Enzymatic transesterification for biodiesel production: a comprehensive review
B. Norjannah
a,
Hwai Chyuan Ong*a,
H. H. Masjukia,
J. C. Juanb and
W. T. Chonga
aDepartment of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: onghc@um.edu.my; ong1983@yahoo.com; Fax: +60-3-7967-5317; Tel: +60-16-590-3110
bNanotechnology & Catalysis Research Centre (NanoCat), Institute of Postgraduate Studies, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 15th June 2016
Abstract
Biodiesel is a type of renewable fuel and a potential alternative for continuously consumed fossil resources. Currently, the method applied for biodiesel production is transesterification which is divided into non-catalyzed reaction, chemical-catalyzed reaction and enzymatic reaction. Enzymatic reaction is more advantageous than the other methods because of its mild reaction conditions, easy product recovery, no wastewater generation, no saponification and higher quality of products. The main component in this reaction is an enzyme called lipase which can catalyze wide variety of substrate including free fatty acids. Two other main raw materials for biodiesel synthesis are oil and acyl acceptor such as alcohol. Biodiesel catalyzed by enzyme is affected by many factors such as lipase specificity, lipase immobilization, oil composition and purity, oil to acyl acceptor molar ratio, acyl acceptors, temperature, and water content. Many methods have been tested to manipulate these factors and improve the enzymatic reaction for biodiesel production. These methods include combination of lipases, enzyme pretreatment, enzyme post treatment, methanol addition technique, use of solvent and silica gel, and reactor design. This paper will critically discuss the three major components of enzymatic production of biodiesel and the methods used to improve enzymatic reaction, as well as a review on its economic evaluation and industrial scale production.
B. Norjannah, Hwai Chyuan Ong, H. H. Masjuki and J. C. Juan The research on biofuels has been established and developed by the group members from Centre for Energy Sciences, Faculty of Engineering, University of Malaya, Malaysia. Professor Dr Masjuki Hj Hassan is Director of the Centre for Energy Sciences and leading the biofuel research in University of Malaya. Dr Hwai Chyuan Ong, Ms B. Norjannaha and Dr J. C. Juan are the academic staff and Researcher in Faculty of Engineering, University of Malaya which are involved actively in biofuel research. |
1. Introduction
The demand of fuel for transportation and industry has been increasing each day and causes the depletion of non-renewable energy such as petroleum and natural gas. In addition, the burning of fossil fuels contributes to carbon dioxide and methane gas emissions that have been associated with global warming and harming the Earth. These problems have become the reasons to find alternative sources of energy that are sustainable and also environmental friendly. One of the potential alternatives is biofuel produced from renewable sources such as plant biomass and animal fats.According to the International Energy Agency,1 10.2% of world total primary energy supply in the year 2013 was contributed by biofuels and waste while 3.6% from other renewable sources such as hydro, geothermal, solar, wind, and heat. This data shows that biofuel has been used widely as energy source together with oil (31.1%), coal (28.9%) and natural gas (21.4%). Furthermore, data from BP Statistical Review of World Energy2 shows that the world total biofuel production in 2014 was 70.8 Mtoe (million tonnes of oil equivalent) and the largest producer was United States at 30.1 Mtoe. About 10.6% of the biofuels were produced by Asia Pacific countries such as China, Indonesia, and Thailand. In Malaysia, its National Biofuel Policy has introduced biodiesel fuel blend in 2009 and the main feedstock for the biodiesel production is palm oil and its residues such as empty fruit bunches, shells and fibers.3 Other countries also have their own biodiesel mandates for example United States and Brazil that use soybean oil as their main feedstock.4
Biofuels are produced in three different states: solid (bio-char), liquid (bioethanol, biodiesel) and gaseous (biohydrogen, biogas).5 As shown in Fig. 1, biodiesel can be categorized into three generations: 1st generation which derived from edible vegetable oils; 2nd generation from non-edible vegetable oils and waste cooking/frying oil; and 3rd generation from algae and other microorganisms.5,6 The benefit of using biodiesel from plants is that its combustion will not increase the net atmospheric levels of carbon dioxide.7 To avoid food versus fuel controversy that is caused by using edible plant oils, the appropriate alternative would be to use non-edible plant oils. Non-edible plants are pest and disease resistant, and able to grow at arid land, higher rainfall, or non-agricultural areas.8 In addition, biodiesel production from non-edible oil could create jobs in rural places and produce useful by-product (seed cakes) that can be used as fertilizers.8
Even though plant oils is a good source of fuel due to its high cetane number of over 40, it cannot be used for a long-term engine performance. This is because they would cause buildup of carbon deposit and sticking of piston rings.9 A solution proposed is blending diesel with non-edible oil which does not involve any chemical process and able to reduce viscosity, gas and smoke emission, as well as improve engine performance.8,10 Other than blending the oil with diesel, another solution is by converting plant oils into alkyl esters that has lower viscosity and improved heating value.9
Generally, biodiesel is produced in form of fatty acid alkyl ester (FAAE) through esterification reaction of fatty acids with short chain alcohols or transesterification reaction of triglyceride (TG) with short chain alcohol that generate glycerol as byproduct.11 For biodiesel production using enzymatic reaction, three components that play important roles in biodiesel synthesis are lipase, oil and acyl acceptor. The selection of these three components, along with the operating conditions will affect the efficiency of the process. Furthermore, many additional measures have been tested to improve the enzymatic reaction such as combination of lipases, enzyme pretreatment, enzyme post treatment, methanol addition technique, use of solvent and silica gel, and reactor design. Therefore, three main components and the methods applied to improve enzymatic production of biodiesel are critical and will be discussed.
2. Biodiesel production process
There are three methods to produce biodiesel through transesterification/esterification: (i) non-catalyzed reaction; (ii) chemical-catalyzed reaction; and (iii) enzyme-catalyzed reaction. The comparison of advantages and disadvantages of these methods are listed in Table 1. Non-catalyzed reaction usually involved transesterification in supercritical conditions (methanol or ethanol). Non-catalyzed reaction has high reaction rate, easy separation of products and no waste generation.12 This reaction can complete in a short time as fast as 2 minutes but requires high temperature and pressure ranges from 280 to 400 °C and 10 to 30 MPa, consumes great energy, and involves high cost.8,13,14| Methods | Advantages | Disadvantages |
|---|---|---|
| Enzymatic reaction (immobilized lipase) | Medium yield, can convert FFA, low energy usage, high product and by-product purity, reusable catalyst, no wastewater | Inhibition by alcohol or by-product, high cost of enzyme, slow reaction |
| Non-catalyzed reaction (supercritical alcohol) | Super-fast reaction, high yield, can convert FFA, no catalyst, easy product purification, no waste | High temperature and pressure, high cost of reactor, high alcohol to oil molar ratio |
| Chemical-catalyzed reaction (homogenous) | High yield, low cost | Wastewater, need product purification steps, difficult catalyst recovery |
| Can convert FFA (acid catalyst) | Saponification (alkali catalyst) | |
| Chemical-catalyzed (heterogeneous) | Fast reaction, high yield, reusable catalyst, medium cost, can be used in continuous process | High energy, difficult catalyst preparation, catalyst leaching |
| Can convert FFA (acid catalyst) | Saponification (alkali catalyst) |
Chemical-catalyzed reaction is divided into homogenous- and heterogeneous-catalyzed reaction. Homogenous-catalyzed reactions involve the usage of acid or alkali catalysts in liquid form. The examples of homogenous acid catalysts are hydrochloric, sulphuric, sulfonic and phosphoric acids, while for homogenous alkali catalysts are sodium hydroxide, sodium methoxide, potassium hydroxide, and potassium methoxide.14–17 Biodiesel productions from non-edible feedstocks such as Jatropha curcas, Ceiba pentandra, Sterculia foetida, and Calophyllum inophyllum using homogenous catalysts have been done previously together with the tests on fuel properties and engine performance.16,18,19 Heterogeneous-catalyzed reactions involve the usage of acid or alkali catalysts in solid form. Examples of heterogeneous acid catalysts are sulphated zirconia, tungstated zirconia, heteropoly acids (HPAs), and Nafion-NR50 while for heterogeneous alkali catalysts are calcium based mixed metal oxides (CaO–MgO), alkaline earth metal oxides, hydrotalcites, and basic zeolites.14,15,20 New heterogeneous catalysts such as binary metal oxide CaO–La2O3 that has both acid and base properties and can catalyze esterification and transesterification simultaneously have also been synthesized.21
The advantage of using acid catalyst, either in solid or liquid form is its capability to convert FFA. Alkali catalysts are not suitable for converting oil with high amount of FFA because it can lead to soap formation (saponification). There are many disadvantages associated with chemical-catalyzed method such as high energy consumption, high cost of recovery and purification of catalysts and glycerol, and the need of wastewater treatment.22,23 Wastewater is mainly generated from the washing step to remove soap and glycerin impurities from biodiesel product that can cause engine and fuel storage problems.24
Because of the mentioned problems, researchers have started to explore enzyme-catalyzed reaction. In this reaction, the raw materials needed are oil, acyl acceptor (usually an alcohol), and an enzyme called lipase as catalyst. The advantages of biodiesel production using enzyme is that it has high specificity toward substrate, wide substrate variation, complete catalysis of free fatty acids, high quality of products, mild reaction temperatures, low alcohol to oil ratio, no saponification, and no generation of wastewater.22,25 However, enzyme transesterification is not widely used due to its high cost, slow reaction rates, enzyme inhibition and loss of activity.22,25 Therefore, further improvement to reduce the price, increase the reaction rate, or reduce enzyme deactivation will be revolutionary.
There are several factors that will affect the yield of biodiesel produced using enzymatic reaction. The factors include lipase specificity and efficiency, lipase immobilization, substrate fatty acid composition and types of acyl acceptor used. Furthermore, different enzyme might need different operating conditions for its optimum activity.28 The main parameters to be controlled for the operating condition include temperature, acyl acceptor to oil molar ratio, lipase amount, reaction time, and stirring speed. Other factors that could also affect enzyme activity are water content, pH and solvent.
The temperature for biodiesel production using enzyme ranges from 20 °C to 60 °C (ref. 29) and the optimum temperature in solvent-free system ranges from 30 °C to 50 °C.30 Low temperature may cause the enzyme to be inactive while high temperature may cause denaturation of its molecular structure. Optimum pH and water content is needed to maintain enzyme structure and keeping it active. The amount of water needed depends on the types of lipase, immobilized support, and the organic solvent used in the reaction system.31 Water content needs to be controlled because excessive water will cause hydrolysis reaction (production of fatty acids) being favored more than transesterification (production of FAAE) thus reduces the yield.7,32 Besides, water also involves in several mechanisms that could cause lipase inactivation.33
Biodiesel production through enzymatic reaction usually consumes long period of time. Many reactions need about 12 to 24 hours to achieve complete conversion and some may take up to 72 hours. Although high amount of lipase is capable of shortening the reaction time, it is not advisable because enzyme is very costly. Moderate amount of lipase that able to produce optimum conversion yield is more preferred. Many tests have been done to reduce the reaction period of enzymatic reaction including lipase pretreatment and adding of solvent. Furthermore, the configuration of reactor may also affect the reaction period and productivity. These matters will be discussed further in next sections.
The common problem associated with enzymatic production of biodiesel is inhibitory effect by alcohol and glycerol. Methanol is the most used acyl acceptor due to its cheaper price. However, it is toxic and may cause enzyme deactivation especially at higher concentration. To avoid enzyme deactivation, it is necessary to control the molar ratio of acyl acceptor to oil (acyl acceptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil) used in the reaction. Glycerol is the by-product of transesterification reaction and could cause mass transfer limitation and reaction rate reduction.34 Glycerol is usually removed during biodiesel synthesis or separated from the product at the end of the reaction by mere standing (glycerol in bottom layer).35
oil) used in the reaction. Glycerol is the by-product of transesterification reaction and could cause mass transfer limitation and reaction rate reduction.34 Glycerol is usually removed during biodiesel synthesis or separated from the product at the end of the reaction by mere standing (glycerol in bottom layer).35
There are several things to look at in choosing the method for biodiesel production. Free fatty acid (FFA) content in substrate is important in determining the type of process to be used.23 Oil with FFA content more than 1% can be processed by using two-step transesterification (acid-catalyzed esterification followed by alkali-catalyzed transesterification). Meanwhile enzymatic reaction can be used for oil with FFA content lower or higher than 1%. Non-catalyzed reaction is usually chosen for a fast-output biodiesel production while chemical-catalyzed reaction is the most common method used due to its high yield.
Even though currently enzyme-catalyzed reaction is not the first choice for biodiesel production industry, it has a big potential to become one. One of the important tasks to do is to design a good enzymatic reaction, not only to reduce operational cost but also to get an optimum amount of biodiesel yield. High-yield enzymatic transesterification can be obtained by controlling the reaction conditions, manipulating the factors affecting the reaction, designing a good bioreactor, and also applying additional methods that can reduce enzyme inhibition or loss of activity during transesterification process. Above all else, it will depend on the selection of three major components of the process: lipase, oil and acyl acceptor.
3. Enzymatic reaction for biodiesel production
3.1 Lipase
The type of enzyme that is used for biodiesel production is lipase (triacylglycerol acylhydrolase EC 3.1.1.3) and this enzyme will convert oil to biodiesel in the form of fatty acid alkyl ester and glycerol as its by-product. Lipases can be extracted from several sources such as fungi, bacteria and yeast (Table 2) and they possess different regioselectivity, specificity and catalytic activity.| Fungi | Bacteria | Yeasts |
|---|---|---|
| Alternaria brassicicola | Achromobacter lipolyticum | Candida deformans |
| Aspergillus niger | Aeromonas hydrophila | Candida parapsilosis |
| Candida antarctica | Bacillus subtilis | Candida rugosa |
| Mucor miehei | Burkholderia glumae | Candida quercitrusa |
| Rhizomucor miehei | Chromobacterium viscosum | Pichia burtonii |
| Rhizopus chinensis | Pseudomonas aeruginosa | Pichia sivicola |
| Rhizopus oryzae | Pseudomonas cepacia | Pichia xylosa |
| Streptomyces exfoliates | Staphylococcus aureus | Saccharomyces lipolytica |
| Thermomyces lanuginosus | Staphylococcus carnosus | Geotrichum candidum |
In terms of regioselectivity, lipases can be divided into four groups:30,36,37
i. Sn-1,3-specific: hydrolyze ester bonds at position sn-1 and sn-3.
ii. Sn-2-specific: hydrolyze ester bond at position sn-2.
iii. Fatty acid specific: hydrolyze ester bonds of long-chain fatty acids with double bonds in between C9 and C10.
iv. Non-specific: hydrolyze ester bonds at any positions.
The product of the enzymatic reaction can be monoglyceride, and/or diglyceride or glycerol (complete breakdown). Non-specific lipase is widely used for biodiesel transesterification for a complete hydrolysis of triglyceride. Examples of non-specific lipases are lipases from C. antarctica, C. rugosa, P. cepacia, and P. fluorescens.38 Sn-1,3-specific lipases such as lipases from R. oryzae, M. miehei and T. lanuginosa are also good biocatalysts.38–40 Studies conducted using immobilized T. lanuginosa lipase obtained up to 100% conversion which is more than its theoretical yield (66%) due to acyl migration from position 2.41–43
Each lipase has different specificity towards its substrates, both triglyceride and alcohol. For triglyceride, the preferences include types of fatty acids, length of fatty acids, presence of double bonds and branching.30,36 For example, C. antarctica lipase prefers short- and medium-chain length fatty acids while R. miehei lipase prefers longer fatty acids.37 For alcohol, most lipases prefer primary alcohols compared to secondary and tertiary alcohols, with the tertiary as the least preferred.36 For example, P. cepacia immobilized on diatomaceous earth reacts slower with 2-butanol compared to 1-butanol when converting triolein to oleic acid ester.33 Furthermore, different lipases show highest enzymatic activity with different alcohols or acyl acceptors. C. antarctica lipase immobilized on macroporous resin (Novozym 435) produced highest yield with methanol, T. lanuginosus lipase immobilized on acrylic resin (Lipozyme TL IM) reacted best with ethanol, while R. miehei lipase immobilized on anion-exchange resin (Lipozyme RM IM) preferred butanol.44
The mechanism for enzymatic transesterification follows ping–pong bi–bi mechanism.25,27 Ping–pong bi–bi mechanism can be described as two substrates react to produce two products through formation of enzyme–substrate intermediates.26 There are three kinetic pathways proposed in the literature: (1) direct alcoholysis of glycerides (triglycerides, diglycerides and monodiglycerides) into fatty acid alkyl esters; (2) two consecutive steps which consist of hydrolysis (conversion of glycerides into free fatty acid) and followed by esterification (conversion of free fatty acids into esters); and (3) simultaneous reactions of both alcoholysis and hydrolysis followed by esterification.45–49
Lipase has two different conformations: inactive closed form and active open form.50 These forms are differentiated by the position of a polypeptide chain called lid which will either block or expose the lipase active site. Strategies that can be applied to immobilize lipase with open form include adsorption on hydrophobic support and cross linking or lyophilization in the presence of detergent.50,51 Immobilized lipase is much more preferred than free lipase because it promotes easy recovery and enables reuse of enzyme. It may also increase enzyme stability in the presence of organic solvents.52 Immobilization of enzyme may affect enzyme activity, specificity and selectivity and also alter its structural form. These changes may not always give positive effects to the enzyme properties. Some may cause improvement while some may lead to impoverishment. The improvement may be caused by stabilization of enzyme hyperactivated form, dispersion of enzyme on the support surface, protection against drastic conditions due to rigidification, and/or promotion of diffusional limitation and component partition by porous support.51
Immobilization method and support material may affect the enzymatic activity of lipase. For example, P. cepacia lipase immobilized on diatomaceous earth has faster reaction rate than P. cepacia lipase immobilized on ceramic particles or kaolinite.33 There are many types of supports that are good for lipase immobilization such as decaoctyl sepabeads, chitosan beads, glyoxyl activated agarose gels, green coconut fiber, mesoporous carbon beads, styrene–divinylbenzene beads, and periodic mesoporous organosilica.53,54 There are four common methods for enzyme immobilization: adsorption, cross-linking, entrapment, and encapsulation.55 Other immobilization technologies invented are cross-linked enzyme aggregates (CLEA), protein-coated microcrystals (PCMC), cross-linked PCMC (CL-PCMC), magnetic particles carrier, and electrospun nanofibers.26,56 Kumari et al.56 had compared the efficiency between different types of immobilized P. cepacia lipase. P. cepacia CLEAs and PCMC produced 92% and 99% conversion respectively in 2.5 h, while free P. cepacia lipase and P. cepacia lipase immobilized on polypropylene support gave 98% and 96% conversion respectively in 6 h.
The enzymes mostly used for large scale industrialization are Candida antarctica lipase immobilized on acrylic resin (commercial name: Novozym 435) and Candida sp. 99-125 lipase immobilized on textile membranes.57 Novozym 435 is commonly used due to its non-specificity, biocatalytic efficiency and availability. Several previous studies have shown that Novozym 435 produced the highest amount of yield or conversion when compared with other several lipases.7,35,58 However, even though Novozym 435 is more favorable compared with other lipases, this immobilized lipase is not tolerant with high concentration of methanol or high content of water. Other lipases that have higher methanol tolerant are lipases from P. lipolyticum and P. cepacia. In a system with 10% methanol concentration and using one step methanol addition, P. lipolyticum produced up to 70% yield while Novozym 435 produced almost no yield.59 Kaieda et al.38 reported that P. cepacia has about the same efficiency both with or without 10% water and is more methanol tolerant even at two to three molar equivalent of methanol compared to other lipases such as C. antarctica and R. oryzae.
Other than free lipase and immobilized lipase, there is also whole cell catalyst. The benefit of using whole cell catalyst is that there is no need for lipase extraction and purification steps, thus reduces its cost.26 Tamalampudi et al.60 compared whole cells R. oryzae immobilized onto biomass support particles (BSPs) with Novozym 435 and found that whole cell catalysts converted Jatropha oil to methyl esters more efficiently than Novozym 435: whole cell produced 80% methyl ester after 60 h, Novozym 435 produced 75% methyl ester after 90 h. In addition, the immobilization process is not complicated since the R. oryzae cells immobilized spontaneously onto BSPs during its cultivation in air-lift bioreactor. Other examples of whole-cell catalysts are whole-cell R. chinensis that produced 93% yield from soybean oil,61 whole-cell A. nomius with 95.3% yield from palm oil,62 and whole-cell A. niger with 90.82% yield from microalgal lipid.63
The quest for the best lipase as biocatalyst in biodiesel production has never ended. Lipase with characteristics such as high tolerance in temperature, organic solvent, pH, and mechanical stress could promote enzymatic biodiesel production to a more feasible industry. Example of new type of lipase with desired properties is Burkholderia ubonensis SL-4 lipase that had good stability in non-ionic detergent and organic solvent, and maintained good activity at high temperature (50 °C) and pH (pH 8.5).64 Another example is lipase from Bacillus safensis DVL-43 has great stability in organic solvents as it able to retain 100% activity after 24 h incubation in xylene, DMSO, and toluene (25% v/v).65
3.2 Feedstock (oil)
Oils that are currently used as sources of triglyceride (also known as triacylglyceride) for biodiesel production include edible vegetable oil, non-edible vegetable oil, algae oil, waste frying/cooking oil and animal fats. List of potential sources for edible oil, non-edible oil and algae oil for biodiesel production is tabulated in Table 3. Each oil feedstock will have different fatty acid composition. Both fatty acid composition of feedstock oil and alcohol moieties play important roles in determining biodiesel properties including cetane number, viscosity, lubricity, melting point, heat of combustion, oxidation stability, cold flow and also exhaust emission of the biofuel produced.44,66| Non-edible oils8,14 | Edible oils14,67 | Algae oils68,69 |
|---|---|---|
| Jatropha curcas L. | Glycine max (soybean) | Chlorella protothecoides |
| Pongamia pinnata L. (karanja) | Elaeis guineensis (palm) | Chlorella vulgaris |
| Simmondsia chinensis (jojoba) | Arachis hypogaea (groundnut) | Chlorella pyrenoidosa |
| Sterfulia foetida (poon) | Olea europaea (olive kernel) | Dunaliella tertiolecta |
| Hevea brasiliensis (rubber seed) | Brassica campestris (canola) | Ankistrodesmus TR-87 |
| Sapindus mukorossi (soapnut) | Helianthus annuus (sunflower) | Botryococcus braunii |
| Ricinus communis (castor) | Gossypium spp. (cottonseed) | Tetraselmis suecica |
| Azadirachta indica (neem) | Zea mays (corn) | Nannochloris |
| Calophyllum inophyllum L. | Cocos nucifera (coconut) | Scenedesmus TR-84 |
| Madhuca indica (mahua) | Sesamum indicum (sesame) | Phaeodactylum tricornutum |
According to G. Knothe,66 the fatty acid properties that affect biodiesel properties are unsaturation degree, chain length and branching of the chain. Cetane number, viscosity, heat of combustion and melting point will increase with increasing chain length and decrease with increasing degree of unsaturation.66 For example, feedstock oil such as soybean oil, sunflower oil, and rice bran oil has low oxidation stability due to high amount of linoleic acid that has double bonds.44 Presence of cyclopropene chain carbon (malvalic and sterculic acid) also causes high viscosity and density and low oxidation stability of Sterculia foetida oil (poon oil).19
Reaction using different substrate but same operating conditions, lipase and acyl acceptor will produce different yield%. Modi et al.70 used Novozym 435 and ethyl acetate, with three different oils: Jatropha curcas, Pongamia pinnata, and Helianthus annuus and obtained highest yield of 92.7% with H. annuus oil. Su et al.7 used Candida sp. lipase immobilized on cellulose fabric and dimethyl carbonate with variety of oil: olive oil, rapeseed oil, sunflower oil, soybean oil, corn oil, cottonseed oil, peanut oil, castor oil and sesame oil. All have different conversions with the highest using soybean oil (22.8%) and lowest with castor oil (0.13%). This might be caused by several factors such as oil's water content and fatty acid composition.7,71
Other than vegetable oil, waste oil has also been studied to be the substrate for biodiesel production. Other than its low price, using waste oil for biodiesel production may reduce the amount of waste thrown to the environment. It was estimated that countries such as United States and China generate large amount of waste cooking oil each year (about 10 million tonnes and 4.5 million tonnes respectively).72 In addition, waste oil has different properties than that of refined or crude oils; waste oil usually has higher water content and free fatty acid40,41 that may affect biodiesel yield.
Other type of oil feedstock is oil extracted from microalgae. Examples of microalgae species used for biodiesel production are Chlorella, Botryococcus, Scenedesmus, Dunuliell, Chlamydomonas, and Nannochloropsis.73 High yield up to 98% was obtained using Chlorella protothecoides, Candida sp. 99-125 lipase and methanol.74 Algae is divided into two categories: (i) microalgae which is unicellular microscopic photosynthetic organism that are found in saltwater and freshwater environments; and (ii) macroalgae which is multicellular and form root, stem and leave structures of higher plants.5,68 Both macro- and micro-algae can be used as raw material for biodiesel production. Microalgae have many advantages such as contains high oil content (25–75% of its dry weight), fast growth rate, high photosynthetic efficiency, high biomass production, and can grow on land unsuitable for agriculture.5,75
Despite these advantages, microalgae oil is different than vegetable oil since it has high content of polyunsaturated fatty acids with four or more double bonds and higher content of phospholipid (more than 10%).68 Fatty acids composition could affect the physicochemical properties of biodiesel produced while high phospholipid can cause negative effect on the reaction system in terms of yield, reaction rate and also biodiesel quality.6,66,68 Besides, biodiesel production from microalgae needs large quantity of algal biomass and its oil extraction process is still costly and energy intensive. Other issues relating to microalgae biofuels have been critically reviewed in other papers.76,77
Quality of the oil is also one of the factors that could affect biodiesel yield. Extraction of oil especially using mechanical expeller and pressing machine will produce oil that usually contains impurities such as solid particles and phospholipids. To remove these impurities, degumming steps that can be applied are by adding 20% concentration of phosphoric acid (H3PO4) to the crude oil followed by density separation and simple filtration.17,19 Watanabe et al.78 have conducted a study on Novozym 435, methanol and soybean oil and found that the degumming process able to remove inhibitory substance in oil and produce about the same conversion as refined oil. The conversion obtained after 6 h are 10.3%, 28.1%, and 30.2 for crude, degummed, and refined soybean oil, respectively. After 48 h, conversion for degummed oil is 93.8% while for refined oil is 95.9%. In addition, lipase activity using degummed oil was maintained even after 25 cycles. This shows that degummed oil has the same quality as refined oil and the degumming step able to remove inhibitory impurities in the crude soybean oil.
The choice of substrate to be used is generally depends on the region. Even though non-edible oil is preferred to avoid food versus fuel controversy, countries like USA and Malaysia use edible oil (soybean oil and palm oil respectively) as their feedstock due to its availability in the country. Non-edible oils are usually chosen by countries that do not have any surplus of edible oils. For example, India has chosen Jatropha oil as biodiesel feedstock since the tree can grow on arid land and resistant to drought. Other choice of substrate like microalgae requires a large area or ponds for its cultivation. There was also an attempt to conduct co-production of biodiesel and ethanol from wheat straw.79 Compared to others, waste cooking oil seems to be a better choice as it can promote the use of waste as a source of energy.
3.3 Acyl acceptor
The common acyl acceptor used for biodiesel synthesis is alcohol. Table 4 lists previous tests done using different types of acyl acceptor. The general equation for the synthesis of biodiesel or fatty acid alkyl ester using alcohol is shown in Fig. 2.| Lipase | Lipase weight% based on oil weigh | Substrate | Acyl acceptor, acyl acceptor to oil molar ratio | Reaction conditions | Other details | Yield/conversion (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a App., approximately.b Imm., immobilized. | |||||||
| Candida sp. 99-125 | 30 | Chlorella protothecoides | Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 h, 38 °C, 180 rpm, pH 7.0 | Water content 10 wt% | 98.15 | 74 |
| Novozym 435 | 5 | Soybean oil | Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 37 °C | One-step alcohol addition | 40 | 80 |
| Two-step alcohol addition | 60 | ||||||
| Three-step alcohol addition | 90 | ||||||
Ethanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
One-step alcohol addition | 95 | |||||
| Two-step alcohol addition | App.a 96 | ||||||
| Three-step alcohol addition | App. 98 | ||||||
| Novozym 435 | 4 | Soybean oil | Methanol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 h, 40 °C, 150 rpm | Three-step alcohol addition, crude oil | App. 88 | 81 |
| Three-step alcohol addition, refined oil | App. 98 | ||||||
| 30 | Methyl acetate, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 h, 40 °C, 150 rpm | One-step methyl acetate addition, crude oil | 92 | |||
| One-step methyl acetate addition, refined oil | 92 | ||||||
| Imm.b P. cepacia | 1.46 | Triolein | Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 40 °C, 80 oscillation per min | Water activity aw = 0.432 | 40 | 33 |
Ethanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | ||||||
Propanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | ||||||
1-Butanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | ||||||
2-Butanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 | ||||||
2-Methyl-1-propanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | ||||||
Pentanol isomers, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | ||||||
| Imm. M. miehei | 10 | Tallow | Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 h, 45 °C, 200 rpm | Solvent hexane (8 mL) | 77.8 | 39 |
Ethanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.3 | ||||||
Propanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.3 | ||||||
Butanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.6 | ||||||
Isobutanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.5 | ||||||
| Novozym 435 | 10 | J. curcas | Ethyl acetate, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 h, 50 °C, 150 rpm | One-step ethyl acetate addition | 91.3 | 70 |
| P. pinnata | 90 | ||||||
| H. annuus | 92.7 | ||||||
| Novozym 435 | 10 | Olive oil | Dimethyl carbonate (DMC), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 40 °C, 150 rpm | Solvent n-heptane (4 mL) | 81.2 | 7 |
| Rapeseed oil | 78.5 | ||||||
| Sunflower oil | 77.1 | ||||||
| Soybean oil | 59.4 | ||||||
| Corn oil | 74.8 | ||||||
| Cottonseed oil | 67.7 | ||||||
| Peanut oil | 75.6 | ||||||
| Castor oil | 33.9 | ||||||
| Sesame oil | 39.7 | ||||||
| Novozym 435 | 10 | Cotton seed oil | DMC, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 40 °C, 150 rpm | Solvent n-heptane (4 mL), one-step acyl acceptor addition | 62.1 | 7 |
Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
37.4 | ||||||
Methyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28.2 | ||||||
| Soybean oil | DMC, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65.8 | |||||
Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24.5 | ||||||
Methyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28.3 | ||||||
| Rapeseed oil | DMC, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76.5 | |||||
Methanol, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.3 | ||||||
Methyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30.5 | ||||||
| Cotton seed oil | DMC, 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 50 °C, 150 rpm | Solvent petroleum ether (4 mL) | 96.4 | |||
| Novozym 435 | 20 | Palm oil | DMC, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 55 °C | 90.5 | 28 | |
| Novozym 435 | 50 | Chlorella sp. KR-1 | DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) biomass to DMC 10 (w/v) biomass to DMC |
6 h, 60 °C | Water 0.2 vol% | 75.5 | 82 |
| Novozym 435 | 10 | Waste cooking oil | DMC, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 60 °C, 200 rpm | 77.87 | 83 | |
 | ||
| Fig. 2 Reactions for synthesis of fatty acid alkyl ester (FAAE).11 (a) Transesterification of TAG (triacylglyceride) with alcohol producing FAAE and glycerol as by-product, (b) esterification of fatty acid with alcohol producing FAAE and water as by-product. R1–4 are acyl residues, R′ is alcohol moiety. | ||
The widely used alcohols for this reaction are methanol and ethanol because of their cheap price. The biodiesel product is fatty acid methyl ester (FAME) and fatty acid ethyl ester (FAEE) if methanol and ethanol is used, respectively. Methanol's high polarity and short chain length make it the most efficient alcohol for transesterification reaction.71 However, one of the problems of using methanol is that it can cause lipase deactivation, denaturation or inhibition. These may be caused by the blocking of triglycerides entry, conformational change or unfolding of enzyme, immiscibility between triglycerides and alcohol, and/or adsorption of alcohol onto polar immobilized material (acrylic resin, polyurethane foam).29,71,84,85 Despite the fact that three molars of alcohol are needed for complete transesterification, lipase will deactivate in the presence of more than one molar equivalent of methanol.35 To solve this problem, several previous studies have suggested stepwise addition or continuous addition of methanol into the system.22,35,71
Ethanol is synthesized from renewable source thus it is much greener compared to methanol that is synthesized from fossil fuels (Table 5). It also causes less inhibitory effect on lipase compared to methanol. Ethanol does not require stepwise addition of alcohol to achieve high conversion of substrate, but the stepwise addition may increase its reaction rate.80 In addition, even though methyl esters give higher maximum engine performance compared to ethyl esters, ethyl ester has its own advantages: higher cetane number and energy content; lower density, pour point, and cloud point; and produce lower NOx and smoke emission.11,80,86 It is also more favorable than methyl ester because it has higher flash and combustion points and lower exhaust temperatures.70
| Type of acyl acceptor | Advantages | Disadvantages |
|---|---|---|
| Methanol | Cheap, fast reaction, high maximum engine performance | Cause enzyme deactivation, require stepwise addition, synthesized from fossil fuel |
| Ethanol | Synthesized from biomass (green), improve fuel properties, low harmful emission | More expensive than methanol, FAEE has higher kinematic viscosity than FAME |
| Other alcohols | Better miscibility with oil | Slow reaction, expensive |
| Ester (methyl or ethyl acetate) | High yield even with unrefined oil, high reusability of enzyme, higher value by-product (triacetin) | High amount of ester and lipase needed for optimum reaction |
| Dimethyl carbonate (DMC) | Non-toxic, can be used as both extraction solvent and transesterification substrate | Expensive, high amount of DMC and lipase needed for optimum reaction |
Other than choosing only one alcohol, both methanol and ethanol can be mixed and used in a single reaction. Zhao et al.87 tested several proportions of methanol/ethanol for conversion of soybean oil using Novozym 435. They found that 0% (100 mol% ethanol), 20%, 40%, and 60% methanol in blended alcohols produced high yield up to 95% (one step addition, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio of oil to alcohol). In addition, methanol is consumed faster in the reaction than ethanol, thus leaving ethanol in the system which improve the solubility between oil and alcohol and reduce lipase deactivation by methanol.87 The esters produced (combination of ethyl and methyl esters) from mixed alcohols using chemical catalyst has shown to have improved characteristics in term of low temperature properties, oxidative stability and lubricity compared to methyl ester alone, but decreased kinematic viscosity as ethanol proportion increased.88
3 molar ratio of oil to alcohol). In addition, methanol is consumed faster in the reaction than ethanol, thus leaving ethanol in the system which improve the solubility between oil and alcohol and reduce lipase deactivation by methanol.87 The esters produced (combination of ethyl and methyl esters) from mixed alcohols using chemical catalyst has shown to have improved characteristics in term of low temperature properties, oxidative stability and lubricity compared to methyl ester alone, but decreased kinematic viscosity as ethanol proportion increased.88
Other types of alcohol that can be used include secondary alcohols, long chain alcohols and branched alcohol. It was observed that secondary alcohols react slower than primary alcohols which might due to steric hindrance and also the specificity of lipase used.33 However, fatty acid esters of secondary or branched-chain alcohols have their own advantages. Instead of adding additives like butyl oleate, adding of these esters can improve low temperature properties such as cloud point and pour point of the fuel.33 Furthermore, higher molecular-weight alcohols such as propanol and butanol have better miscibility with oil compared to methanol and ethanol.44 Maceiras et al.29 conducted transesterification reaction with waste frying oil and Novozym 435, and found that the enzymes has higher relative activity when using 2-propanol compared to methanol. Even though isopropyl alcohol is more expensive than methanol, it was found that isopropyl ester has better biodiesel properties than methyl ester.66
Another acyl acceptor for biodiesel production is ester such as methyl acetate and ethyl acetate. Methyl and ethyl acetate do not cause negative effect on lipase activity compared to methanol or ethanol (thus no need for stepwise addition) and will produce higher value by-product called triacetin or triacetylglycerol which has no negative effect on the reaction (Fig. 3).70,81 Triacetin is a useful product that can be used in many fields such as in medicine, food, cosmetic, pesticide, cigarette and many more.70
 | ||
| Fig. 3 Interesterification reaction of TAG with methyl acetate producing FAME and triacetin as by-product.81 | ||
Other benefit of using esters as acyl acceptor is its high yield even when using crude oil. Du et al.81 showed that high yield was obtained when using methyl acetate in both crude and refined oil. Meanwhile, with methanol, reaction with crude oil had slower reaction rate and lower yield compared with refined oil. In addition, the lipase activity dropped to less than 70% on the fourth cycle for methanol, but maintained its activity (>90%) even after 100 cycles for methyl acetate. Another study was conducted by Modi et al.70 to compare the reusability of enzyme between ethanolysis (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 4
oil = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4-step addition, 8 h period) and interesterification (ethyl acetate
1, 4-step addition, 8 h period) and interesterification (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil = 11
oil = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1-step addition, 12 h period). Ethanolysis had zero enzyme activity at 6th cycle, but interesterification maintained enzyme activity above 90% even at 12th cycle. In spite of these advantages, there are also several drawbacks involved. The reaction may require high acyl acceptor to oil molar ratio and high amount of lipase for an optimum reaction.7,58,70,81
1, 1-step addition, 12 h period). Ethanolysis had zero enzyme activity at 6th cycle, but interesterification maintained enzyme activity above 90% even at 12th cycle. In spite of these advantages, there are also several drawbacks involved. The reaction may require high acyl acceptor to oil molar ratio and high amount of lipase for an optimum reaction.7,58,70,81
Other than alcohol, it was also discovered that dimethyl carbonate (DMC) can be a suitable acyl acceptor for biodiesel production. DMC is odorless, non-toxic, and heat-stable solvent which can be used as extraction solvent as well as substrate for transesterification reaction.82 Su et al.7 proposed that reaction with DMC is irreversible due to immediate decomposition of intermediate compound (carbonic acid monoacyl ester) to carbon dioxide and alcohol as by-products. Meanwhile Zhang et al.28 proposed that the products of this reaction are FAME and glycerol dicarbonate after conducting GC and GC-MS analysis. The overall reaction for interesterification of triglycerides with DMC was explained by Calero et al.89 and shown in Fig. 4.
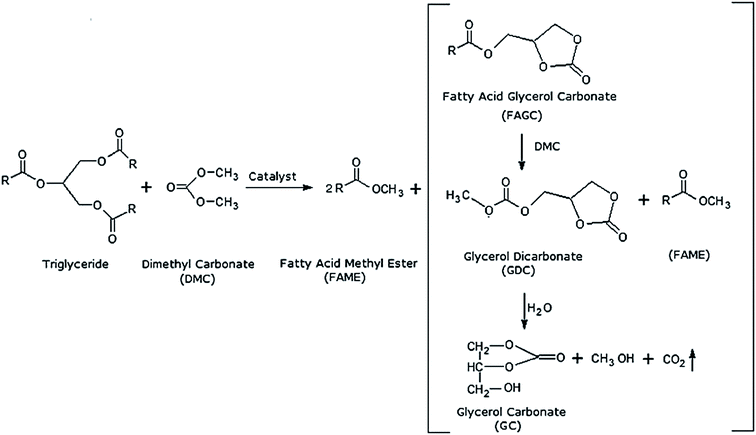 | ||
| Fig. 4 Interesterification of triglyceride with dimethyl carbonate (DMC) producing FAME and Fatty Acid Glycerol Carbonate (FAGC).89 FAGC will be further broken down into glycerol dicarbonate (GDC) and glycerol carbonate (GC). | ||
Biodiesel production using DMC as acyl acceptor has some advantages over alcohols or esters. Experiment conducted by Su et al.7 using DMC as substrate and petroleum ether as solvent shows that the initial activity for one-step, two-step, three-step, four-step and five-step addition are slightly different but all achieved about 90% conversion after 24 h. Thus, there is no need for complex step of multiple acyl acceptor addition. The highest conversion was achieved with molar ratio for cottonseed oil to DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5, which is not as high compared to when using esters.
4.5, which is not as high compared to when using esters.
4. Techniques to improve enzymatic transesterification
4.1 Combination of lipases
Several lipases can be mixed and used as catalysts in a single reaction to increase its biodiesel yield (Table 6). As was previously stated, lipases have different specificities, regioselectivities and catalytic capabilities. Using mixed lipases in a single reaction will allow each lipase to attack different targets and thus achieves a complete conversion. In addition, lipases with good catalytic efficiency but expensive, can be mixed with lower efficiency lipase but cheaper, to reduce the enzyme cost while still maintaining the amount of yield produced when single lipase is used.40| Lipase | Lipase amount | Substrate | Acyl acceptor (alcohol to oil molar ratio) | Solvent/water | Reaction conditions/details | Yield/conversion (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a All wt% is based on oil weight.b App., approximately.c Imm., immobilized. | |||||||
| Lipozyme TL IM | 20 wt%a | Rapeseed oil | Methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
t-Butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent to oil vol ratio) 1 solvent to oil vol ratio) |
12 h, 35 °C, 130 rpm | 85 | 40 |
| Novozym 435 | 2 wt% | 90 | |||||
| Lipozyme TL IM, and Novozym 435 | 3 wt% | 95 | |||||
| 1 wt% | |||||||
| Novozym 435 | 13.7 wt% | Olive oil | Ethanol (7.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Water 4 wt% | 18 h, 35.9 °C, 180 rpm | App.b 43 | 37 |
| Lipozyme TL IM | 13.7 wt% | App. 46 | |||||
| Lipozyme RM IM | 13.7 wt% | App. 45 | |||||
| Novozym 435, Lipozyme TL IM, and Lipozyme RM IM | 58.5% | 95 | |||||
| 29.0% | |||||||
| 12.5% (of 13.7 wt%) | |||||||
| Lipozyme TL IM | 15 wt% | Palm oil | Ethanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Water 4 wt% | 18 h, 37.7 °C, 180 rpm | App. 42 | 37 |
| Lipozyme RM IM | 15 wt% | App. 40 | |||||
| Lipozyme TL IM, and Lipozyme RM IM | 52.5% | 80 | |||||
| 47.5% (of 15 wt%) | |||||||
| Imm.c T. lanuginosus | 25 wt% | Soybean oil | Ethanol (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Water 4 wt% | 10 h, 30 °C, 200 rpm | App. 80 | 96 |
| Lipozyme RM IM | 25 wt% | App. 40 | |||||
| Imm. T. lanuginosus, and Lipozyme RM IM | 80% | 90 | |||||
| 20% (of 25 wt%) | |||||||
| Imm. P. fluorescens | 10 wt% | Palm oil | Ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Water 10% | 12 h, 45 °C, 500 rpm, two-step hydrolysis esterification reaction | App. 85 | 41 |
| Imm. P. fluorescens and Imm. C. rugosa | 5 wt% | App. 85 | |||||
| 5 wt% | |||||||
| Imm. C. rugosa and Novozym 435 | 5 wt% | App. 45 | |||||
| 5 wt% | |||||||
Some lipases are a good match not only due to their different specificities, but also because they have different rate-limiting step. For example, C. antarctica lipase and T. lanuginosus lipase is a good combination because the rate-limiting step for C. antarctica is the conversion of diglyceride to monoglyceride, while for T. lanuginosus is the conversion of triglyceride to diglyceride.26 Because of this, they complement each other and able to overcome the rate-limiting steps.
The most effective combination of lipases is most likely through a relatively modern technology: genetic engineering. This includes the expression of different lipases in a single host organism. In recent years, there have been many studies conducted on recombinant lipases.90–92 Yan et al.93 used whole-cell Pichia pastoris displaying both C. antarctica and T. lanuginosus lipases on its surface for converting soybean oil to biodiesel. They managed to get 95.4% conversion after 12.6 h, which is relatively short period of time. Furthermore, they found that the conversion percentage is about the same with the reaction combining same quantity of two immobilized lipases, Novozym 435 and Lipozyme TL IM (97.3%). This is believed to be able to lower the cost of buying different lipases separately. Another study was done by Guan et al.94 using R. miehei lipase (1,3-specific) and P. cyclopium lipase (non-specific) both expressed in and extracted from Pichia pastoris. They converted soybean oil to biodiesel and obtained 95.1% conversion after 12 h. Recombinant Pichia pastoris whole cell with intracellular overexpression of T. lanuginosus lipase was used as biocatalyst in biodiesel production from waste cooking oil and had produced 82% yield within 84 h.95
Other example of mixing two lipases with different specificities was done by Li et al.40 They added both Lipozyme TL IM and Novozym 435 into the reaction and obtained 95% yield which was higher than yield obtained with either of the lipase alone. Rodrigues and Ayub96 used both lipase of T. lanuginosus immobilized in Lewatit VP OC 1600 and Lipozyme RM IM (R. meihei) to convert soybean oil into biodiesel. The yield obtained (90%) was about 10% higher than using T. lanuginosus alone and 50% higher than using R. miehei alone. Meanwhile Poppe et al.37 tested combination of three lipases on olive oil and palm oil that have different composition of fatty acids: olive oil has high content of C18:1 while palm oil has high content of C16. The result showed that mixed lipases produced higher conversion compared to single lipase and best conversion was obtained using three mixed lipases (Novozym 435, Lipozyme TL IM and Lipozyme RM IM) for olive oil and only two mixed lipases (Lipozyme TL IM and Lipozyme RM IM) for palm oil. The conversions are 95% and 80% for olive oil and palm oil, respectively.
Combination can be also done with lipases of different catalytic capabilities such as combining lipase having high hydrolysis activity with lipase having high esterification activity, or transesterification activity. Tongboriboon et al.41 combined immobilized C. rugosa lipase (AY) which has high hydrolysis with immobilized P. fluorescens lipase (AK) that has high transesterification and lipase AY with Novozym 435 that has high esterification activity. Even though combination of 5 wt% AK + 5 wt% AY gave same yield (about 85%) as 10 wt% AK, it is still better in terms of cost because lipase AY is cheaper than AK. Unexpectedly, pairing of Novozym 435 + AY has lower yield than AK + AY, even though combining high hydrolysis lipase with high esterification lipase should theoretically produce high yield as they complement each other. Tongboriboon et al.41 assumed this situation happened due to esterification of free fatty acid to triacylglycerol instead of ethyl ester due to the presence of hydrolysis by-product, glycerol.
4.2 Enzyme pretreatment
Pretreatment of lipase before the start of enzymatic reaction can restore enzyme deactivation and increase biodiesel yield and enzymatic activity.28,29,84,97 Pretreatment usually involves immersion, incubation, or washing of lipase with substrates, organic solvents, salts, or enzyme lycoprotectants.22,28 Table 7 shows biodiesel production with different types of pretreatment applied.| Lipase | Oil | Pretreatment solution | Pretreatment method | Transesterification | Yield/conversion/relative activity (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Single step | Stepwise | ||||||
| a All wt% is based on oil weight.b App., approximately.c Imm., immobilized. | |||||||
| Novozym 435 | Soybean oil | None | None | Lipase 5.26 wt%a, methanol 0.26 mL. Incubate for 30 min at 30 °C, 200 rpm | 2.5 | — | 84 |
| Hexane | Immerse in hexane 1 h, then in oil overnight | 3.5 | — | ||||
| Methyl ester (ME) | Immerse in ME for 0.5 h, then in oil 4 h | 4.1 | — | ||||
| Immerse in ME 1 h, then in oil 4 h | 9.5 | — | |||||
| Immerse in ester ME 1.5 h, then in oil 4 h | 8.9 | — | |||||
| Immerse in ME 2 h, then in oil 4 h | 9.1 | — | |||||
| Immerse in ME 1 h, then in oil overnight | 11.1 | — | |||||
| Soybean oil | Immerse 4 h | 8.6 | — | ||||
| Immerse overnight | 10.0 | — | |||||
| Isopropanol | Immerse in alcohol 1 h, then in soybean oil 1 h | 16.8 | — | ||||
| 2-Butanol | Immerse in alcohol 1 h, then in soybean oil 1 h | 17.6 | — | ||||
| t-Butanol | Immerse in alcohol 1 h, then in soybean oil 1 h | 24.5 | — | ||||
| Novozym 435 | Soybean oil | None | None | Lipase 4 wt%, methanol to oil molar ratio App.b 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction at 30 °C, 150 oscillations per min 1. Reaction at 30 °C, 150 oscillations per min |
— | 97 (after 24 h) | 97 |
| Methyl oleate | Incubate in methyl oleate for 0.5 h, wash with soybean oil, then incubate in soybean oil for 12 h. | — | 97 (after 3.5 h) | ||||
| C. antarctica (free lipase) | Waste frying oil | Methanol | Immersion for 72 h, then remove enzyme by vacuum filtration | Lipase 10 wt%, methanol to oil ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. Incubate for 8 h at 50 °C, 150 rpm 40. Incubate for 8 h at 50 °C, 150 rpm |
Decreased relative activity | — | 29 |
| 2-Propanol | Decreased relative activity | — | |||||
| Novozym 435 | Waste frying oil | Methanol | Immersion for 72 h, then remove enzyme by vacuum filtration | Lipase 10 wt%, methanol to oil ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. Incubate for 8 h at 50 °C, 150 rpm 40. Incubate for 8 h at 50 °C, 150 rpm |
Decreased relative activity | — | 29 |
| 2-Propanol | Decreased relative activity | — | |||||
| Imm.c Candida sp. 99-125 | Soybean oil | Control | Immersion in 30 mL solution at 4 °C for 24 h, then dried at room temperature | Lipase 10 wt%, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm |
1.20 | 74.5 | 28 |
| Water | 29.0 | 74.5 | |||||
| Methanol 10% | 30.2 | 79.9 | |||||
| Methanol 20% | 39.6 | 76.9 | |||||
| Methanol 40% | 5.88 | 71.5 | |||||
| Control | Immersion in 30 mL solution at 4 °C for 24 h, then dried at room temperature | Lipase 10 wt%, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm |
2.44 | 74.4 | |||
| n-Propyl alcohol | 0.98 | 77.1 | |||||
| n-Butanol | 4.34 | 80.7 | |||||
| Isopropyl alcohol | 1.18 | 78.4 | |||||
| t-Butanol | 2.02 | 79.0 | |||||
| Isobutyl alcohol | 2.33 | 77.7 | |||||
| Control | Immersion in 30 mL solution at 4 °C for 24 h, then dried at room temperature | Lipase 10 wt%, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm 1, 2 mL n-hexane, and 200 μL water. Reaction for 12 h at 40 °C, 180 rpm |
1.54 | 75.2 | |||
| (NH4)2SO4 | 57.2 | 82.1 | |||||
| CaCl2 | 71.2 | 84.3 | |||||
| KCl | 56.1 | 84.4 | |||||
| K2SO4 | 47.2 | 83.6 | |||||
| MgCl2 | 74.5 | 81.9 | |||||
| Imm. R. oryzae whole-cell | Soybean oil | None | None | Oil 9.65 g, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1 M phosphate buffer (pH 6.8). Incubate at 35 °C, 150 oscillation per min for 7 days to test stability 1, 0.1 M phosphate buffer (pH 6.8). Incubate at 35 °C, 150 oscillation per min for 7 days to test stability |
— | 16 residual activity | 98 |
| 0.05–1.0 vol% glutaraldehyde solution | Incubate at 25 °C for 1 h, filter, shake in phosphate buffer at 4 C for 5 min, washed with tap water for 1 min, then dried for 24 h at room temperature | — | 74–78 residual activities | ||||
| None | None | Oil 9.65 g, methanol to oil molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1 M phosphate buffer (pH 6.8). Reaction at 35 °C, 150 oscillation per min, for 6 cycles (72 h each cycle) 1, 0.1 M phosphate buffer (pH 6.8). Reaction at 35 °C, 150 oscillation per min, for 6 cycles (72 h each cycle) |
— | 50 | |||
| 0.1 vol% glutaraldehyde solution | Incubate at 25 C for 1 h, filter, shake in phosphate buffer at 4 C for 5 min, washed with tap water for 1 min, then dried for 24 h at room temperature | — | 70–83 | ||||
| Novozym 435 | J. curcas | None | None | Lipase 10 wt%, ethyl acetate to oil molar ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction for 12 h at 50 °C, 150 rpm 1, reaction for 12 h at 50 °C, 150 rpm |
91.3 | — | 70 |
| Ethyl acetate | Immerse in ethyl acetate for 72 h | 91.1 | — | ||||
| P. pinnata | None | None | 90 | — | |||
| Ethyl acetate | Immerse in ethyl acetate for 72 h | 89.6 | — | ||||
| H. annuus | None | None | 92.7 | — | |||
| Ethyl acetate | Immerse in ethyl acetate for 72 h | 92.4 | — | ||||
Alcohols are frequently tested for lipase pretreatment. However, it is important to know that different lipases may react differently to different alcohol and not all alcohols are suitable as enzyme pretreatment. For example, t-butanol pretreatment increases the initial reaction rate of immobilized C. Antarctica lipase,84 but it does not give any improvements on immobilized Candida sp.99-125 lipase.28 This result may be due to the distinct characteristic of the lipases, influence of the immobilized support or the presence of solvent in the system.
Example of pretreatment using alcohol was done by Chen and Wu84 using Novozyme 435 and soybean oil feedstock. They pretreated Novozym 435 with alcohol of 3 or 4 carbons: isopropanol, 2-butanol and tert-butanol by immersing it in the alcohol for 1 h, and then immersed in soybean oil for another 1 h. They obtained highest yield of 24.5% (30 min reaction time) using tert-butanol pretreatment with an increase of almost tenfold. Another study conducted by Maceiras et al.29 on Novozyme 435 and C. Antarctica lipase B (free lipase) using methanol and propanol pretreatments, but both resulted with decrease in relative activity.
Meanwhile Lu et al.28 tested pretreatment of Candida sp. 99-125 immobilized on textile membrane with different concentration of methanol (10%, 20% and 40%) using both one-step and three-step addition. They found that 10% and 20% methanol gave positive result especially with one-step methanol addition with a yield increase of about 30 fold. In addition, they also tested pretreatment using other short chain alcohols such as n-propyl alcohol, n-butanol, isopropyl alcohol, t-butanol, and isobutyl alcohol. These pretreatments did not show much improvement and caused decrease in yield for one-step addition.
Previous studies have conducted several tests on enzyme pretreatment using solutions such as its substrate (vegetable oil and ethyl acetate), product (methyl ester), and others such as hexane, glutaraldehyde, methyl oleate, salt solution, and water. Pretreatment with hexane, methyl ester and soybean oil did increase the yield of 30 min reactions using Novozym 435.84 Pretreatment with methyl oleate reduce the reaction period for Novozym 435 from 24 h to 3.5 h to obtain 97% methyl ester content97 while immersion in ethyl acetate gave no improvement on enzyme activity.70 In addition, immersion of immobilized Candida sp. 99-125 in water increases the yield for one-step methanol addition.28 This might because water pretreatment has affected the water distribution in the immobilized lipase and thus improved lipase flexibility.28
Studies by Ban et al.98 showed that glutaraldehyde-pretreatment of whole-cell R. oryzae immobilized on biomass support particles (BSPs) increased the stability of the lipase, protected it from the negative impact of high concentration of methyl ester, and also prevented lipase leakage from the cells. Residual activities are more than 70% with incubation in 0.05–1.0 vol% glutaraldehyde solution for 7 days compared to 16% residual activity of untreated lipase. Furthermore, residual activities after 6 cycles were around 70–83% compared to 50% of untreated lipase. These results show that glutaraldehyde-pretreatment can improve lipase stability.
Other pretreatment solution that was proved to improve the yield of enzyme is salt solution. Lu et al.28 tested pretreatment of Candida sp. 99-125 with salt solution of low saturation salt solution: 1 mM of potassium chloride (KCl), calcium chloride (CaCl2), magnesium chloride (MgCl2), potassium sulfate (K2SO4) and ammonium sulfate ((NH4)2SO4). These pretreatments gave slight increase for three-step methanol addition but significant impact to one-step addition. The best result was obtained using MgCl2 with an increase from 1.54% yield (control) to 74.5%, almost comparable with the yield when using three-step addition.
