Xue, L. Li and B. Tang, Anal. Chem., 2016, 88, 12177–12182 CrossRef CAS PubMed.
- Z. Zhang, Y. Jiao, Y. Wang and S. Zhang, Sci. Rep., 2016, 6, 29872 CrossRef CAS PubMed.
- D. He, X. He, X. Yang and H.-W. Li, Chem. Sci., 2017, 8, 2832–2840 RSC.
- T. M. Allen, Nat. Rev. Cancer, 2002, 2, 750–763 CrossRef CAS PubMed.
- S. M. Douglas, I. Bachelet and G. M. Church, Science, 2012, 335, 831–834 CrossRef CAS PubMed.
- C. Wu, D. Han, T. Chen, L. Peng, G. Zhu, M. You, L. Qiu, K. Sefah, X. Zhang and W. Tan, J. Am. Chem. Soc., 2013, 135, 18644–18650 CrossRef CAS PubMed.
- S. Kruspe and U. Hahn, Angew. Chem., Int. Ed., 2014, 53, 10541–10544 CrossRef CAS PubMed.
- A. D. Keefe, S. Pai and A. Ellington, Nat. Rev. Drug Discovery, 2010, 9, 537–550 CrossRef CAS PubMed.
- G. V. Dubacheva, T. Curk, R. Auzély-Velty, D. Frenkel and R. P. Richter, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 5579–5584 CrossRef CAS PubMed.
- Z. Xiao, D. Shangguan, Z. Cao, X. Fang and W. Tan, Chem. – Eur. J., 2008, 14, 1769–1775 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |