Open Access Article
Open Access ArticleCarbon quantum dots: synthesis, properties and applications
Youfu
Wang
and
Aiguo
Hu
*
Shanghai Key Laboratory of Advanced Polymeric Materials, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: hagmhsn@ecust.edu.cn; Fax: +86-21-64253037; Tel: +86-21-64253037
First published on 17th June 2014
Abstract
Carbon quantum dots (CQDs, C-dots or CDs), which are generally small carbon nanoparticles (less than 10 nm in size) with various unique properties, have found wide use in more and more fields during the last few years. In this feature article, we describe the recent progress in the field of CQDs, focusing on their synthetic methods, size control, modification strategies, photoelectric properties, luminescent mechanism, and applications in biomedicine, optronics, catalysis and sensor issues.
1. Introduction
Carbon-based quantum dots consisting of graphene quantum dots (QGDs) and carbon quantum dots (CQDs, C-dots or CDs) are a new class of carbon nanomaterials with sizes below 10 nm. They were first obtained during the purification of single-walled carbon nanotubes through preparative electrophoresis in 2004,1 and then via laser ablation of graphite powder and cement in 2006.2 Carbon-based quantum dots with fascinating properties have gradually become a rising star as a new nanocarbon member due to their benign, abundant and inexpensive nature.3 Carbon is commonly a black material, and was generally considered to have low solubility in water and weak fluorescence. Wide attention has been focused on carbon-based quantum dots because of their good solubility and strong luminescence, for which they are referred to as carbon nanolights.3During the past few years, much progress has been achieved in the synthesis, properties and applications of carbon-based quantum dots, as reviewed by Baker et al.,3 Lee et al.,4 and Zhu et al.5 Compared to traditional semiconductor quantum dots and organic dyes, photoluminescent carbon-based quantum dots are superior in terms of high (aqueous) solubility, robust chemical inertness, facile modification and high resistance to photobleaching. The superior biological properties of carbon-based quantum dots, such as low toxicity and good biocompatibility, entrust them with potential applications in bioimaging, biosensor and biomolecule/drug delivery. The outstanding electronic properties of carbon-based quantum dots as electron donors and acceptors, causing chemiluminescence and electrochemical luminescence, endow them with wide potentials in optronics, catalysis and sensors.
As the properties and applications of GQDs have been systematically summarized elsewhere,5–7 we will focus our horizons on CQDs due to their unique properties and great potential in various applications (Fig. 1). In this feature article, we describe the recent progress in the field of CQDs, focusing on their synthetic methods, size control, modification strategies, optical properties, luminescent mechanism, and applications in biomedicine, optronics, catalysis and sensor issues.
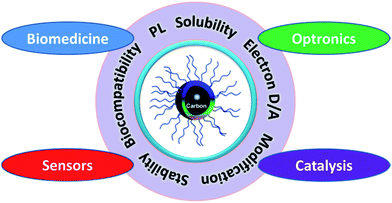 | ||
| Fig. 1 CQDs with unique properties have great potential in biomedicine, optronics, catalysis and sensors. | ||
2. Synthesis, size control and modification
Many methods have been proposed to prepare CQDs during the last decade, which can be roughly classified into “Top-down” and “Bottom-up” approaches, and they can be modified during preparation or post-treatment (Fig. 2). Three problems facing CQDs preparation need to be noticed: (i) carbonaceous aggregation during carbonization, which can be avoided by using electrochemical synthesis, confined pyrolysis or solution chemistry methods, (ii) size control and uniformity, which is important for uniform properties and mechanistic study, and can be optimized via post-treatment, such as gel electrophoresis, centrifugation, and dialysis and (iii) surface properties that are critical for solubility and selected applications, which can be tuned during preparation or post-treatment. We will discuss the main methods for CQDs synthesis, the size control via confined pyrolysis and the modification of CQDs, including functionalization, doping and nanohybrids. The features of different synthetic methods for the preparation of CQDs are summarized in Table 1.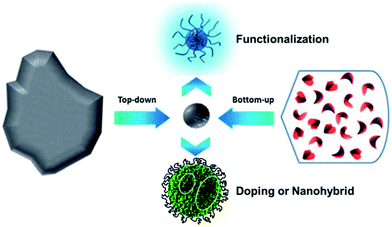 | ||
| Fig. 2 Schematic illustration of CQDs preparation via “top-down” and “bottom-up” approaches, and modification including functionalization, doping and nanohybrids. Adapted with permission.8 Copyright 2013, Royal Society of Chemistry. | ||
| Synthetic Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Chemical ablation | Most accessible, various sources | Harsh conditions, drastic processes, multiple-steps, poor control over sizes | 9–14 |
| Electrochemical carbonization | Size and nanostructure are controllable, stable, one-step | Few small molecule precursors | 15–22 |
| Laser ablation | Rapid, effective, surface states tunable | Low QY, poor control over sizes, modification is needed | 2 and 23–27 |
| Microware irradiation | Rapid, scalable, cost effective, eco-friendly | Poor control over sizes | 28–31 |
| Hydrothermal/solvothermal treatment | Cost effective, eco-friendly, non-toxic | Poor control over sizes | 34–40 |
2.1. Synthetic methods
Photoluminescent CQDs were synthesized in one-pot using polyethylenimine (PEI), a cationic branched polyelectrolyte, as both a carbon source and passivating agent via HNO3 oxidation.14 In contrast to the commonly reported pH-insensitive CQDs, the PL of these CQDs was highly pH-sensitive, i.e., the PL intensity decreased with increasing pH from pH 2 to 12. In addition, the pH response of the PL behaviour was reversible. This property endows them the potential to serve as proton sensors in monitoring cell metabolization processes with proton release. When incubated with HeLa cells, the CQDs could readily penetrate the cell membrane and exhibit low cytotoxicity and favorable biocompatibility, which is essential for HeLa cell imaging.