4.3 Enzyme post treatment
Adsorption of glycerol and formation of layer containing heterogeneous mixture of oil and biodiesel on the enzyme surface during reaction could block lipase activity.44,99 To solve this, post-treatment such as enzyme washing after each reaction cycle is needed to maintain the yield amount and enzyme activity.The most common solvent used for enzyme washing is hexane. Since the heterogeneous layer formed is non-polar, non-polar hexane is believed to be effective in washing away the layer.44 A study by Rodrigues et al.44 washed three types of immobilized lipases (Novozym 435, Lipozyme TL IM and Lipozyme RM IM) with n-hexane, ethanol, propanol, water after each reaction cycle. Enzyme washed with n-hexane shows greater relative conversion yield (Novozym 435 = 90%, Lipozyme TL-IM = 80%, Lipozyme RM IM = 75%) after 7 cycles compared with other solvents. Meanwhile the control is almost deactivated for Novozym 435 and less than 20% relative conversion yield for the other two enzymes. Poppe et al.37 washed mixed lipase (Novozym 435, Lipozyme TL IM and Lipozyme RM IM) with hexane and maintained around 80% conversion after 7 cycles. The same process was done by Rodrigues and Ayub96 to mixed T. lanuginosus and R. meihei immobilized lipases after each cycle. The enzyme activity was above 90% after 10 cycles compared to around 30% without hexane wash.
Other than hexane, alcohols also can be used for enzyme washing. Yu et al.100 washed immobilized P. cepacia lipase with tert-butanol after each cycle and the lipase retained about 80% of its initial conversion after three repeated uses (unwashed retained only about 70%). Chen and Wu84 reactivate completely deactivated Novozym 435 with 2-butanol and tert-butanol to 56% and 75% of its original activity respectively. They washed the enzyme with the alcohol three times, washed with soybean oil once, and then immersed it in soybean oil in incubator at 30 °C overnight. This washing process was tested not only on batch system, but also on continuous system. Chen and Wu84 has succeeded in maintaining the conversion above 70% in a continuous stirred tank reactor for more than 70 days by washing the lipases using tert-butanol each time the methyl ester conversion decreased to below 70%.
Other solvents such as acetone and DMC have also been used previously. Su et al.7 conducted a study to convert cottonseed oil to biodiesel using Novozym 435, DMC as acyl acceptor, and petroleum ether as organic solvent, for 24 h. After each batch, used Novozym 435 was recovered by filtration, washed with 25 mL acetone 3 times, and dried at room temperature. After five batches, treated lipase showed 80% relative conversion meanwhile untreated lipase showed only 3.2% relative conversion. Lee et al.82 performed reaction using Novozym 435, microalgae Chlorella sp. KR-1 and DMC and managed to maintain relative yield above 90% for 10 batches by performing enzyme washing with DMC after each batch.
There are several things that should be taken into account for choice of solvent to be used. This include its effectiveness in removing the heterogeneous layer and glycerol on the enzyme surface, its effect on the structure of lipase, as well as its effect on the immobilization support.99 Solvent like hexane could dissolve macroporous resin support, and polar solvent such as ethanol and butanol could change the morphology of a gel of granulated silica support.99 In addition, some solvent may be toxic and flammable, which can cause harm to the biodiesel plant workers who are exposed to it.
4.4 Methanol addition technique
Methanol may be the cheapest and widely used acyl acceptor for biodiesel production. However, the drawback of using this shortest chain alcohol is lipase deactivation. This effect can be reduced by controlling the amount of methanol added to the reaction system at a specified time. The most common method used for methanol addition is three-stepwise methanol addition which able to obtain high conversion of more than 90% (Table 8). Shimada et al.35 gained a high conversion of 97.4% using Novozym 435 and mixture of soybean and rapeseed oil, while Watanabe et al.78 achieved 95.9% conversion with Novozym 435 and soybean oil.| Lipase | Lipase weight% based on oil weigh | Substrate | Methanol to oil molar ratio | Reaction conditions | Methanol addition technique | Yield/conversion (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a Imm., immobilized.b App., approximately. | |||||||
| Novozym 435 | 4 | Soybean and rapeseed oils | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 h, 30 °C, 130 oscillation per min | Three-step (1 molar equivalent added at 0 h, 10 h and 24 h) | 97.4 | 35 |
| Novozym 435 | 4 | Soybean oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 h, 30 °C, 130 oscillations per min | Three-step (1/3 molar equivalent added at 0 h, 10 h and 24 h) | 95.9 | 78 |
| Imm.a R. oryzae whole-cell | 4 | Jatropha oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 h, 30 °C, water 5% (v/v) | Three-step (1/3 molar equivalent added at 0 h, 4 h and 17 h) | 80 | 60 |
| Novozym 435 | 5 | Soybean oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 37 °C | Three-step (1/3 at molar equivalent added at 0 h, 7 h and 14 h) | 90 | 80 |
| Two-step (1/3 molar equivalent added at 0 h, 2/3 molar equivalent added at 7 h) | 60 | ||||||
| One-step | 40 | ||||||
| Candida sp. 99-125 | 10 | Soybean oil | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 h, 40 °C, 180 rpm, solvent n-hexane (2 mL), water 200 μl | Three-step (1/3 molar equivalent added at 0 h, 4 h and 8 h) | 74.4 | 28 |
| One-step | 2.44 | ||||||
| Novozym 435 | 4 | Soybean oil | 2.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 30 °C, 150 oscillations per min | Multiple-step (1 molar equivalent at 0 h, then 0.33 molar equivalent at 1 h, 3 h, 5 h, 7 h, and 9 h to maintain methanol content at around 30 g l−1) | 97 | 97 |
| Imm. B. cepacia | 8 | Jatropha oil | 6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 h, 30 °C, 150 rpm, water content 7% (v/w) | 3 h intervals | 89 | 101 |
| 5 h intervals | App.b 88 | ||||||
| 8 h intervals | App. 82 | ||||||
| One-step | App. 62 | ||||||
| Novozym 435 | 10 | Jatropha oil | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h, 40 °C, 700 rpm | Gradient (continuous addition for first 3 h) | 63.91 | 71 |
| Stepwise addition (three equal portions at the beginning and then two at 30 min intervals) | 37.73 | ||||||
| 24 h, 45 °C, 700 rpm | Gradient (continuous addition for first 3 h) | 46.88 | |||||
| Stepwise addition (three equal portions at the beginning and then two at 30 min intervals) | 41.96 | ||||||
Further tests were done to compare three steps with one or two steps methanol addition. Cerveró et al.80 obtained 40%, 60%, and 90% conversions for one-step, two-step, and three-step addition respectively. Meanwhile Lu et al.28 obtained 74.4% yield for three-step and 2.44% yield for one-step addition. These results show that three-step methanol addition is better than one-step or two-step methanol addition.
Furthermore, methanol addition of more than 3 steps is also effective to give high yield. Samukawa et al.97 used six and nine stepwise addition of methanol to maintain maximum initial reaction rate based on Michaelis–Menten equation for pretreated and non-treated lipase respectively and obtained over 97% methyl ester content for both methods. Methanol addition using 3 h, 5 h or 8 h intervals would also give high yield up to 89%.101
Other than stepwise addition, continuous addition of alcohol can also be applied. Ko et al.71 conducted a study to compare the FAME conversion obtained between stepwise (3 equal portions at the beginning and following two 30 min intervals) and gradient (continuous addition for first 3 h) methanol addition strategies at several different temperatures. It was found that the highest conversion was obtained using gradient method (63.91% at 40 °C) compared to when using stepwise method (41.96% at 45 °C). This study suggested that continuous addition achieved higher conversion than stepwise due to decreased methanol intoxication, higher empirical first-order rate constants and favorable energetics for the first 6 hours of reaction.71
Many recent studies have applied stepwise or continuous addition of methanol in their biodiesel production to avoid lipase deactivation.63,102,103 This method can also be applied to the design of biodiesel reactor, both batch and continuous, for a large scale biodiesel production.
4.5 Effect of silica gel
Adding silica gel into the reaction system has four purposes: (i) to remove water; (ii) to add methanol; (iii) to absorb glycerol by-product; and (iv) to promote acyl migration. Silica gel can be used to remove water produced from the esterification of FFA and alcohol (acid-catalyzed), or absorb remaining water left after biodiesel washing. Babaki et al.104,105 demonstrated that adding blue silica gel can increase the FAME yield, but an excess amount may result in decreased yield.Silica gel can also be used for methanol addition, as an alternative to methanol stepwise-addition technique that may complicate the process. Silica gel swelled with methanol will release the methanol in a controlled manner, thus this prevents enzyme deactivation. Lee et al.34 used silica gel swelled with methanol and obtained high conversion of 99.9% and 96.8% by Novozym 435 and Lipozyme RM IM, respectively at three or more molar equivalent of methanol.
Glycerol absorbing properties of silica gel (0.13 g glycerol per g silica gel) can also affect the conversion yield.34 Glycerol is a by-product of transesterification reaction that may interfere with enzyme activity by affecting the mass transfer limitation.34 Thus, if the glycerol is continuously removed during the reaction, it would likely to increase the enzymatic activity. Stevenson et al.106 added silica gel for conversion of tallow oil with three molar equivalents of butanol using immobilized M. meihei and obtained 98% yield compared to 70% yield when no silica gel was added. They also tested the reaction with other adsorbents such as starch, cellulose, celite, and charcoal and found that silica gel was more effective in adsorbing glycerol.
Other than that, it was discovered that silica gel can increase rate of acyl migration. Du et al.42 discovered that silica gel, either used as immobilized support or added to the reaction system, will promote acyl migration from position 2. They conducted an analysis using thin layer chromatography to analyze the reaction intermediates and confirmed that there was acyl migration activity. By using sn-1,3-specific T. lanuginosus immobilized on silica gel (Lipozyme TL), the yield obtained was about 92% which is significantly much higher than 63% when using free lipase with the same lipase activity.42 Theoretically, the maximum yield that can be achieved is only 66% for lipase with this specificity. Furthermore, adding of silica gel into the system did increase the yield. This was clearly demonstrated when 66% and 90% yields were obtained when using 4% Lipozyme TL and 4% Lipozyme TL + 6% silica gel, respectively.42
4.6 Effect of solvent
Biodiesel production using enzyme as catalyst can be done with or without solvent. Solvent is used as a way to decrease the effect of lipase inhibition or intoxication by methanol or glycerol. Other than increased production yield, there are many advantages of using solvent in reaction system. Solvent can help reduce viscosity and ensure homogeneity of reaction mixture due to immiscibility of alcohol and triglyceride.25,80 It also keeps the water around the enzyme which consequently helps increase water activity and enzyme stability.25There have been many studies conducted to gain more insights about the effect of solvent in enzymatic transesterification (Table 9). Among the fastest reactions and highest yields was obtained when using diesel as solvent. Kojima et al.107 achieved complete conversion within 3 hours by using diesel as solvent, and within 7 hours when using n-hexane or kerosene. This study was conducted using C. cylindracea (free lipase) on waste activated bleaching earth that would absorb about 40 wt% vegetable oil during refining process of crude vegetable oil. Furthermore, using diesel does not require solvent separation and removal at the end of the reaction as the diesel can be used directly in diesel engine.
