Open Access Article
Open Access ArticleCovalent triazine-based frameworks (CTFs) from triptycene and fluorene motifs for CO2 adsorption†
Subarna
Dey
a,
Asamanjoy
Bhunia
a,
Dolores
Esquivel
b and
Christoph
Janiak
*a
aInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de
bDepartment of Organic Chemistry, Nanochemistry and Fine Chemistry Research Institute (IUIQFN), Faculty of Sciences, University of Córdoba, Campus de Rabanales, Marie Curie Building, Ctra. Nnal. IV, 14071 Córdoba, Spain
First published on 31st March 2016
Abstract
Two microporous covalent triazine-based frameworks (CTFs) with triptycene (TPC) and fluorene (FL) backbones have been synthesized through a mild AlCl3-catalyzed Friedel–Crafts reaction, with the highest surface area of up to 1668 m2 g−1 for non-ionothermal CTFs. CTF-TPC and CTF-FL show an excellent carbon dioxide uptake capacity of up to 4.24 mmol g−1 at 273 K and 1 bar.
The burning of fossil fuels increases the CO2 level in the atmosphere which may contribute to global warming and is more imminent to ocean acidification.1,2 Therefore, research on carbon capture and storage/sequestration (CCS) has been receiving great attention over the last few decades.3 It may be necessary to develop CO2 capture technologies with as little energy penalty as possible. Typically, a flue gas contains N2 (75–76%), CO2 (15–16%), H2O (5–7%), O2 (3–4%), CO (20 ppm), SOx (<800 ppm), and NOx (<500 ppm) with the emission temperatures of 50–75 °C at 1 bar but its exact composition highly depends on the design of the power plant and the source of natural gas or coal.4 To capture CO2 at such concentration requires separating CO2 in the presence of N2 and H2O in post-combustion systems. Known CO2 separations in a post-combustion process are amine scrubbing (amine washing) and chilled ammonia technologies5 in which the Lewis acid CO2 interacts with Lewis basic solution resulting in carbamate formation. Drawbacks include regeneration, fouling of the equipment and solvent boil-off. Alternative methods, e.g., chemisorption on solid oxide surfaces or physical adsorption on porous solids, are attracting increasing attention.
Solid adsorbents are zeolites,6 metal–organic frameworks (MOFs),4 silica,7 activated carbons8 and porous organic polymers (POPs).9 MOFs and POPs have gained attention due to their high surface area, ability to be functionalized and selectivity for CO2 over other gases. Classes of POPs are often differentiated according to their building units such as benzimidazole-linked polymers (BILPs),10 hyper-crosslinked polymers (HCPs),11 polymers of intrinsic microporosities (PIMs),12 porous aromatic frameworks (PAFs),13 conjugated microporous polymers (CMPs),14 covalent organic frameworks (COFs),15 Schiff base networks (SNWs),16 covalent imine polymers (CIP),17 porous polymer frames/networks (PPFs/PPNs),18 element–organic frameworks (EOFs)19 and nitrogen-doped porous carbon materials (NPCs).20 POPs often have higher stability towards moisture than MOFs which is crucial for post-combustion CO2 capture materials.
Covalent triazine-based frameworks (CTFs) are another subclass of POPs that possess high chemical and thermal stability with a high specific surface area.21 CTFs are interesting because of cheap and readily available starting materials, nitrogen content, facile synthesis and certain hydrophilicity. The following strategies for porous CTF preparation have been reported: (1) ionothermal conditions (ZnCl2),21 (2) strong Brønsted acid conditions (CF3SO3H),22 and (3) Friedel–Crafts (AlCl3) reaction.23,24 Kuhn, Antonietti and Thomas et al. developed CTFs with permanent porosity.21 By (1) and (2) these materials are made by the trimerization reaction of carbonitriles to form triazine rings. From their elemental composition, we view CTFs from ionothermal reaction with ZnCl2 as in-between well-defined COFs and porous carbon materials. CTFs with surface areas of up to 1152 m2 g−1 were synthesized by using strong Brønsted acid conditions (CF3SO3H) under room temperature and microwave conditions.22 Brønsted acid conditions usually provide lower surface areas but they avoid decomposition and condensation reactions such as C–H bond cleavage and carbonization and, hence, result in less structural defects. Recently, anhydrous aluminum chloride catalyzed Friedel–Crafts reactions have been used to prepare rather well-defined CTF materials with surface areas up to 1452 m2 g−1.23,24 Friedel–Crafts catalysis offers the significant advantages of cheap, easy handling, low reaction temperature, facile synthesis conditions and high yield materials with high surface areas.