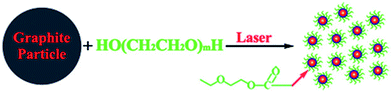 | ||
| Fig. 3 A one-step synthesis of CQDs in PEG200N solvent via laser irradiation. Adapted with permission.23 Copyright 2009, Royal Society of Chemistry. | ||
Solvothermal carbonization followed by extraction with an organic solvent is a popular approach to prepare CQDs.40,41 Typically, carbon-yielding compounds were subjected to heat treatment in high boiling point organic solvents, followed by extraction and concentration. Bhunia et al. synthesized two kinds of the CQDs, hydrophobic and hydrophilic with diameters less than 10 nm from the carbonization of carbohydrates.40 The hydrophobic ones were produced by mixing different amounts of carbohydrate with octadecylamine and octadecene before being heated up to 70–300 °C for 10–30 minutes. The hydrophilic ones can be synthesized by heating an aqueous solution of carbohydrate within wide pH ranges. The hydrophilic CQDs with yellow and red emissions can also be synthesized by mixing an aqueous solution of carbohydrate with concentrated phosphoric acid followed with heating at 80–90 °C for 60 min (Fig. 4).
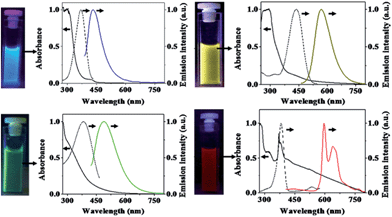 | ||
| Fig. 4 Digital images of CQDs solutions under appropriate excitations and their absorption (solid black lines), excitation (dashed black lines) and emission (color lines) spectra. Emission spectra were measured by excitation at 370 nm, 400 nm, 425 nm, and 385 nm for CQD – blue, CQD – green, CQD – yellow, and CQD – red, respectively. All excitation spectra were recorded in respective emission maxima. Adapted with permission.40 Copyright 2013, Nature Publishing Group. | ||
2.2. Size control-confined pyrolysis
For particular applications and mechanistic study, it is important to control the sizes of CQDs to get uniform properties. Many approaches have been proposed to obtain uniform CQDs during preparation or post-treatment. In most of the reports, the as-synthesized CQDs fragments were purified via post-treatments like filtration, dialysis, centrifugation, column chromatography and gel-electrophoresis. It is of great importance to control the size during the preparation process. Discrete CQDs with tunable and uniform sizes can be prepared via confined pyrolysis of an organic precursor in nanoreactors (Fig. 5). Three steps were used as follows: (i) absorbing the organic precursor into porous nanoreactors via capillary force, (ii) pyrolysis of the organic precursor confined in the nanoreactors into carbonaceous matter, (iii) release of the as-synthesized CQDs by removing the nanoreactors. The size and size-distribution of the CQDs produced from this method are dictated by the texture parameters of the nanoreactors.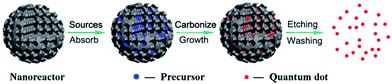 | ||
| Fig. 5 Schematic illustration of the preparation of CQDs via confined pyrolysis of an organic precursor in nanoreactors. Adapted with permission.5 Copyright 2012, Royal Society of Chemistry. | ||
Porous silicas are the most widely used nanoreactors for their various, tunable and easily obtained textures, thermal stability and easy removal.43–45 Zhu et al. synthesized hydrophilic CQDs with mesoporous silica nanospheres as nanoreactors by impregnation of a citric acid precursor (Fig. 5).5,45 After pyrolysis at 300 °C for 2 hours in air, followed by silica removal and dialysis, CQDs with a uniform size of 1.5–2.5 nm were prepared and showed good photostability, low toxicity, excellent luminescence, and up-conversion properties.
Polymeric core–shell nanoparticles are also effective nanoreactors with thermally cross-linkable core and thermally removable shell.42,46,47 Recently, we prepared CQDs via pyrolysis of PAN@PMMA core–shell nanoparticles prepared using a one-pot micro-emulsion polymerization process (Fig. 6).42 The core PAN domain was crosslinked and chipped at 270 °C under air with protection from the shell PMMA domain. Furthermore, elevating the temperature caused the carbonization of the PAN fragments and decomposition of the PMMA domains to release the N-doped CQDs. The prepared CQDs showed dual emission at about 410 nm and 450 nm and stable PL in moderate pH solutions, which is essential for bioimaging.
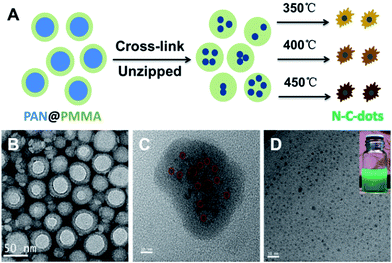 | ||
| Fig. 6 Scheme of preparing CQDs from polymeric nanoparticles (A) and the TEM images of the core–shell polymeric nanoparticles (B), core cross-linked polymeric nanoparticles (C) and final CQDs (D). The digital image of the CQDs solution is shown in the inset. Adapted with permission.42 Copyright 2013, Royal Society of Chemistry. | ||
Thermally unstable polymers are also used as block matter to avoid carbonaceous aggregation during thermal treatment, especially when the carbon-yielding domain is a cross-linkable polymer.8,48 We prepared well-defined CQDs with tunable and uniform sizes from single-chain polymeric nanoparticles, which were formed using a Bergman cyclization-intermediated chain collapse49–51 of linear polymers containing enediyne units (Fig. 7).8 The polynaphthylene-containing polymeric nanoparticles formed were transformed to CQDs via a bijective way (one-to-one correspondence). The sizes of the final CQDs can be easily tuned by changing the length of the polymeric chains or the grafting degrees of the enediyne in each chain. The prepared CQDs showed size-dependant luminescent properties and the PL peak blue-shifted with an increase of size. The PL mechanism of CQDs was also investigated based on experimental results and theoretical simulation, and it will be discussed in detail in Section 3.2.
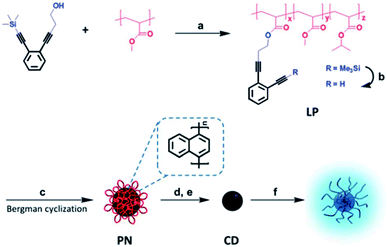 | ||
| Fig. 7 Preparation of soluble CQDs with tunable sizes from single-chain polymeric nanoparticles. Adapted with permission.8 Copyright 2013, Royal Society of Chemistry. | ||
2.3. Surface functionalization
Surface modification is a powerful method to tune the surface properties of materials for selected applications. There are many approaches for functionalizing the surface of CQDs through the surface chemistry or interactions, such as covalent bonding,36,52–54 coordination,55 π–π interactions,56 and sol–gel technology.57,58The majority of CQDs are rich in oxygen-containing groups, which endows them with feasibility in covalent bonding. Surface passivation via covalent bonding of amine-containing agents is a common method to improve the PL of CQDs, which showed an important influence on the properties of CQDs. Fluorescent CQDs with diameters of about 3 nm emitting blue-green light were synthesized using a hydrothermal carbonization of 2Na·EDTA. Then, the CQDs were functionalized with spiropyrans to obtain surface-functionalized CQDs. The emission of the functionalized CQDs centered at 510 nm could be switched off, while being turned on at 650 nm via energy transfer between the CQDs and spiropyrans after irradiation with ultraviolet (UV) light (Fig. 8).59 The process could be reversed using irradiation with visible light. The functionalized CQDs show excellent photo-reversibility and high stability.
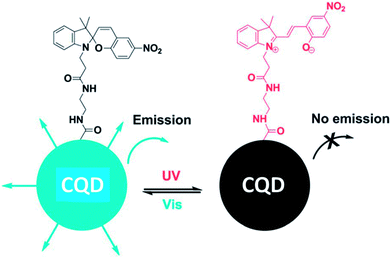 | ||
| Fig. 8 Schematic illustration of the light-induced fluorescence modulation of spiropyran-functionalized CQDs. Adapted with permission.59 Copyright 2013, Royal Society of Chemistry. | ||
In addition to covalent bonding to CQDs, coordination is another useful strategy. A simple method for phosphate (Pi) detection (Fig. 9) was established by developing an off-on fluorescence probe using europium-adjusted CQDs, which was successfully applied to the detection of Pi in complicated matrixes such as an artificial wetlands system.55 When the surface carboxyl groups on the CQDs were coordinated with Eu3+, the fluorescence of the CQDs was turned off. The fluorescence can, however, be switched on when Eu3+ was specifically coordinated with Pi.
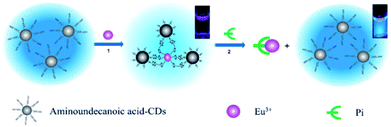 | ||
| Fig. 9 Schematic representation of Pi detection based on the off-on fluorescence probe of CQDs adjusted by Eu3+. Adapted with permission.55 Copyright 2011, Royal Society of Chemistry. | ||
The Sol–gel technique is also a promising approach for decorating the surface of CQDs with functional molecules. Liu et al. reported a method to synthesize highly luminescent (QY = 47%) amorphous CQDs in one minute using organo-silane as a coordinating solvent.57 The CQDs, benefited from the surface methoxysilyl groups, have a diameter of ∼0.9 nm and can easily be fabricated into pure CQDs fluorescent films or monoliths by simply heating at 80 °C for 24 h. Moreover, the water-insoluble CQDs can be further transformed into water-soluble CQDs/silica particles with good biocompatibility and low toxicity. CQDs@MIP (molecularly imprinted polymer) was synthesized by a one-pot, room-temperature, sol–gel polymerization and was applied as a fluorescence sensor for the detection of dopamine in an aqueous solution.58
2.4. CQDs doping
Doping is a widely used approach to tune the PL properties of photoluminescent materials. Various doping methods with dozens of elements such as N,35,36,41,42,60–62 S,63,64 and P65 have been reported to tune the properties of CQDs.N-doping is the most studied way to enhance the emission of the CQDs by inducing an upward shift in the Fermi level and electrons in the conduction band.66 It was demonstrated that only the nitrogen bonding to carbon can really enhance the PL emission of CQDs. The N-CQDs show nitrogen content-dependent PL intensities with multicolor and two-photon up-conversion properties.29,41 A Mg/N co-doping strategy to fabricate highly luminescent CQDs with QY of 83% was studied by Liu et al. (Fig. 10).67 After hydrothermal treatment of a homogeneous solution containing citric acid and Mg(OH)2 at 200 °C for 3 h, the raw products were treated by filtration, dialysis and lyophilization to obtain CQDs denoted as Mg-CQDs. The Mg-citric acid chelate in the carbon source was utilized to introduce Mg and preserve the majority of the carboxyl groups, which together with the N-passivation contributed to a dramatic increase in the PL enhancement of the final CQDs.
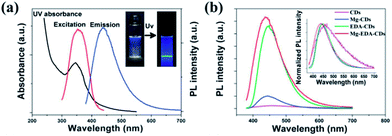 | ||
| Fig. 10 (a) UV-Vis and PL spectra (λex = 360 nm) of the Mg–EDA-CQDs in an aqueous solution (insets: photographs under natural light and UV light). (b) PL spectra (λex = 360 nm) of CQDs with different doping strategies. Adapted with permission.67 Copyright 2014, Royal Society of Chemistry. | ||
P- and N-co-doped CQDs were synthesized via microwave irradiation of DMF in the presence of phosphoric acid.65 These CQDs exhibited excellent photoluminescent characteristics with high electrocatalytic activity and good tolerance to the methanol crossover effects in the oxygen reduction reaction (ORR) in an alkaline medium due to heteroatom functionalization. Sulfur- and nitrogen-co-doped CQDs (S–N-CQDs) were synthesized on a large-scale using sulfuric acid to carbonize and etch hair fiber.63 It was found that S and N can form different binding configurations in the S–N-CQDs framework, such as a –C–S– covalent bond of the thiophene-S and –C–SOx– (x = 2, 3, 4, sulfate or sulfonate) for S-doped, pyridinic N and pyrrolic N for N-doped, respectively. Moreover, a higher reaction temperature leads to the formation of S–N-CQDs with smaller size, higher S content, and longer wavelength of photoluminescent emissions. The resultant S–N-CQDs also exhibited good luminescence stability, low toxicity, excellent biocompatibility, and high solubility.
2.5. CQDs nanohybrids
More recently, many efforts have been focused on the preparation of novel hybrids comprised of CQDs and inorganic nanoparticle cores (e.g., iron oxide,68 zinc oxide, silica,21 and titania21,69). The resultant hybrids integrate the fluorescence properties of the CQDs with the magnetic, optical or mechanical properties of the oxide cores. Such hybrids hold great promise as magneto-optical biolabeling agents or efficient photocatalysts.TiO2/CQDs composites were synthesized in situ from bidentate TiO2/vitamin-C (VC) complexes via a hydrothermal method.69 The effects of the amounts of VC, hydrothermal temperatures and reaction times were explored for the H2 generation from photocatalytic water splitting catalyzed by the TiO2/CQDs nanohybrids. The TiO2-NPs/CQDs nanohybrids obtained at 200 °C for 2 h with a VC amount of 0.001 g showed a 9.7-fold higher H2 production rate than bare TiO2 NPs and showed good cycling performance (Fig. 11). The TiO2-NWs/CQDs nanohybrids prepared at 90 °C for 4 h also produced hydrogen at a rate of 1189.7 μmol g−1 h−1, which is 4.2 times higher than that of bare TiO2 NWs. The superior photocatalytic performance of these nanohybrids may be attributed to the synergetic effects of the hydrothermal treatment along with the favourable electron transfer ability and up conversion of the CQDs.
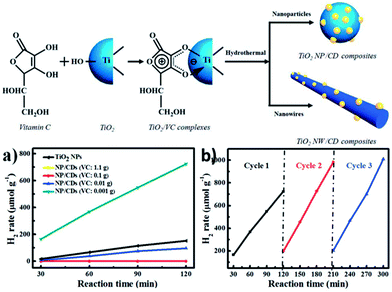 | ||
| Fig. 11 (Up) Schematic illustration of the in situ hydrothermal synthesis of TiO2/CQD nanohybrids. (Down) (a) Time course of H2 production from TiO2 NPs and NP/CQDs composites synthesized at different VC amounts: 1.1, 0.1, 0.01 and 0.001 g. (b) A typical time course of H2 production from NP/CQDs composites for 3 cycles. Adapted with permission.69 Copyright 2014, Royal Society of Chemistry. | ||
Au NPs were used to enhance the fluorescence of CQDs by forming Au-PAMAM-CQD conjugates using an amidation reaction.70 In this process, PAMAM serves as a spacer of a suitable size to keep the Au NPs and CQDs at an appropriate distance from one another for PL enhancement. Both the amount of Au NPs and CQDs can influence the fluorescence enhancement. An appropriate amount of Au NPs and CQDs linked to PAMAM leads to an optimum fluorescence enhancement. The PL intensity of CQDs can be enhanced as much as 62-fold by conjugating Au NPs and CQDs with an optimized molar ratio to PAMAM, which is desirable for many applications.
3. Properties and luminescent mechanism
3.1. Optical properties
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, the n–π* transition of C
C bonds, the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and/or others.
O bonds and/or others.
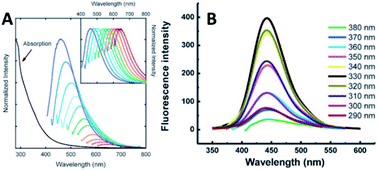 | ||
| Fig. 12 (A) Absorbance and PL spectra with increasingly longer excitation wavelengths (in 20 nm increments starting from 400 nm) of 5 nm PPEI-EI CQDs in an aqueous solution formed using laser ablation methods (inset shows the normalized PL spectra). Adapted with permission.2 Copyright 2006, American Chemical Society. (B) PL of 1.9 nm CQDs at different excitation wavelengths of 290–380 nm. Adapted with permission.19 Copyright 2008, Royal Society of Chemistry. | ||
The PL properties of the CQDs can be tuned via modification as described in Section 2 or via electron/energy transfer as demonstrated in Fig. 8 and Section 3.1.7.
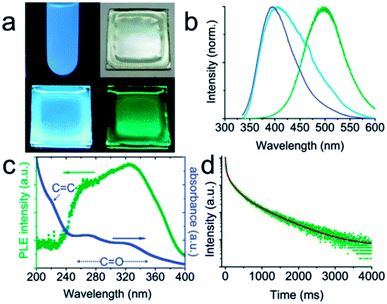 | ||
| Fig. 13 (a and b) Digital photographs and the corresponding spectra of CQDs: dispersed in water under UV light (a: upper left; b: blue line); dispersed in a PVA matrix under daylight (a: upper right), UV light (a: lower left; b: cyan line) and right after UV light has been turned off (a: lower right; b: olive line). The UV excitation for the photographs is 365 nm, while that for the spectra is 325 nm. (c) Phosphorescence excitation spectrum (olive dots) and absorption spectrum (blue dots) of CQDs dispersed in water. (d) Time-resolved phosphorescence spectrum. Adapted with permission.72 Copyright 2013, Royal Society of Chemistry. | ||
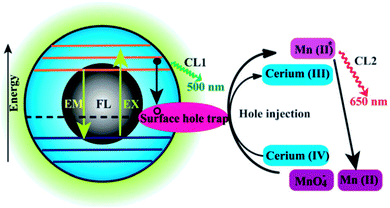 | ||
| Fig. 14 Schematic illustration of the FL and CL mechanisms in the CQDs–KMnO4 and CQDs–cerium(IV) systems. CL1 and CL2 represent two CL routes in the system. Adapted with permission.73 Copyright 2012, Royal Society of Chemistry. | ||
A novel CL phenomenon was also observed for the as-prepared CQDs in a strong alkaline solution (NaOH or KOH). The CQDs exhibited excellent electron donor ability towards dissolved oxygen to form the superoxide anion radical (O2˙−) in a solution of NaOH. These results directly provided evidence for the excellent electron-donating ability of CQDs.75,76 Radiative recombination of the injected electrons by “chemical reduction” of the CQDs and thermally excited generated holes was suggested to account for the CL behaviour in strong alkaline solutions.76
The CL of CQDs opens up new opportunities for their potential in the determination of reductive substances.77 The dual role of CQDs as an electron donor and acceptor offers great potential in optronics and catalysis as described in Section 4.2 and 4.3.
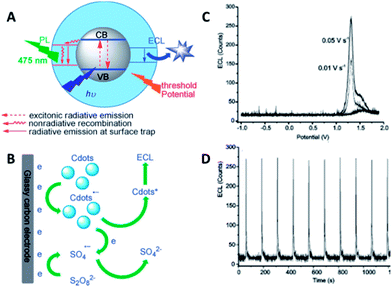 | ||
| Fig. 15 (A) Schematic illustration of the PL and ECL emissions of CQDs. (B) The mechanism of ECL in the o-CQDs/K2S2O8 system. (C) ECL profiles of o-CQDs at a scan rate of 0.01 and 0.05 V s−1. (D) The reproducibility of ECL in a continuous scan mode. Adapted with permission.78 Copyright 2013, Wiley-VCH. | ||
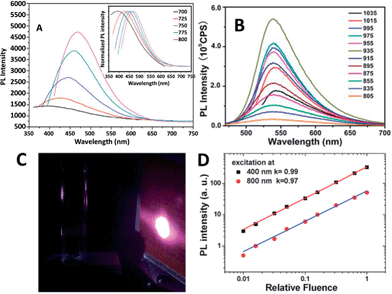 | ||
| Fig. 16 (A) UCPL spectra of the CQDs dispersed in water at excitation wavelengths progressively increasing from 700 nm in 25 nm increments. Inset: the corresponding normalized PL spectra. Adapted with permission.45 Copyright 2011, Royal Society of Chemistry. (B) UCPL properties of CQDs dispersed in water at excitation wavelengths from 805 nm to 1035 nm. Adapted with permission.79 Copyright 2012, Royal Society of Chemistry. (C) The photographs of the CQD solution excited at 800 nm with 100 mW laser (the bright spot is laser on an infrared detection paper). (D) Excitation intensity dependence of fluorescence with excitation at 400 nm (black squares) and 800 nm (red circles) in a fluorescence spectrophotometer. Adapted with permission.80 Copyright 2014, Royal Society of Chemistry. | ||
The UCPL of CQDs is a fascinating feature, however, very recently, a UCPL study on five differently synthesized CQDs and GQDs demonstrated that the CQDs and GQDs did not exhibit observable UCPL.80 Under the experimental conditions reported earlier, so-called UCPL in five differently synthesized CQDs and GQDs in a commercial fluorescence spectrophotometer was observed. However, the UCPL actually originates from the normal fluorescence excited by the leaking component from the second diffraction in the monochromator of the fluorescence spectrophotometer (Fig. 16C). The leaking component and thus UCPL can be eliminated by adding a suitable long-pass filter in the excitation pathway of a fluorescence spectrophotometer. Intensity dependent experiments clearly confirmed that the so-called UCPL is actually the normal fluorescence with linear response rather than a multiple phonon process (Fig. 16D). These experiments suggested that most of the CQDs and GQDs may not have detectable UCPL. Note that it is necessary to eliminate the normal fluorescence and measure the excitation intensity dependence of the fluorescence when observing UCPL of CQDs.
Sun et al. found that the PL from a CQDs solution could be efficiently quenched in the presence of either electron acceptors such as 4-nitrotoluene and 2,4-dinitrotoluene or electron donors such as N,N-diethylaniline.81 Namely, the photoexcited CQDs are excellent as both electron donors and electron acceptors. They also found efficient PL quenching in CQDs by surface-doped metals through disrupting the excited state redox processes.82 Electron transfer in nanocomposites of CQDs–GO, CQDs–MWNTs and CQDs–TiO2 NPs without linker molecules was also studied.83 Significant PL quenching was observed in the CQD–GO system, which was attributed to the ultrafast electron transfer from CQDs to GO with a time constant of 400 fs. In comparison, addition of carbon nanotubes resulted in static quenching of fluorescence in CQDs. No charge transfer was observed in either CQD–MWNT or CQD–TiO2 nanocomposites. These interesting PET properties of CQDs as an electron donor/acceptor may offer new opportunities for light-energy conversion, catalysis and related applications, as well as mechanistic elucidation.
3.2. PL mechanism
Although there have been many efforts focused on the physicochemical properties of CQDs, the origin of the observed optoelectronic behaviour is a topic of discussion to date.The origin of the PL of CQDs has been assigned to several reasons in the literature: optical selection of differently sized nanoparticles (quantum effect),3,21 defects and surface states,23 surface groups,84 surface passivation,3 fluorophores with different degrees of π-conjugation,85,86 and the recombination of electron–hole pairs localized within small sp2 carbon clusters embedded within a sp3 matrix.87
A systematic investigation on the formation mechanism of the CQDs prepared from pyrolysis of citric acid (CA)–ethanolamine (EA) precursor at different temperatures was presented recently.62 Pyrolysis at 180 °C leads to a CQD precursor with an intense PL and high QY formed by the dehydration of CA–EA. At higher temperatures (230 °C) a carbonaceous core starts to form. The PL at this stage is contributed from the presence of both molecular fluorophores and the carbonaceous core (Fig. 17). CQDs that exhibit mostly or exclusively PL arising from carbonaceous cores were obtained at even higher temperatures (300 and 400 °C, respectively). Since the molecular fluorophores predominate at low pyrolysis temperatures while the carbonaceous core starts forming at higher temperatures, the PL behaviour of CQDs strongly depends on the conditions used in their synthesis.