| Lipase | Substrate | Solvent | Transesterification | Yield/conversion (%) | Ref. |
|---|---|---|---|---|---|
| a All wt% is based on oil weight.b App., approximately.c Imm., immobilized.d SC., supercritical. | |||||
| C. cylindracea | Waste activated bleaching earth (ABE) | Diesel | Lipase 10 wt%a, waste ABE 390 g, methanol to oil molar ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction at 37 °C, 250 rpm. One step methanol addition 1. Reaction at 37 °C, 250 rpm. One step methanol addition |
App.b 100% after 2–3 h | 107 |
| n-Hexane | App. 100% after 7 h | ||||
| Kerosene | App. 100% after 7 h | ||||
| Candida sp. 99-125 | Glycerol trioleate | Dimehylsulfoxide | Lipase 20 wt%, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, water 10 wt%. Reaction for 24 h at 40 °C, 180 rpm. Three-step methanol addition at 0 h, 8 h, and 16 h 1, water 10 wt%. Reaction for 24 h at 40 °C, 180 rpm. Three-step methanol addition at 0 h, 8 h, and 16 h |
9.01 | 32 |
| Acetonitrile | 2.95 | ||||
| Acetone | 1.67 | ||||
| Tetrahydrofuran | 3.31 | ||||
| t-Butanol | 31.3 | ||||
| CH2Cl2 | 28.08 | ||||
| Benzene | 76.68 | ||||
| CHCl3 | 24.4 | ||||
| Toluene | 78.98 | ||||
| CCl4 | 79.7 | ||||
| Cyclohexane | 75.83 | ||||
| n-Hexane | 80.91 | ||||
| R. chinensis whole-cell | Soybean oil | Acetone | Lipase 238 U, methanol to oil molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, soybean oil 0.65 mol L−1. Reaction for 72 h at 30 °C, 150 rpm 1, soybean oil 0.65 mol L−1. Reaction for 72 h at 30 °C, 150 rpm |
73.4 | 61 |
| tert-Butyl alcohol | 65.8 | ||||
| Tertiary | 67.9 | ||||
| Cyclohexane | 71.1 | ||||
| Petroleum ether | 73.5 | ||||
| n-Hexane | 76.5 | ||||
| n-Heptane | 86.7 | ||||
| Isooctane | 82.4 | ||||
| n-Octane | 84.2 | ||||
| Novozym 435 | Cottonseed oil | None | Lipase 1.67 wt%, methanol to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Reaction for 10 h at 50 °C 6. Reaction for 10 h at 50 °C |
0 | 108 |
| t-Butanol | 90 | ||||
| Novozym 435 | Cottonseed oil | Petroleum ether | Lipase 10 wt%, DMC to oil molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction for 24 h at 40 °C, 150 rpm. One-step DMC addition 1. Reaction for 24 h at 40 °C, 150 rpm. One-step DMC addition |
78.3 | 7 |
| Acetone | 2.5 | ||||
| Petroleum ether | Lipase 10 wt%, DMC to oil molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5. Reaction for 24 h at 50 °C, 150 rpm. One-step DMC addition (optimum conditions) 4.5. Reaction for 24 h at 50 °C, 150 rpm. One-step DMC addition (optimum conditions) |
96.4 | |||
| Lipozyme TL IM | Rapeseed oil | None | Lipase 5 wt%, methanol to oil molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction for 12 h at 35 °C, 130 rpm 1. Reaction for 12 h at 35 °C, 130 rpm |
10 | 40 |
| t-Butanol | 75 | ||||
| Novozym 435 | Chlorella pyrenoidosa | t-Butanol | Lipase 10 wt%, methanol to oil molar ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, no water added. Reaction for 48 h at 40 °C 1, no water added. Reaction for 48 h at 40 °C |
44.4 | 113 |
| [BMIm][PF6] | Lipase 10 wt%, methanol to oil molar ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, water 5 μL. Reaction for 48 h at 50 °C 1, water 5 μL. Reaction for 48 h at 50 °C |
86.2 | |||
| Imm.c P. expansum | Chlorella pyrenoidosa | t-Butanol | Lipase 20 wt%, methanol to oil molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, water 15 μL. Reaction for 48 h at 40 °C 1, water 15 μL. Reaction for 48 h at 40 °C |
48.6 | 113 |
| [BMIm][PF6] | Lipase 20 wt%, methanol to oil molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, water 15 μL. Reaction for 48 h at 50 °C 1, water 15 μL. Reaction for 48 h at 50 °C |
90.7 | |||
| Novozym 435 | Soybean oil | t-Butanol | Lipase 2 wt%, methanol. Reaction for 12 h at 50 °C, 250 rpm | 65.8 | 111 |
| [Emim][TfO] | 80 | ||||
| Novozym 435 | Corn oil | SC.d carbon dioxide | Lipase 15 wt%, methanol to oil molar ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction for 4 h at 60 °C and 10 MPa 1. Reaction for 4 h at 60 °C and 10 MPa |
81.3 | 117 |
| Lipozyme RM IM | Chicken feather meal oil | SC. carbon dioxide | Lipase 84 g, methanol to oil molar ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction at 40 °C, 250 bar, 30 g min−1 flow rate 1. Reaction at 40 °C, 250 bar, 30 g min−1 flow rate |
98.8 | 115 |
| Novozym 435 | Soybean oil | SC. carbon dioxide | Lipase 40 g, ethanol to oil molar ratio 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction for 1 h at 70 °C and 200 bar 1. Reaction for 1 h at 70 °C and 200 bar |
94 | 116 |
| Novozym 435 | Scenedesmus sp. | SC. carbon dioxide | Lipase 35 wt%, methanol to oil molar ratio 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reaction for 4 h at 47 °C, 200 bar 1. Reaction for 4 h at 47 °C, 200 bar |
80 | 118 |
Lu et al.32 have tested the conversion of glycerol trioleate to biodiesel using immobilized Candida sp. 99-125 with twelve different organic solvents. From this study, they have made several important points: (i) there might be a correlation between hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) value with yield obtained; (ii) hydrophilic solvents need less water while hydrophobic solvents need more water in the system to be effective; and (iii) solubility of methanol in reaction system does not affect production yield. The result obtained from their study was immobilized Candida sp.99-125 produced higher yield in hydrophobic solvents such as n-hexane, benzene, toluene, CCl4, and cyclohexane. This result is also supported by He et al.61 who tested nine kinds of solvents and found that organic solvents with log
P) value with yield obtained; (ii) hydrophilic solvents need less water while hydrophobic solvents need more water in the system to be effective; and (iii) solubility of methanol in reaction system does not affect production yield. The result obtained from their study was immobilized Candida sp.99-125 produced higher yield in hydrophobic solvents such as n-hexane, benzene, toluene, CCl4, and cyclohexane. This result is also supported by He et al.61 who tested nine kinds of solvents and found that organic solvents with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P between 4.0 and 4.5 produced better results than the others. Kojima et al.107 tested with eighteen solvents and found that C. cylindracea activity was stable in solvents with hydrophobicity index higher than 1.3 such as chloroform, toluene, tetrachloromethane, n-hexane, kerosene and diesel.
P between 4.0 and 4.5 produced better results than the others. Kojima et al.107 tested with eighteen solvents and found that C. cylindracea activity was stable in solvents with hydrophobicity index higher than 1.3 such as chloroform, toluene, tetrachloromethane, n-hexane, kerosene and diesel.
In addition, Su et al.7 produced high conversion in non-polar organic solvent as compared to that of polar organic solvent. This is because polar solvent may interfere with lipase hydrogen bonding and hydrophobic interactions, and thus cause alteration of its molecular structure.7 tert-Butanol, an amphiphilic and moderately polar solvent is also known to give positive results. Several experiments conducted using immobilized lipase with and without t-butanol as solvent show that the yield or conversion increased when t-butanol was added.40,108,109 Many researchers may argue that the t-butanol may participate in the transesterification as acyl acceptor but Royon et al.108 found that t-butanol was not a substrate in the reaction since there is no alcoholysis took place without methanol addition.
Even though addition of solvent can improve production yield, the amount added into the reaction mixture need to be controlled. Li et al.40 conducted experiments using Lipozyme TL IM, rapeseed oil and t-butanol as solvent and discovered that the yield decreased with high volume of t-butanol due to excessive dilution. Furthermore, differences in lipase origin or immobilization method would affect how the enzymes will react in organic solvents.32 For example, n-hexane gave positive result to Candida sp. 99-125 (ref. 32) but it did not affect P. cepacia lipase. In research conducted by Kumari et al.56 on mahua oil using P. cepacia lipase and different solvents such as hexane, octane, and acetonitrile, only octane gave slightly higher conversion compared to solvent-free reaction. The other two solvents did not give any positive results.
Another potential solvent is ionic liquid. Ionic liquid has unique properties such as low vapor pressure, high thermal stability, good solubility in both organic and inorganic materials, and its ability to form multiple phase systems.110 Physical and chemical properties of ionic liquid such as melting point, acidity and basicity, viscosity, density and hydrophobicity can be tuned by altering the combination of cations and anions in it.110,111 Despite all these advantages, ionic liquid is considered expensive and hazardous if contain hexafluorophosphate (PF6) anion.26 Not all ionic liquids are suitable for enzymatic biodiesel production; hydrophobic ionic liquid is more efficient than hydrophilic ionic liquid and some ionic liquid showed lower yield than when using organic solvents such as t-butanol or isopropanol.26 Nonetheless, using ionic liquid as solvent in biodiesel production may increase the yield and reduce the rate of decreasing lipase activity while recycle.112 Experiment conducted by Lai et al.113 on Chlorella pyrenoidosa showed that reactions using [BMIm][PF6] produced about twice the yield of reactions using t-butanol for both Novozym 435 and immobilized P. expansum lipases. Ha et al.111 tested 23 ionic liquids for methanolysis of soybean oil using Novozym 435 and the highest yield of 80% was obtained in [Emim][TfO], which was 15% higher than yield in t-butanol and eight times higher than yield in solvent-free system. Other ionic liquids such as [Hmim][PF6] increased the efficiency of Candida rugosa lipase to convert Chinese tallow kernel oil from about 35–95.4%.114
Supercritical carbon dioxide has the advantage to be used as a solvent due to its non-toxic and non-flammable properties. Biodiesel production using this solvent is capable of producing high biodiesel yield in a short reaction time and the separation is much easier since the products do not dissolve in carbon dioxide at room conditions.12 Compared to non-catalyzed reaction that uses very high temperature, supercritical carbon dioxide is used in a moderate temperature thus make it suitable for enzyme reaction. By using this supercritical fluid, Gameiro et al.115 obtained 98.8% yield at 40 °C and 250 bar, and Colombo et al.116 obtained 94% yield at 70 °C and 200 bar.
Usage of solvent for biodiesel production has several issues related to it. Some solvents are toxic, flammable, and volatile which makes them dangerous to human. Biodiesel production using solvent may also need elimination or recovery steps, larger reactor volume and additional production cost.25,35,80 Nonetheless, the high product yield, better enzyme performance, and shorter reaction time given by the addition of solvent may justify these disadvantages.
4.7 Reactor design
Biodiesel is produced in a reactor in either batch or continuous system. There are many types of reactor that have been developed such as fluidized beds, expanding beds, recirculation, and membrane reactors.27 Among these, the common types of reactor used for biodiesel production are stirred tank reactor (STR) and packed bed reactor (PBR) (Fig. 5). STR generally uses agitation/stirring to disperse the enzyme in the reaction mixture, while PBR contains packed enzyme in a column. | ||
| Fig. 5 Reactor design: batch STR (stirred tank reactor), continuous STR, and PBR (packed bed reactor).53 | ||
The simplest reactor configuration would be the batch STR which consists of temperature measurement devices and control, and stirring system such as propeller or impeller.53 At the end of the process, the lipase will be collected by centrifugation or filtration. Since batch STR often takes up a long of reaction time, it may need high volume tanks for large scale biodiesel production. An alternative to batch STR is a continuous STR, which retain enzyme in the reactor by placing a filter at the reactor outlet.22 A common problem associated with STR is the susceptibility of lipase to disruption due to high shear stress from the agitation. The stirring speed needs to be adjusted at an optimum rate that will balance between a high productivity and a good preservation of lipase stability. Examples of biodiesel production using STR were done Keng et al.119 with a yield of 97.2% after 5 h using 75 liters STR (50 L working volume), and Ognjanovic et al.120 with 99.7% yield after 24 h reaction time using STR equipped with six-bladed turbine impeller.
The packed lipase in PBR has lower exposure to mechanical shear stress compared to STR. PBR is commonly used with continuous process as it allows reuse of enzyme without prior separation. In continuous process, the temperature and pressure will be controlled and the reaction medium is pumped throughout the column under a specific flow rate, which will determine the residence time.53 The advantage of a continuous reactor over a batch reactor is that there is no need to unload, clean and reload the reactor after each batch. There have been many tests conducted on biodiesel production using PBR.121,122 An example of PBR was tested by Xu et al.123 who developed a two-stage ethanol-based biodiesel production and obtained overall productivity of 1.56 kg FAEE per (kg catalyst) per h. Ognjanovic et al.120 obtained 96.25% conversion after 8 h using PBR and had almost no loss of productivity after 72 h (8 cycles) of operation.
The stability of immobilized lipase in term of mechanical and operational determines its suitability to be used in a reactor. For example, the immobilized support needs to have high resistance towards friction and shear stress in STR, and high resistance towards compression in high flow rates PBR.53 To avoid lipase deactivation, methanol can be added into the reaction system in a continuous or stepwise manner. There is also a need to include glycerol removal system in a continuous biodiesel production to avoid accumulation of glycerol. Accumulation of glycerol would not only inhibit the enzymatic reaction, but also increase the mass transfer resistance of substrates to immobilized lipase and may cause column clogging and pressure dropping.124 For this reason, Hama et al.125 constructed a PBR integrated with glycerol-separating system in a solvent free biodiesel production. In addition, instead of the long single PBR, Tran et al.124 designed a serial PBRs (three columns in series) with subsequent removal of glycerol and managed to increase biodiesel conversion up to 85%.
5. Economic evaluation and industrial scale production
When developing a biodiesel production process, one of the major concerns for enzymatic biodiesel production is its economical aspect. The higher cost of enzyme makes the enzyme-catalyzed reaction to be less favorable compared to chemical-catalyzed production. Nonetheless, this drawback can be managed through repeatable use of enzyme, which directs to the application of immobilized lipase. There have been a few studies that measured the economical aspect of enzymatic biodiesel production. For example, Jegannathan et al.126 conducted an economic assessment of biodiesel production between three catalysts: alkali, soluble enzyme, and immobilized enzyme. This assessment was calculated for batch mode (stirred tank) with a production capacity of 103 tonne. The price estimated for the lipase was $150 per kg. It was calculated that alkali catalysts had the lowest production cost ($1166.67 per tonne) compared to immobilized lipase catalyst ($2414.63 per tonne) and soluble lipase catalyst ($7821.37 per tonne). The higher production cost when using immobilized enzyme was due to higher cost of lipase and longer reaction time. However, it has to be mentioned that this assessment included washing process in the production line which is not necessarily needed for enzyme catalyst. In addition, it also used transesterification reaction time of 72 hours which can be shortened depending on the efficiency of the lipase used.Since the enzymatic production of biodiesel can be done with or without solvent, an economical comparison between these two processes had also been done. Sotoft et al.127 evaluated the production of 8 and 200 mio. kg biodiesel per year from rapeseed oil and methanol, and made a comparison between solvent free and cosolvent (t-butanol) production. They used two prices of enzyme that account for the current price (762.71€ per kg enzyme) and estimated price in the future (7.63€ per kg enzyme). The product price for solvent free production was estimated to 0.73–1.49€ per kg biodiesel and 0.05–0.75€ per kg biodiesel for enzyme price of 762.71€ per kg enzyme and 7.63€ per kg enzyme respectively. Meanwhile, the product price for cosolvent production was estimated as 1.50–2.38€ per kg biodiesel. The total capital investment for cosolvent production was calculated to be higher due the installation costs of solvent recovery column, which was higher than the cost of extra number of reactors and decanters needed for solvent free operation. They concluded that cosolvent production process was too expensive and not a viable choice.
An economic analysis of a biodiesel production plant from waste cooking oil (WCO) using supercritical carbon dioxide was done by Lisboa et al.128 It was estimated that the biodiesel cost was 1.64€ per L and 0.75€ per L (for a WCO price of 0.25€ per kg and enzyme prices of 800€ per kg and 8€ per kg, respectively). This production cost was calculated based on conversion of 8000 ton WCO per year, using immobilized lipase Thermomyces lanuginosus (Lipozyme TL IM) and ethanol.