Here, the porous frameworks CTF-TPC and CTF-FL were synthesized by Friedel–Crafts reactions between the “linkers” triptycene or fluorene with cyanuric chloride as the “node” in the presence of anhydrous AlCl3 as the catalyst in dichloromethane (Scheme 1, see ESI† for details). After purification, the insoluble materials (Fig. S1 in the ESI†) were reproducibly collected in yields greater 80%. The strong triazine electrophile formed by AlCl3, results in the electrophilic aromatic substitution on triptycene or fluorene to give the triazine frameworks. Network formation was supported by FT-IR (Fig. 1) and 13C MAS or 13C cross-polarization with magnetic angle spinning (CP-MAS) NMR spectroscopy (Fig. S2 and S3, ESI†). The absence of a C–Cl stretching band at 849 cm−1 suggested that there was no cyanuric chloride left in the materials.24 The absorption band of the triazine ring is shifted from 1352 cm−1 in cyanuric chloride to 1384 cm−1 in CTF-TPC and 1346 cm−1 in CTF-FL (see Section 6 in the ESI† for comparative literature data).25
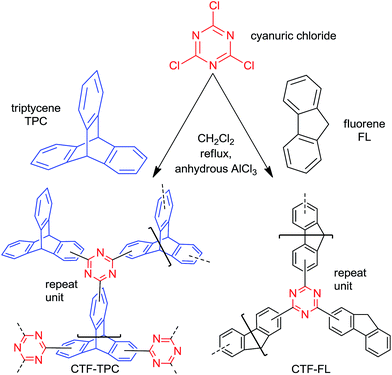 | ||
| Scheme 1 Synthesis route and model structures for CTF-TPC and CTF-FL (based on elemental analysis, see Section 5 in the ESI†). Both models have a terminal and two bridging ligands for each triazine C3N3 ring. For CTF-TPC we do not assume the formation of a symmetric network because of the different possible positions for acylation on the TPC aryl rings. For CTF-FL we assume the formation of a polymer chain which can be crosslinked if the terminal FL occasionally also becomes bridged. | ||
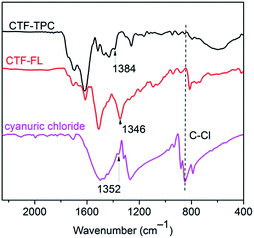 | ||
| Fig. 1 FT-IR spectra of CTF-TPC and CTF-FL in comparison to that of cyanuric chloride, with proposed triazine bands labelled. | ||
The signals in the solid-state 13C CP/MAS NMR spectra are in the expected range (Fig. S2 and S3, ESI†),26–28 which suggests the formation of triazine-based frameworks. CTF-TPC shows a chemical shift at 51 ppm, corresponding to the bridgehead carbons of the triptycene unit. The signals of the phenyl ring of triptycene are in the range of 120–150 ppm, the triazine ring carbons appear at 164 ppm. CTF-FL shows a peak at 37 ppm, which can be assigned to the methylene bridge carbon in the fluorene unit. The other major peaks from 115 ppm to 150 ppm correspond to the aromatic carbons in the fluorene unit; a peak for the triazine ring carbons is detected at 172 ppm.
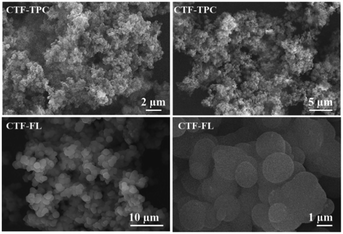
The amorphous nature of the products was assessed by PXRD (Fig. S4, ESI†). The diffractograms show only three broad bands around 17, 30 and 40° 2theta. From TGA, it can be observed that CTF-TPC and CTF-FL start to decompose under an O2 atmosphere at 380 and 360 °C, respectively (Fig. S5, ESI†). Scanning electron microscopy (SEM) reveals aggregation of spherical particles (Fig. 2).
The porosities of the activated CTFs (degassing at 200 °C for 24 h) were characterized by N2 sorption measurements at 77 K. The N2 adsorption isotherm of CTF-TPC exhibits a steep increase in N2 uptake at low p/p0 values, and continues to increase at high pressure values. Therefore, the curve could be classified as a combination of types I and IV. The H4 type hysteresis is often associated with narrow slit-like pores but the type I character indicates microporosity.29 On the other hand, the isotherm of CTF-FL is only of type I with H4 type hysteresis. The Brunauer–Emmett–Teller (BET) surface areas (pressure range of p/p0 = 0.01–0.05) of CTF-TPC and CTF-FL are 1668 and 773 m2 g−1, respectively. BET measurements of three other batches each confirmed the reproducibility of the results (Table S4, ESI†). The surface areas are comparable with those of other CTFs (Table S8, ESI†). To the best of our knowledge, the surface area of CTF-TPC, synthesized by AlCl3, exceeds all reported values for similar networks. The surface area of CTF-FL is lower than that of the previously reported fluorene-based CTFs synthesized by ZnCl2.27 In agreement with the type I isotherms the ratio of micropore volume to total pore volume (V0.1/Vtot) is in the range of 70–79% (Table 1). Pore size distributions by non-local density functional theory (NLDFT) using the model of “N2 on carbon, slit-pores method” gave a narrow distribution of micropores centered at 5.9, 7.3, 11.8 and 14.8 Å for CTF-TPC, and 5, 6.4, 8 and 11.8 Å for CTF-FL (Fig. 3). However, a low proportion of mesopores was observed around ∼27 Å for both CTFs in agreement with the H4 hysteresis of the N2 isotherms.
| Compound | S BET (m2 g−1) | S Lang (m2 g−1) | V 0.1 (cm3 g−1) | V tot (cm3 g−1) | V 0.1/Vtot | V micro(CO2)e (cm3 g−1) |
|---|---|---|---|---|---|---|
| a Calculated BET surface area over the pressure range of 0.01–0.05p/p0 values for batches from repeated syntheses are given in Table S4 in the ESI. b Langmuir surface area over the pressure range of 0–110 torr. c Micropore volume calculated from the N2 adsorption isotherm at p/p0 = 0.1 for pores ≤2 nm (20 Å). d Total pore volume at p/p0 = 0.95 for pores ≤20 nm. e Total pore volume for pores with diameters smaller than 1 nm (10 Å, cf. Fig. S7, ESI) from the CO2 NL-DFT model at 273 K. | ||||||
| CTF-TPC | 1668 | 2041 | 0.65 | 0.93 | 0.70 | 0.11 |
| CTF-FL | 773 | 936 | 0.31 | 0.39 | 0.79 | 0.11 |
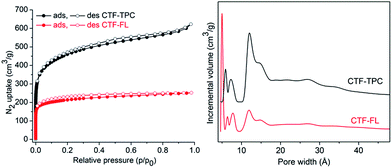 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms at 77 K (left) of CTF-TPC and CTF-FL and NLDFT pore size distribution (PSD) curve (right) of CTF-TPC and CTF-FL. | ||
The H2 sorption of CTF-TPC and CTF-FL (Table 2 and Fig. 4) is comparable to that of many reported CTF materials.21a,27,30,31 The CO2 adsorption of 95.2 cm3 g−1 (4.24 mmol g−1) for CTF-TPC and of 73.2 cm3 g−1 (3.26 mmol g−1) for CTF-FL (Tables 2 and S5, ESI†) agrees with the published data for other CTFs such as FL-CTF (1.27–4.28 mmol g−1),26a FCTF-1 (4.67–5.53 mmol g−1),32 CTF-0 (4.22 mmol g−1),33 CTF-1 (2.47–3.82 mmol g−1),32 CTF-Pl–P6 (1.88–3.39 mmol g−1),22 CTF-P1M–P6M (0.94–4.42 mmol g−1),22 MCTF-300–500 (2.25–3.16 mmol g−1),34 PCTF-1–7 (1.85–3.26 mmol g−1)31 and NOP-1–6 (1.31–2.51 mmol g−1)30 (see Table S8 in the ESI† for details). The isosteric heats of adsorption (Qst) from the CO2 adsorption isotherms at 273 and 298 K near zero loading are 32 and 35 kJ mol−1 for CTF-TPC and -FL, respectively (Fig. S6, ESI†), and are again comparable to those of other CTFs (see Section 11 in the ESI†). Upon increasing the loading the Qst values decrease to 26 and 28 kJ mol−1 for CTF-TPC and CTF-FL, respectively, which are, however, still well above the heat of liquefaction of bulk CO2 with 17 kJ mol−1. The high Qst value can be attributed to the high polar framework and the pore size effect. The high adsorption enthalpy at zero coverage is explained by the initial filling of the small ultramicropores with 4 Å diameter (Fig. S7†) with adsorbate–surface interactions to both sides or ends of the CO2 molecules.
| Compound | H2a (cm3 g−1) | CO2b (cm3 g−1) | Q 0ads(CO2)c (kJ mol−1) | CH4b (cm3 g−1) | N2b (cm3 g−1) | CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2d Henry N2d Henry |
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2d IAST N2d IAST |
|---|---|---|---|---|---|---|---|
| a Gas uptake at 77 K. b At 273 K. c Heat of adsorption for CO2 at zero loading from adsorption isotherms acquired at 273 and 293 K. d Gas selectivity calculated at 273 K. | |||||||
| CTF-TPC | 195.8 | 95.2 | 32 | 30 | 9.2 | 20 | 30 |
| CTF-FL | 146.6 | 73.2 | 35 | 24.9 | 7.1 | 25 | 48 |
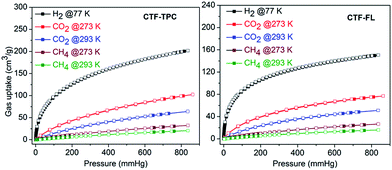 | ||
| Fig. 4 Gas sorption isotherms of CTF-TPC (left) and CTF-FL (right) (filled symbols for adsorption, empty symbols for desorption). | ||
The CH4 uptake capacities of CTF-TPC and CTF-FL (Fig. 4, Tables 2 and S5 in the ESI†) are comparable with the values for PCTF-1 to -7 from our previous work as well as other CTFs.24,31 The gas sorption capacity for CH4 of CTFs follows the increase in surface area and pore volume (Table 1).
From the available single-gas adsorption isotherms, the CO2 selectivities over N2 or CH4 for CTF-TPC and CTF-FL were calculated at 273 and 293 by using the Henry equation and the ideal adsorbed solution theory (IAST). From the Henry equation, the adsorption selectivity for CO2 over N2 is 20 and 25 at 273 K (16 and 21 at 293 K) for CTF-TPC and CTF-FL, respectively, whereas the corresponding CO2/CH4 selectivities were in the range of 4–5 at 273 K and 293 K (Fig. S8 and S9 in the ESI; Tables 2 and S5 in the ESI†). From IAST (Fig. S10, S11 and Tables S6, S7 in the ESI†), the CO2/N2 selectivities of CTF-TPC and CTF-FL are 30 and 48 at 273 K (Table 2), which are higher than from the Henry calculation. The selectivity of CTF-FL is high compared with recent work by others.27 A trade-off relation is observed for the CO2/N2 selectivity versus porosity: a higher surface area of CTF-TPC (compared to CTF-FL) exhibits lower selectivity, as can be generalized for other CTF-based polymers (see Table S8 in the ESI†).
In summary, the AlCl3 catalyzed Friedel–Crafts reaction can be used for the aromatic linkers triptycene and fluorene to construct triazine-based frameworks. By using this approach, the compound CTF-TPC with 1668 m2 g−1 gave the highest BET surface area found for such AlCl3-catalyzed cyanuric-chloride based CTFs, so far. It is suggested that the triptycene unit is especially amenable to construct high surface-area porous materials.35 CTF-TPC and CTF-FL adsorbed 4.24 mmol g−1 and 3.26 mmol g−1 (273 K/1 bar) of CO2, respectively. Such high CO2 uptake together with cost-effective synthesis make CTFs a promising candidate for porous adsorbents. Porosity and gas adsorption behavior can be tuned by changing the linker in this cyanuric-acid/AlCl3-catalysis approach.