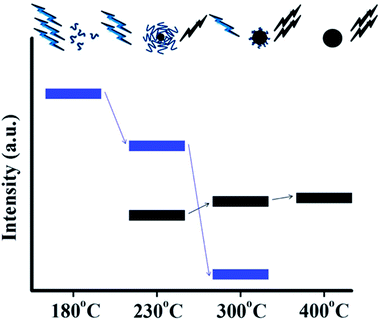 | ||
| Fig. 17 Schematic representation of the emission characteristics of three photoactive species produced from the thermal treatment of a mixture of CA and EA. During pyrolysis, the organic fluorophores (blue groups) are consumed for the build-up of the carbonaceous core (black sphere) so that the PL component, which corresponds to the carbonaceous core (black bars), increases at the expense of the component that arises from the organic fluorophores (blue bars). Adapted with permission.62 Copyright 2011, American Chemical Society. | ||
Multiple fluorescence intensity levels of individual CQDs were observed recently.88 Imaging of single CQDs at different excitation energies revealed significant heterogeneity in the lower energy trap sites between particles. It is also found that individual CQDs exhibit single-step photobleaching and transient blinking to the background level suggesting single-molecular behaviour. These observations suggest the possibility that single CQDs can possess multiple chromophoric units associated with the CQDs core and oxygenated defect-related emissive traps. Interestingly, the majority of the reduced CQDs showed multiple levels, while the oxidized CQDs predominantly showed a single level. A possible reason for this is that after the initial excitation the energy is transferred from the higher energy absorbing site to a lower energy emissive site in the oxidized CQDs. In contrast, the low-energy emissive traps were entirely or partially removed when CQDs are reduced, blocking the energy-transfer pathways. Consequently, the emission for reduced CQDs is likely to be from the originally excited chromophores. The presence of the emissive traps or quenching states would likely be dependent on the synthetic methods and the post-treatment of CQDs.
Fig. 18 shows the optical images of CQDs of four typical sizes, illuminated by white and UV light, and the corresponding emission spectra from left to right.21 The PL properties vary sensitively with the size of the CQDs (Fig. 18c), and with increasing size, the emissions were red-shifted. The theoretical calculations (Fig. 18d) showed the dependence of the HOMO–LUMO gap on the size of the graphene fragments. As the size of the fragment increases, the gap decreases gradually, and the gap energy in the visible spectral range was obtained from graphene fragments with a diameter of 14–22 Å, which agrees well with the visible emission of CQDs with diameters of <3 nm. Thus, it is deduced that the strong emission of CQDs comes from the quantum-sized graphite structure instead of the carbon–oxygen surface.
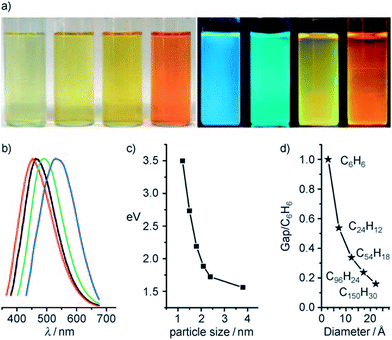 | ||
| Fig. 18 (a) Typically sized CQDs optical images illuminated under white (left; daylight lamp) and UV light (right; 365 nm); (b) PL spectra of typically sized CQDs: the red, black, green, and blue lines are the PL spectra for blue-, green-, yellow-, and red-emission CQDs, respectively; (c) the relationship between the CQDs size and PL properties; (d) HOMO–LUMO gap dependence on the size of the graphene fragments. Adapted with permission.21 Copyright 2010, Wiley-VCH. | ||
We recently prepared soluble CQDs with tunable sizes from single-chain polymeric nanoparticles (Fig. 7).8 PL study shows that the optimal emission wavelength of CQDs is red-shifted when the size of CQDs decreases, which is different from the trends typically found in semiconductor quantum dots and CQDs prepared from graphitized materials.21 To clarify the PL mechanism of CQDs prepared from different sources, a theoretical study based on density functional theory was performed. Two series of model compounds, fused aromatic rings and cyclo-1,4-naphthylenes, were chosen for CQDs with different microstructures. The calculation data indicated that the PL energy of CQDs is dictated by the size and microstructure of the sp2 carbon core. For CQDs with a graphitized core (Class I), the smaller the size of the core (Fig. 18c and d), the higher the PL energy, while for CQDs with an amorphous core (Class II), an inverse trend is observed (Fig. 19). CQDs exhibit a higher PL intensity after surface reduction but no obvious emission shift, which further shows that the QY of CQDs is controlled by the surface chemistry.89
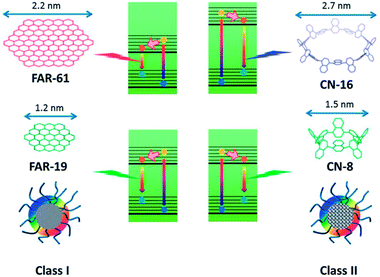 | ||
| Fig. 19 Schematic illustration of the PL mechanism of Class I and Class II CQDs. Adapted with permission.8 Copyright 2013, Royal Society of Chemistry. | ||
CQDs synthesized by electrochemical ablation and small molecule carbonization, as well as GQDs fabricated by solvothermally cutting graphene oxide, are three typical green fluorescence carbon-based quantum dots. Ultrafast spectroscopy was used to investigate the PL origin in these fluorescent carbon-based quantum dots. According to the change of surface functional groups during chemical reduction and the obvious emission-type transformation, these green luminescence emissions are unambiguously assigned to special edge states consisting of several carbon atoms on the edge of carbon backbone and functional groups with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality (carbonyl and carboxyl groups).90 These findings suggest that the competition among various emission centers (bright edge states) and traps dominate the optical properties of fluorescent carbon-based quantum dots.
O functionality (carbonyl and carboxyl groups).90 These findings suggest that the competition among various emission centers (bright edge states) and traps dominate the optical properties of fluorescent carbon-based quantum dots.