The current issue of falling crude oil prices will definitely give negative impacts to the global biofuel industry. The current price for crude oil is around $31.05 per barrel (February 2016) which has decreased dramatically to less than a third of its price in Feb 2014 (ref. 129) (Table 10). This situation has caused the preference of petroleum-based fuel over biodiesel. Nonetheless, this problem can be put under control by government intervention. The regulation of domestic fuel price by the government may control the gasoline and diesel fuel prices at the pump and thus maintain biofuel as a competitive choice. In addition, many countries, especially the major biofuel producing countries, have implemented biofuel policies to boost the growth of their biofuel sector. For example, in United States, Energy Policy Act of 2005 established a renewable fuel standard (RFS) that required the increase of renewable fuel usage from 9 billion per year in 2008 to 36 billion per year in 2022.130 In 2012, the US president announced the establishment of “All-of-the-above energy” policy to make a long-term plan that uses every available sources of energy including wind, solar and biofuels. Other incentives such as tax credits of $1.01 per gallon and $1 per gallon were given to cellulosic biofuel and biodiesel productions respectively from December 2011 to December 2013. Meanwhile in Brazil, invention of flexible fuel vehicle that can run on any gasoline-ethanol blend has increased the growth of its national ethanol market. Brazil also gives taxes exemption (PIS and CONFIN) for ethanol industries and provides low-interest loans and subsidies to sugarcane farmers for land expansion.130 The increasing proportion of biofuel blends in the market that is supported by government mandates also helps to sustain biofuel industry.
| Commodity | Feb 2011 | Feb 2012 | Feb 2013 | Feb 2014 | Feb 2015 | Feb 2016 |
|---|---|---|---|---|---|---|
| Crude oil (petroleum) | $97.73 | $112.7 | $107.66 | $104.82 | $54.93 | $31.05 |
| Rapeseed oil | $1.42k | $1.30k | $1.22k | $0.97k | $0.75k | $0.78k |
| Palm oil | $1.25k | $1.05k | $0.79k | $0.81k | $0.63k | $0.60k |
| Soybean oil | $1.27k | $1.17k | $1.13k | $0.87k | $0.70k | $0.69k |
Furthermore, the price for vegetable oils such as soybean oil, rapeseed oil and palm oil also has decreased to half its price these past 5 years (Table 10). For example, soybean oil price has decreased from $1.27k per metric ton in February 2011 to $0.69k per metric ton in February 2016. Since cost of biodiesel is about 30% higher than petroleum diesel due to the feedstock price of plant oils,22 the decrease of this feedstock price may as well reduce the biodiesel price and thus alleviate the impact of fallen crude oil price. Other than that, choosing a cheaper feedstock such as waste cooking oil is also an attractive option.
The plan for industrial scale production of biodiesel using enzyme as catalyst is no longer conceptual. In recent years, enzyme manufacturers and biodiesel producers have collaborated with each other to develop new technology of enzymatic biodiesel production that is more feasible and economical. For example, Novozymes (an enzyme maker company from Denmark) has collaborated with many biodiesel producer companies such as Piedmont Biofuels, Blue Sun Biodiesel, WB services, Buster Biofuels, including Viesel Fuel LLC that has a enzymatic biodiesel production line with a capacity of 5 million gallons output per year.131,132
There are already many biodiesel plants that produced biodiesel using enzymatic reaction presently. In 2007, Lvming Co. Ltd. built an enzymatic production line with capacity of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in Shanghai, China.57 The factory used immobilized lipase Candida sp. 99-125 as catalyst (0.4% to the weight of oil) and waste cooking oil as raw material. About 90% FAME yield was obtained under optimal condition. The process was conducted in a stirred tank reactor, and a centrifuge was used to separate glycerol and water. In 2012, Piedmont Biofuels, North Carolina, developed a new technology (FAeSTER) for a continuous biodiesel production using immobilized or liquid enzyme.22 They established an enzymatic biodiesel process that can utilize high free fatty acids feedstocks, as high as 100% FFA.133 Another factory, Hainabaichuan Co. Ltd. in Hunan Province, China, applied the technology from Tsinghua University and used commercial Novozyme 435 as catalyst.57
000 tons in Shanghai, China.57 The factory used immobilized lipase Candida sp. 99-125 as catalyst (0.4% to the weight of oil) and waste cooking oil as raw material. About 90% FAME yield was obtained under optimal condition. The process was conducted in a stirred tank reactor, and a centrifuge was used to separate glycerol and water. In 2012, Piedmont Biofuels, North Carolina, developed a new technology (FAeSTER) for a continuous biodiesel production using immobilized or liquid enzyme.22 They established an enzymatic biodiesel process that can utilize high free fatty acids feedstocks, as high as 100% FFA.133 Another factory, Hainabaichuan Co. Ltd. in Hunan Province, China, applied the technology from Tsinghua University and used commercial Novozyme 435 as catalyst.57
6. Conclusion
Due to high cost of enzyme, slow reaction rate and enzyme inhibition, enzymatic reaction still need further improvement to be used for biodiesel production as compared to that of chemical-catalyzed reactions. Enzymatic reaction is more advantageous than chemical methods because of its mild reaction conditions, easy product recovery, wide variation of substrate including free fatty acids, no wastewater generation, no saponification and higher quality of products.The three main components for biodiesel synthesis using enzymatic reaction are lipase, oil and acyl acceptor. Lipase, the catalysts for enzymatic transesterification has unique characteristics and plays a major role in determining the production yield. Many methods have been tested to improve biodiesel production through enzymatic reaction such as combination of lipases, enzyme pretreatment, enzyme post treatment, methanol addition technique, use of solvent, and adding of silica gel. These methods are applied to get the best possible yield and to reduce the effect of enzyme inhibition during transesterification process. The positive effects of these additional methods were proven, but there are some matters that need to be thought of: additional method will cause extra cost and may also complicate the production steps. One of the effective ways is to create new strain of lipase or recombinant lipase that has high tolerant to methanol, high catalytic efficiency, good resistance towards harsh condition, and able to maintain its efficiency after many uses. Accordingly, genetic engineering involving lipase and its expression is an area that needs to be further developed. Other important areas are techniques for lipase immobilization and the design of biodiesel reactor. Reactor design is very crucial to control the input and output of the reaction system. Features that can be included and improved in the design will include glycerol removal system and continuous/stepwise addition of methanol.
A great deal of energy and efforts have been invested to improve feasibility and efficiency of biodiesel production. By improving the enzymatic reaction for biodiesel production, it is most likely for this method to be used in the industry for big-scale biodiesel production and may contribute towards a greener and environmental friendly energy production.
Acknowledgements
The authors would like to thank Ministry of Higher Education Malaysia and University of Malaya for the financial support under High Impact Research Grant (UM.C/625/1/HIR/MOHE/ENG/07), Postgraduate Research Fund (PPP: PG106-2015A), UMRG: RP022A-13AET and FRGS: FP009-2014A.References
- International Energy Agency, 2015.
- BP, 2015.
- M. H. M. Ashnani, A. Johari, H. Hashim and E. Hasani, Renewable Sustainable Energy Rev., 2014, 35, 244–257 CrossRef.
- D. Drabik, H. de Gorter and G. R. Timilsina, Energy Economics, 2014, 44, 80–88 CrossRef.
- M. Mubarak, A. Shaija and T. V. Suchithra, Algal Res., 2015, 7, 117–123 CrossRef.
- B. Singh, A. Guldhe, I. Rawat and F. Bux, Renewable Sustainable Energy Rev., 2014, 29, 216–245 CrossRef CAS.
- E.-Z. Su, M.-J. Zhang, J.-G. Zhang, J.-F. Gao and D.-Z. Wei, Biochem. Eng. J., 2007, 36, 167–173 CrossRef CAS.
- A. E. Atabani, A. S. Silitonga, H. C. Ong, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin and H. Fayaz, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS.
- P. K. Devan and N. V. Mahalakshmi, Fuel Process. Technol., 2009, 90, 513–519 CrossRef CAS.
- P. K. Devan and N. V. Mahalakshmi, Fuel, 2009, 88, 861–867 CrossRef CAS.
- A. Röttig, L. Wenning, D. Bröker and A. Steinbüchel, Appl. Microbiol. Biotechnol., 2010, 85, 1713–1733 CrossRef PubMed.
- O. S. Stamenković, A. V. Veličković and V. B. Veljković, Fuel, 2011, 90, 3141–3155 CrossRef.
- G. Madras, C. Kolluru and R. Kumar, Fuel, 2004, 83, 2029–2033 CrossRef CAS.
- E. F. Aransiola, T. V. Ojumu, O. O. Oyekola, T. F. Madzimbamuto and D. I. O. Ikhu-Omoregbe, Biomass Bioenergy, 2014, 61, 276–297 CrossRef CAS.
- B. Bharathiraja, M. Chakravarthy, R. R. Kumar, D. Yuvaraj, J. Jayamuthunagai, R. P. Kumar and S. Palani, Renewable Sustainable Energy Rev., 2014, 38, 368–382 CrossRef CAS.
- H. C. Ong, H. H. Masjuki, T. M. I. Mahlia, A. S. Silitonga, W. T. Chong and T. Yusaf, Energy, 2014, 69, 427–445 CrossRef CAS.
- A. S. Silitonga, H. C. Ong, H. H. Masjuki, T. M. I. Mahlia, W. T. Chong and T. F. Yusaf, Fuel, 2013, 111, 478–484 CrossRef CAS.
- H. C. Ong, H. H. Masjuki, T. M. I. Mahlia, A. S. Silitonga, W. T. Chong and K. Y. Leong, Energy Convers. Manage., 2014, 81, 30–40 CrossRef CAS.
- H. C. Ong, A. S. Silitonga, H. H. Masjuki, T. M. I. Mahlia, W. T. Chong and M. H. Boosroh, Energy Convers. Manage., 2013, 73, 245–255 CrossRef CAS.
- Y. H. Taufiq-Yap, H. V. Lee, R. Yunus and J. C. Juan, Chem. Eng. J., 2011, 178, 342–347 CrossRef CAS.
- H. V. Lee, J. C. Juan and Y. H. Taufiq-Yap, Renewable Energy, 2015, 74, 124–132 CrossRef CAS.
- L. P. Christopher, K. Hemanathan and V. P. Zambare, Appl. Energy, 2014, 119, 497–520 CrossRef CAS.
- J. C. Juan, D. A. Kartika, T. Y. Wu and T.-Y. Y. Hin, Bioresour. Technol., 2011, 102, 452–460 CrossRef CAS PubMed.
- J. Wall, J. V. Gerpen and J. Thompson, Trans. ASABE, 2011, 54, 535–541 CrossRef.
- L. Fjerbaek, K. V. Christensen and B. Norddahl, Biotechnol. Bioeng., 2009, 102, 1298–1315 CrossRef CAS PubMed.
- A. Guldhe, B. Singh, T. Mutanda, K. Permaul and F. Bux, Renewable Sustainable Energy Rev., 2015, 41, 1447–1464 CrossRef CAS.
- A. Gog, M. Roman, M. Toşa, C. Paizs and F. D. Irimie, Renewable Energy, 2012, 39, 10–16 CrossRef CAS.
- J. Lu, L. Deng, R. Zhao, R. Zhang, F. Wang and T. Tan, J. Mol. Catal. B: Enzym., 2010, 62, 15–18 CrossRef CAS.
- R. Maceiras, M. Vega, C. Costa, P. Ramos and M. C. Márquez, Chem. Eng. J., 2011, 166, 358–361 CrossRef CAS.
- M. Szczęsna Antczak, A. Kubiak, T. Antczak and S. Bielecki, Renewable Energy, 2009, 34, 1185–1194 CrossRef.
- J. Lu, Y. Chen, F. Wang and T. Tan, J. Mol. Catal. B: Enzym., 2009, 56, 122–125 CrossRef CAS.
- J. Lu, K. Nie, F. Wang and T. Tan, Bioresour. Technol., 2008, 99, 6070–6074 CrossRef CAS PubMed.
- A. Salis, M. Pinna, M. Monduzzi and V. Solinas, J. Biotechnol., 2005, 119, 291–299 CrossRef CAS PubMed.
- M. Lee, J. Lee, D. Lee, J. Cho, S. Kim and C. Park, Enzyme Microb. Technol., 2011, 49, 402–406 CrossRef CAS PubMed.
- Y. Shimada, Y. Watanabe, T. Samukawa, A. Sugihara, H. Noda, H. Fukuda and Y. Tominaga, J. Am. Oil Chem. Soc., 1999, 76, 789–793 CrossRef CAS.
- M. Kapoor and M. N. Gupta, Process Biochem., 2012, 47, 555–569 CrossRef CAS.
- J. K. Poppe, C. R. Matte, M. d. C. R. Peralba, R. Fernandez-Lafuente, R. C. Rodrigues and M. A. Z. Ayub, Appl. Catal., A, 2015, 490, 50–56 CrossRef CAS.
- M. Kaieda, T. Samukawa, A. Kondo and H. Fukuda, J. Biosci. Bioeng., 2001, 91, 12–15 CrossRef CAS PubMed.
- L. Nelson, T. Foglia and W. Marmer, J. Am. Oil Chem. Soc., 1996, 73, 1191–1195 CrossRef CAS.
- L. Li, W. Du, D. Liu, L. Wang and Z. Li, J. Mol. Catal. B: Enzym., 2006, 43, 58–62 CrossRef CAS.
- K. Tongboriboon, B. Cheirsilp and A. H-Kittikun, J. Mol. Catal. B: Enzym., 2010, 67, 52–59 CrossRef CAS.
- W. Du, Y.-Y. Xu, D.-H. Liu and Z.-B. Li, J. Mol. Catal. B: Enzym., 2005, 37, 68–71 CrossRef CAS.
- R. C. Rodrigues, B. C. C. Pessela, G. Volpato, R. Fernandez-Lafuente, J. M. Guisan and M. A. Z. Ayub, Process Biochem., 2010, 45, 1268–1273 CrossRef CAS.
- R. Rodrigues, G. Volpato, K. Wada and M. Ayub, J. Am. Oil Chem. Soc., 2008, 85, 925–930 CrossRef CAS.
- Y. Li, W. Du, L. Dai and D. Liu, J. Mol. Catal. B: Enzym., 2015, 121, 22–27 CrossRef CAS.
- A. Canet, K. Bonet-Ragel, M. D. Benaiges and F. Valero, Biomass Bioenergy, 2016, 85, 94–99 CrossRef CAS.
- B. Cheirsilp, A. H. Kittikun and S. Limkatanyu, Biochem. Eng. J., 2008, 42, 261–269 CrossRef CAS.
- S. Liu, K. Nie, X. Zhang, M. Wang, L. Deng, X. Ye, F. Wang and T. Tan, J. Mol. Catal. B: Enzym., 2014, 99, 43–50 CrossRef CAS.