Acknowledgements
Support of the work by BMBF project OptiMat 03SF0492C and by the University of Düsseldorf through its strategic research fund (SFF) is gratefully acknowledged. We thank Mrs J. Dechnik, Mrs L. Schmolke, Mr D. Dietrich, Mr S. Glomb, Mr Hastürk and Mr P. Roloff for their help in analytical measurements.Notes and references
- R. S. Haszeldine, Science, 2009, 325, 1647 CrossRef CAS PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed; H. He, W. Li, M. Zhong, D. Konkolewicz, D. Wu, K. Yaccato, T. Rappold, G. Sugar, N. E. David and K. Matyjaszewski, Energy Environ. Sci., 2013, 6, 488–493 Search PubMed.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- J. A. Mason, T. M. McDonald, T.-H. Bae, J. E. Bachman, K. Sumida, J. J. Dutton, S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2015, 137, 4787–4803 CrossRef CAS PubMed.
- G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed; U. Desideri and A. Paolucci, Energy Convers. Manage., 1999, 40, 1899–1915 CrossRef.
- J. Zhang, P. A. Webley and P. Xiao, Energy Convers. Manage., 2008, 49, 346–356 CrossRef CAS.
- (a) G. Qi, Y. Wang, L. Estevez, X. Duan, N. Anako, A.-H. A. Park, W. Li, C. W. Jones and E. P. Giannelis, Energy Environ. Sci., 2011, 4, 444–452 RSC; (b) J. C. Hicks, J. H. Drese, D. J. Fauth, M. L. Gray, G. Qi and C. W. Jones, J. Am. Chem. Soc., 2008, 130, 2902–2903 CrossRef CAS PubMed.
- L. Wang and R. T. Yang, J. Phys. Chem. C, 2012, 116, 1099–1106 CAS.
- V. Guillerm, L. J. Weselinski, M. Alkordi, M. I. H. Mohideen, Y. Belmabkhout, A. J. Cairns and M. Eddaoudi, Chem. Commun., 2014, 50, 1937–1940 RSC; S. Mondal and N. Das, J. Mater. Chem. A, 2015, 3, 23577–23586 Search PubMed; G. Liu, Y. Wang, C. Shen, Z. Ju and D. Yuan, J. Mater. Chem. A, 2015, 3, 3051–3058 Search PubMed.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2012, 24, 1511–1517 CrossRef CAS.
- J. Germain, J. Hradil, J. M. J. Fréchet and F. Svec, Chem. Mater., 2006, 18, 4430–4435 CrossRef CAS.
- B. S. Ghanem, K. J. Msayib, N. B. McKeown, K. D. M. Harris, Z. Pan, P. M. Budd, A. Butler, J. Selbie, D. Book and A. Walton, Chem. Commun., 2007, 67–69 RSC.
- H. Zhao, Z. Jin, H. Su, J. Zhang, X. Yao, H. Zhao and G. Zhu, Chem. Commun., 2013, 49, 2780–2782 RSC.
- J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS PubMed.
- (a) S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC; (b) Introduction to themed issue on COFs: A. I. Cooper, CrystEngComm, 2013, 15, 1483 RSC; (c) S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed; (d) T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS PubMed; (e) S. Dalapati, S. Jin, J. Gao, Y. Xu, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2013, 135, 17310–17313 CrossRef CAS PubMed; (f) S. Nandi, U. Werner-Zwanziger and R. Vaidhyanathan, J. Mater. Chem. A, 2015, 3, 21116–21122 RSC.
- M. Shunmughanathan, P. Puthiaraj and K. Pitchumani, ChemCatChem, 2015, 7, 666–673 CrossRef CAS.
- R. Gomes, P. Bhanja and A. Bhaumik, Chem. Commun., 2015, 51, 10050–10053 RSC.