3.3. Biological properties
Impressive progress has been made in engineering bright CQDs bioprobes with good stability. However, biocompatibility of the functionalized CQDs is still a critical issue for further applications in live cells, tissues, and animals. Systematic cytotoxicity evaluations were carried out on both raw CQDs and passivated CQDs during the last few years. Sun's group employed CQDs produced by the arc-discharge of graphite rods, and then refluxed in HNO3 for 12 h for cytotoxicity assay. The bare CQDs were apparently nontoxic to cells up to a relatively high concentration of 0.4 mg mL−1. Luminescent CQDs synthesized by the electrochemical treatment of graphite were also evaluated in terms of cytotoxicity assay using a human kidney cell line, in which the cell viability was not affected by the dots.19 Furthermore, Ray et al. improved a soot-based approach for CQDs synthesis with diameters of 26 nm.91 The experimental results of cell viability also confirmed that the CQDs showed negligible cytotoxicity at concentrations required for fluorescence bioimaging.The cytotoxicity of the CQDs passivated with functional groups, such as PEG,26 PPEI-EI,25 PEI,92 BPEI (branched poly(ethylenimine)),93 and PAA (poly(acrylic acid)),94 were also evaluated in cytotoxicity assays. The PEGylated CQDs in all available configurations27,95 were non-cytotoxic up to concentrations much higher than that is necessary for cell imaging and related applications. In addition, CQDs functionalized with PEG1500N were injected into mice for toxicity evaluation up to 28 days, and the results suggested no significant toxic effects in vivo.27 Moreover, experimental results indicated that the PPEI-EI-passivated CQDs were mostly nontoxic to the cells below a relatively high threshold of carbon core-equivalent PPEI-EI concentration.25 According to the MTT assay, a PEI free sample was apparently nontoxic to HT-29 cells even at relatively high concentrations. However, the PEI-functionalized CQDs were more cytotoxic than PPEI-EI-functionalized CQDs. The more ethylenimine (EI) units within the PEI may be associated with the lower concentration thresholds for the CQDs to become cytotoxic, as PEI is the homopolymer corresponding to PPEI-EI with an extreme EI fraction of 100%. Free PAA in a nonaqueous solution was found to be harmful to cells even at relatively low concentrations (50 μg mL−1). The PAA-functionalized CQDs were generally comparable to free PAA at the same CQD core-equivalent concentrations, both were toxic to the cells with an exposure time of 24 h, but less so when the exposure time was shortened to 4 h. Overall, molecules with low cytotoxicity even at high concentrations such as PEG and PPEI-EI are suitable for CQDs functionalization for in vivo imaging and biosensing. Molecules with higher cytotoxicity including BPEI and PAA, can still be used to functionalize CQDs used in vivo if their concentrations are maintained low enough and the incubation time short enough.92
4. Applications
4.1. Biomedicine
The pioneering work on CQDs for bioimaging in vitro2 and in vivo26 was reported by Sun's group. Confocal microscopy images of E. coli ATCC 25922 labeled with the PEGylated CQDs were obtained at different excitation wavelengths. Yang et al. were the first to explore the feasibility of CQDs as a fluorescence contrast agent in mice.27 In the experiments, PEGylated CQDs in an aqueous solution were injected subcutaneously into mice, and the fluorescence images at different excitation wavelengths collected. There was sufficient contrast for the imaging in both green and red emissions.26 Tao et al. applied the same protocol to nude mice and obtained similar results.101 More specifically, an aqueous solution of CQDs was injected subcutaneously into mice, followed by fluorescence imaging with excitations at seven different wavelengths from 455 nm to 704 nm. The best fluorescence contrast was obtained at an excitation of 595 nm (Fig. 20).
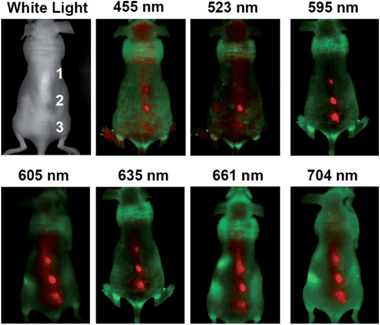 | ||
| Fig. 20 In vivo fluorescence images of a CQDs-injected mice. The images were taken at various excitation wavelengths. Red and green represent fluorescent signals of the CQDs and the tissue autofluorescence, respectively. Adapted with permission.101 Copyright 2012, Wiley-VCH. | ||
It is attractive to integrate multi-imaging technology for one agent for comprehensive understanding of the state of the illness. Most popular nanostructured multimodal imaging probes are combinations of magnetic resonance imaging (MRI) and optical imaging modalities. MRI can offer high spatial resolution and the capacity to simultaneously obtain physiological and anatomical information, whereas optical imaging allows for rapid screening.102 Zboril et al. reported the synthesis of ultrafine Gd(III)-doped CQDs with a dual fluorescence/MRI character through the thermal decomposition of a precursor composed of an organic salt and a gadolinium(III) complex (Fig. 21).97 The dots were water-dispersible, displaying bright fluorescence in the visible range upon light excitation, showing strong T1-weighted MRI contrast comparable to commercial Gadovist and possessing low cytotoxicity. In our opinion, it is possible to combine a common T1 MRI agent with CQDs via robust covalent bonding, which can increase the rotational correlation time (τR) of the T1-imaging probes.103,104
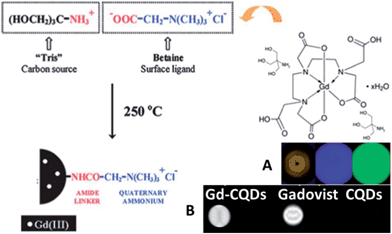 | ||
| Fig. 21 Synthetic scheme for Gd-CQDs. (A) A brown aqueous dispersion of Gd-CQDs displays bright blue and green emission under 360–370 nm and 460–495 nm irradiation, respectively, (B) MRI positive contrast effects in T1-weighted images of Gd-CQDs, the commercial Gd-based contrast agent, i.e., Gadovist (both samples were diluted in water with the same concentration of Gd), and undoped CQDs. All images were obtained using a 1.5 T clinical MRI tomograph. Adapted with permission.97 Copyright 2012, Royal Society of Chemistry. | ||
Srivastava et al. fabricated iron oxide-doped CQDs (IO-CQDs) for multi-modality (MR/fluorescence) bioimaging.105 The IO-CQDs were prepared using the thermal decomposition of organic precursors in the presence of small Fe3O4 nanoparticles (with an average size of 6 nm). The IO-CQDs could be taken by RAW 264.7 cells, and the fluorescence was mainly detected in the cell cytoplasm. For in vivo imaging, the IO-CQDs were introduced into rats through intravenous injection. Fluorescence signals due to the IO-CQDs were observed in the spleen slide samples. The MRI results suggested enhanced signals in the brain blood vessel under both T1 and T2 models. It is also possible to combine other imaging technologies with the fluorescent imaging of CQDs for multi-modal bioimaging due to the biocompatibility of CQDs.