- S. Al-Zuhair, F. W. Ling and L. S. Jun, Process Biochem., 2007, 42, 951–960 CrossRef CAS.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- R. C. Rodrigues, C. Ortiz, A. Berenguer-Murcia, R. Torres and R. Fernandez-Lafuente, Chem. Soc. Rev., 2013, 42, 6290–6307 RSC.
- M. Mohammadi, M. Ashjari, S. Dezvarei, M. Yousefi, M. Babaki and J. Mohammadi, RSC Adv., 2015, 5, 32698–32705 RSC.
- J. K. Poppe, R. Fernandez-Lafuente, R. C. Rodrigues and M. A. Z. Ayub, Biotechnol. Adv., 2015, 33, 511–525 CrossRef CAS PubMed.
- V. Gascon, I. Diaz, R. M. Blanco and C. Marquez-Alvarez, RSC Adv., 2014, 4, 34356–34368 RSC.
- A. E. Ghaly, D. Dave, M. S. Brooks and S. Budge, Am. J. Biochem. Biotechnol., 2010, 6, 54–76 CrossRef CAS.
- V. Kumari, S. Shah and M. N. Gupta, Energy Fuels, 2006, 21, 368–372 CrossRef.
- T. Tan, J. Lu, K. Nie, L. Deng and F. Wang, Biotechnol. Adv., 2010, 28, 628–634 CrossRef CAS PubMed.
- Y. Xu, W. Du, D. Liu and J. Zeng, Biotechnol. Lett., 2003, 25, 1239–1241 CrossRef CAS PubMed.
- K. S. Yang, J.-H. Sohn and H. K. Kim, J. Biosci. Bioeng., 2009, 107, 599–604 CrossRef CAS PubMed.
- S. Tamalampudi, M. R. Talukder, S. Hama, T. Numata, A. Kondo and H. Fukuda, Biochem. Eng. J., 2008, 39, 185–189 CrossRef CAS.
- Q. He, Y. Xu, Y. Teng and D. Wang, Chin. J. Catal., 2008, 29, 41–46 CrossRef CAS.
- M. M. R. Talukder, H. Z. S. Lee, R. F. Low, L. C. Pei-Lyn, D. Warzecha and J. Wu, J. Mol. Catal. B: Enzym., 2013, 89, 108–113 CrossRef CAS.
- A. Guldhe, P. Singh, S. Kumari, I. Rawat, K. Permaul and F. Bux, Renewable Energy, 2016, 85, 1002–1010 CrossRef CAS.
- W. Yang, Y. He, L. Xu, H. Zhang and Y. Yan, J. Mol. Catal. B: Enzym., 2016, 126, 76–89 CrossRef CAS.
- D. Kumar, R. Parshad and V. K. Gupta, Int. J. Biol. Macromol., 2014, 66, 97–107 CrossRef CAS PubMed.
- G. Knothe, Fuel Process. Technol., 2005, 86, 1059–1070 CrossRef CAS.
- M. M. Gui, K. T. Lee and S. Bhatia, Energy, 2008, 33, 1646–1653 CrossRef CAS.
- M. Y. Noraini, H. C. Ong, M. J. Badrul and W. T. Chong, Renewable Sustainable Energy Rev., 2014, 39, 24–34 CrossRef CAS.
- A. Demirbas and M. Fatih Demirbas, Energy Convers. Manage., 2011, 52, 163–170 CrossRef.
- M. K. Modi, J. R. C. Reddy, B. V. S. K. Rao and R. B. N. Prasad, Bioresour. Technol., 2007, 98, 1260–1264 CrossRef CAS PubMed.
- C.-H. Ko, K.-W. Yeh, Y.-N. Wang, C.-H. Wu, F.-C. Chang, M.-H. Cheng and C.-S. Liou, Energy, 2012, 48, 375–379 CrossRef CAS.
- M. K. Lam, K. T. Lee and A. R. Mohamed, Biotechnol. Adv., 2010, 28, 500–518 CrossRef CAS PubMed.
- S.-H. Ho, X. Ye, T. Hasunuma, J.-S. Chang and A. Kondo, Biotechnol. Adv., 2014, 32, 1448–1459 CrossRef CAS PubMed.
- W. Xiong, X. Li, J. Xiang and Q. Wu, Appl. Microbiol. Biotechnol., 2008, 78, 29–36 CrossRef CAS PubMed.
- R. Halim, M. K. Danquah and P. A. Webley, Biotechnol. Adv., 2012, 30, 709–732 CrossRef CAS PubMed.
- M. K. Lam and K. T. Lee, Biotechnol. Adv., 2012, 30, 673–690 CrossRef CAS PubMed.
- R. Slade and A. Bauen, Biomass Bioenergy, 2013, 53, 29–38 CrossRef.
- Y. Watanabe, Y. Shimada, A. Sugihara and Y. Tominaga, J. Mol. Catal. B: Enzym., 2002, 17, 151–155 CrossRef CAS.
- Y. Morikawa, X. Zhao and D. Liu, RSC Adv., 2014, 4, 37878–37888 RSC.
- J. M. Cerveró, J. R. Álvarez and S. Luque, Biomass Bioenergy, 2014, 61, 131–137 CrossRef.
- W. Du, Y. Xu, D. Liu and J. Zeng, J. Mol. Catal. B: Enzym., 2004, 30, 125–129 CrossRef CAS.
- O. K. Lee, Y. H. Kim, J.-G. Na, Y.-K. Oh and E. Y. Lee, Bioresour. Technol., 2013, 147, 240–245 CrossRef CAS PubMed.
- N. Gharat and V. K. Rathod, J. Mol. Catal. B: Enzym., 2013, 88, 36–40 CrossRef CAS.
- J.-W. Chen and W.-T. Wu, J. Biosci. Bioeng., 2003, 95, 466–469 CrossRef CAS PubMed.
- M. Lotti, J. Pleiss, F. Valero and P. Ferrer, Biotechnol. J., 2015, 10, 22–30 CrossRef CAS PubMed.
- J. M. Encinar, J. F. González, J. J. Rodríguez and A. Tejedor, Energy Fuels, 2002, 16, 443–450 CrossRef CAS.
- T. Zhao, D. S. No, Y. Kim, Y. S. Kim and I.-H. Kim, J. Mol. Catal. B: Enzym., 2014, 107, 17–22 CrossRef CAS.
- H. Joshi, B. R. Moser, J. Toler and T. Walker, Biomass Bioenergy, 2010, 34, 14–20 CrossRef CAS.
- J. Calero, D. Luna, E. D. Sancho, C. Luna, F. M. Bautista, A. A. Romero, A. Posadillo, J. Berbel and C. Verdugo-Escamilla, Renewable Sustainable Energy Rev., 2015, 42, 1437–1452 CrossRef CAS.
- T.-C. Kuo, J.-F. Shaw and G.-C. Lee, Bioresour. Technol., 2015, 192, 54–59 CrossRef CAS PubMed.
- J. Amoah, S.-H. Ho, S. Hama, A. Yoshida, A. Nakanishi, T. Hasunuma, C. Ogino and A. Kondo, Biochem. Eng. J., 2016, 105, 10–15 CrossRef CAS.
- S. H. Duarte, G. L. del Peso Hernández, A. Canet, M. D. Benaiges, F. Maugeri and F. Valero, Bioresour. Technol., 2015, 183, 175–180 CrossRef CAS PubMed.
- Y. Yan, L. Xu and M. Dai, RSC Adv., 2012, 2, 6170–6173 RSC.
- F. Guan, P. Peng, G. Wang, T. Yin, Q. Peng, J. Huang, G. Guan and Y. Li, Process Biochem., 2010, 45, 1677–1682 CrossRef CAS.
- J. Yan, X. Zheng and S. Li, Bioresour. Technol., 2014, 151, 43–48 CrossRef CAS PubMed.
- R. C. Rodrigues and M. A. Z. Ayub, Process Biochem., 2011, 46, 682–688 CrossRef CAS.
- T. Samukawa, M. Kaieda, T. Matsumoto, K. Ban, A. Kondo, Y. Shimada, H. Noda and H. Fukuda, J. Biosci. Bioeng., 2000, 90, 180–183 CrossRef CAS PubMed.
- K. Ban, S. Hama, K. Nishizuka, M. Kaieda, T. Matsumoto, A. Kondo, H. Noda and H. Fukuda, J. Mol. Catal. B: Enzym., 2002, 17, 157–165 CrossRef CAS.
- E. C. G. Aguieiras, D. S. Ribeiro, P. P. Couteiro, C. M. B. Bastos, D. S. Queiroz, J. M. Parreira and M. A. P. Langone, Appl. Biochem. Biotechnol., 2016, 1–12 Search PubMed.
- C.-Y. Yu, L.-Y. Huang, I. C. Kuan and S.-L. Lee, Int. J. Mol. Sci., 2013, 14, 24074–24086 CrossRef PubMed.
- Q. You, X. Yin, Y. Zhao and Y. Zhang, Bioresour. Technol., 2013, 148, 202–207 CrossRef CAS PubMed.
- K. Bonet-Ragel, A. Canet, M. D. Benaiges and F. Valero, Fuel, 2015, 161, 12–17 CrossRef CAS.
- A. Guldhe, B. Singh, I. Rawat, K. Permaul and F. Bux, Fuel, 2015, 147, 117–124 CrossRef CAS.
- M. Babaki, M. Yousefi, Z. Habibi, M. Mohammadi, P. Yousefi, J. Mohammadi and J. Brask, Renewable Energy, 2016, 91, 196–206 CrossRef CAS.
- M. Babaki, M. Yousefi, Z. Habibi, M. Mohammadi and J. Brask, J. Mol. Catal. B: Enzym., 2015, 120, 93–99 CrossRef CAS.
- D. E. Stevenson, R. A. Stanley and R. H. Furneaux, Enzyme Microb. Technol., 1994, 16, 478–484 CrossRef CAS.
- S. Kojima, D. Du, M. Sato and E. Y. Park, J. Biosci. Bioeng., 2004, 98, 420–424 CrossRef CAS PubMed.
- D. Royon, M. Daz, G. Ellenrieder and S. Locatelli, Bioresour. Technol., 2007, 98, 648–653 CrossRef CAS PubMed.
- R. R. Nasaruddin, M. Z. Alam and M. S. Jami, Bioresour. Technol., 2014, 154, 155–161 CrossRef CAS PubMed.
- A. H. Mohammad Fauzi and N. A. S. Amin, Renewable Sustainable Energy Rev., 2012, 16, 5770–5786 CrossRef CAS.
- S. H. Ha, M. N. Lan, S. H. Lee, S. M. Hwang and Y.-M. Koo, Enzyme Microb. Technol., 2007, 41, 480–483 CrossRef CAS.
- R. Manurung, R. Hasibuan, T. Taslim, N. S. Rahayu and A. Darusmy, Procedia - Social and Behavioral Sciences, 2015, 195, 2485–2491 CrossRef.
- J.-Q. Lai, Z.-L. Hu, P.-W. Wang and Z. Yang, Fuel, 2012, 95, 329–333 CrossRef CAS.
- F. Su, C. Peng, G.-L. Li, L. Xu and Y.-J. Yan, Renewable Energy, 2016, 90, 329–335 CrossRef CAS.
- M. Gameiro, P. Lisboa, A. Paiva, S. Barreiros and P. Simões, Fuel, 2015, 153, 135–142 CrossRef CAS.
- T. S. Colombo, M. A. Mazutti, M. Di Luccio, D. de Oliveira and J. V. Oliveira, J. Supercrit. Fluids, 2015, 97, 16–21 CrossRef CAS.
- O. N. Ciftci and F. Temelli, J. Supercrit. Fluids, 2013, 75, 172–180 CrossRef CAS.
- H. Taher, S. Al-Zuhair, A. H. Al-Marzouqi, Y. Haik and M. Farid, Biochem. Eng. J., 2014, 90, 103–113 CrossRef CAS.
- P. S. Keng, M. Basri, A. B. Ariff, M. B. Abdul Rahman, R. N. Z. Abdul Rahman and A. B. Salleh, Bioresour. Technol., 2008, 99, 6097–6104 CrossRef CAS PubMed.
- N. Ognjanovic, D. Bezbradica and Z. Knezevic-Jugovic, Bioresour. Technol., 2009, 100, 5146–5154 CrossRef CAS PubMed.
- S. F. A. Halim, A. H. Kamaruddin and W. J. N. Fernando, Bioresour. Technol., 2009, 100, 710–716 CrossRef CAS PubMed.
- D.-T. Tran, Y.-J. Lin, C.-L. Chen and J.-S. Chang, Appl. Energy, 2014, 126, 151–160 CrossRef CAS.
- Y. Xu, M. Nordblad and J. M. Woodley, J. Biotechnol., 2012, 162, 407–414 CrossRef CAS PubMed.
- D.-T. Tran, C.-L. Chen and J.-S. Chang, Appl. Energy, 2016, 168, 340–350 CrossRef CAS.
- S. Hama, S. Tamalampudi, A. Yoshida, N. Tamadani, N. Kuratani, H. Noda, H. Fukuda and A. Kondo, Biochem. Eng. J., 2011, 55, 66–71 CrossRef CAS.
- K. R. Jegannathan, C. Eng-Seng and P. Ravindra, Renewable Sustainable Energy Rev., 2011, 15, 745–751 CrossRef CAS.
- L. F. Sotoft, B.-G. Rong, K. V. Christensen and B. Norddahl, Bioresour. Technol., 2010, 101, 5266–5274 CrossRef CAS PubMed.
- P. Lisboa, A. R. Rodrigues, J. L. Martín, P. Simões, S. Barreiros and A. Paiva, J. Supercrit. Fluids, 2014, 85, 31–40 CrossRef CAS.
- IndexMundi, Crude Oil (petroleum) Monthly Price - US Dollars per Barrel, http://www.indexmundi.com/commodities/?commodity=crude-oil%26months=60.
- Y. Su, P. Zhang and Y. Su, Renewable Sustainable Energy Rev., 2015, 50, 991–1003 CrossRef.
- R. Hobden, in Biodiesel Magazine, 2014 Search PubMed.
- R. Kotrba, in Biodiesel Magazine, 2014 Search PubMed.
- Piedmont Biofuels, Enzymatic Biodiesel, http://www.biofuels.coop/enzymatic-biodiesel.
| This journal is © The Royal Society of Chemistry 2016 |