- L.-B. Sun, A.-G. Li, X.-D. Liu, X.-Q. Liu, D. Feng, W. Lu, D. Yuan and H.-C. Zhou, J. Mater. Chem. A, 2015, 3, 3252–3256 CAS.
- M. Rose, W. Bohlmann, M. Sabo and S. Kaskel, Chem. Commun., 2008, 2462–2464 RSC.
- M. Nandi, K. Okada, A. Dutta, A. Bhaumik, J. Maruyama, D. Derks and H. Uyama, Chem. Commun., 2012, 48, 10283–10285 RSC.
- (a) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS PubMed; (b) P. Kuhn, A. l. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333–13337 CrossRef CAS PubMed.
- S. Ren, M. J. Bojdys, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Adv. Mater., 2012, 24, 2357–2361 CrossRef CAS PubMed.
- H. Lim, M. C. Cha and J. Y. Chang, Macromol. Chem. Phys., 2012, 213, 1385–1390 CrossRef CAS.
- P. Puthiaraj, S.-M. Cho, Y.-R. Lee and W.-S. Ahn, J. Mater. Chem. A, 2015, 3, 6792–6797 CAS.
- (a) S. K. Kundu and A. Bhaumik, RSC Adv., 2015, 5, 32730–32739 RSC; (b) C. Bai, M. Zhang, B. Li, Y. Tian, S. Zhang, X. Zhao, Y. Li, L. Wang, L. Ma and S. Li, J. Hazard. Mater., 2015, 300, 368–377 CrossRef CAS PubMed; (c) P. Puthiaraj, S.-S. Kim and W.-S. Ahn, Chem. Eng. J., 2016, 283, 184–192 CrossRef CAS.
- (a) C. Zhang, Y. Liu, B. Li, B. Tan, C.-F. Chen, H.-B. Xu and X.-L. Yang, ACS Macro Lett., 2012, 1, 190–193 CrossRef CAS; (b) C. Zhang, T.-L. Zhai, J.-J. Wang, Z. Wang, J.-M. Liu, B. Tan, X.-L. Yang and H.-B. Xu, Polymer, 2014, 55, 3642–3647 CrossRef CAS.
- S. Hug, M. B. Mesch, H. Oh, N. Popp, M. Hirscher, J. Senker and B. V. Lotsch, J. Mater. Chem. A, 2014, 2, 5928–5936 CAS.
- S. Kuecken, J. Schmidt, L. Zhi and A. Thomas, J. Mater. Chem. A, 2015, 3, 24422–24427 CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- S. Xiong, X. Fu, L. Xiang, G. Yu, J. Guan, Z. Wang, Y. Du, X. Xiong and C. Pan, Polym. Chem., 2014, 5, 3424–3431 RSC.
- (a) A. Bhunia, V. Vasylyeva and C. Janiak, Chem. Commun., 2013, 49, 3961–3963 RSC; (b) A. Bhunia, I. Boldog, A. Möller and C. Janiak, J. Mater. Chem. A, 2013, 1, 14990–14999 RSC.
- Y. Zhao, K. X. Yao, B. Teng, T. Zhang and Y. Han, Energy Environ. Sci., 2013, 6, 3684–3692 CAS.
- P. Katekomol, J. Roeser, M. Bojdys, J. Weber and A. Thomas, Chem. Mater., 2013, 25, 1542–1548 CrossRef CAS.
- X. Liu, H. Li, Y. Zhang, B. Xu, S. A, H. Xia and Y. Mu, Polym. Chem., 2013, 4, 2445–2448 RSC.
- (a) G. Zhang, O. Presly, F. White, I. M. Oppel and M. Mastalerz, Angew. Chem., Int. Ed., 2014, 53, 1516–1520 CrossRef CAS PubMed; (b) G. Zhang, O. Presly, F. White, I. M. Oppel and M. Mastalerz, Angew. Chem., Int. Ed., 2014, 53, 5126–5130 CAS; (c) S. M. Elbert, F. Rominger and M. Mastalerz, Chem.–Eur. J., 2014, 20, 16707–16720 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, NMR, powder XRD, elemental analysis, IR, TGA, gas sorption and selectivity. See DOI: 10.1039/c6ta00638h |
| This journal is © The Royal Society of Chemistry 2016 |