CQDs can be used as an effective fluorescent sensing platform for nucleic acid detection with selectivity single-base mismatch. The general concept was based on the adsorption of the fluorescently labeled single-stranded DNA (ssDNA) probe by CQD via π–π interactions, which is accompanied by substantial fluorescence quenching, followed by specific hybridization with its target to form double-stranded DNA (dsDNA).56 This results in desorption of the hybridized dsDNA from the CQD surface accompanied with subsequent recovery of fluorescence, probing the target DNA (Fig. 22).
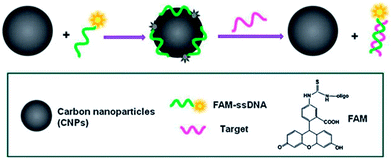 | ||
| Fig. 22 A schematic (not to scale) illustrating the CQD-based fluorescent nucleic acid detection. Adapted with permission.56 Copyright 2011, Royal Society of Chemistry. | ||
A robust and multifunctional CQD-based fluorescence resonance energy transfer (FRET) probe for detecting and imaging mitochondrial H2O2 was demonstrated. The CQDs serve as the donor of energy transfer and the carrier for the sensing system. A boronate-based H2O2 recognition element, boronate-protected fluorescein (Fig. 23), was covalently linked onto the CQDs.110 It can be used for tracking the exogenous H2O2 levels in L929 cells, and can also be used to visualize the endogenously produced H2O2 in RAW 264.7 macrophage cells.
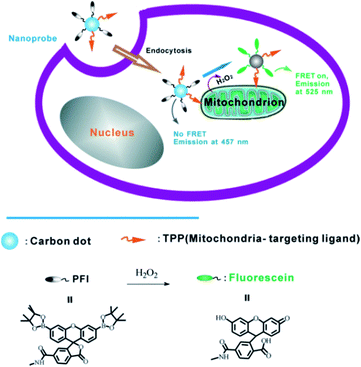 | ||
| Fig. 23 Schematic illustration of FRET-based ratiometric sensing of mitochondrial H2O2 in living cells by the nanoprobe. Adapted with permission.110 Copyright 2014, Wiley-VCH. | ||
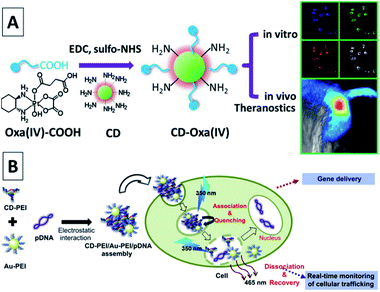 | ||
| Fig. 24 (A) Synthetic scheme for CD-Oxa and its applications in bioimaging and theranostics. Adapted with permission.53 Copyright 2014, Wiley-VCH. (B) A schematic illustration for the gene delivery and real-time monitoring of cellular trafficking utilizing CD-PEI/Au-PEI/pDNA assembled nanohybrids. Adapted with permission.112 Copyright 2013, Elsevier. | ||
Kim et al. coupled CQDs with gold nanoparticles for an assembly, which was then conjugated with PEI–pDNA for delivering DNA to cells.112 Note that fluorescence emissions from the assembly of CQD-gold nanoparticles could be quenched by pDNA; thus, the release of pDNA could be probed by the recovery of the fluorescence signals. The experimental results suggested that the assembly entered the cells with the CQDs located in the cell cytoplasm and the released pDNA entered the cell nuclei, achieving significant transfection efficiency (Fig. 24B). Pandey et al. used CQDs functionalized gold nanorods for the delivery of doxorubicin in a multi-modality fashion, including drug delivery, photothermal therapy, and bioimaging using the same platform.113 The widely used anti-psychotic drug haloperidol (HaLO)-grafted CQDs with cysteamine hydrochloride (CysHCl) as a linker can offer controlled release under physiological conditions for more than 40 h following the Hixson–Crowell model under standardized conditions.114 A broad spectrum antibiotic, ciprofloxacin attached to CQDs with bright green fluorescence can not only pave a way for bioimaging but also provide an efficient new nanocarrier for controlled drug release with high antimicrobial activity under physiological conditions.115
4.2. Optronics
After the first attempt by Ozin, who introduced CQDs as a sensitizer to capture sunlight in DSCs,116 much research work has been performed to use this emergent nanolight to improve the performance of DSCs. As the charge recombination of photogenerated electrons in the porous electrode with either the oxidized dye or the electrolyte will reduce the efficiency of DSCs. Inspired by natural photosystems, Lee et al. developed a CQD-bridged dye/semiconductor complex system for the fabrication of highly efficient photoelectric conversion systems. CQDs not only enhanced the UV-vis absorbance of rhodamine B (RhB) solutions due to the synergistic hyperchromic effect between RhB and CQDs (Fig. 25), but also effectively suppressed the recombination of photogenerated electrons, thereby leading to a significantly enhanced photoelectric conversion efficiency.117 Doping of CQDs into the dye/semiconductor complex significantly improved the photoelectric conversion efficiency of the complex by ∼7 times.
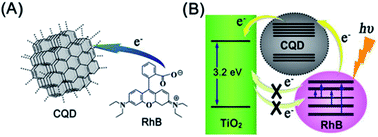 | ||
| Fig. 25 (A) Scheme of electron transfer from RhB to CQDs. (B) CQDs serve as an electron transfer intermediary for bridging the photogenerated electrons and suppressing their recombination. Adapted with permission.117 Copyright 2013, American Chemical Society. | ||
 | ||
| Fig. 26 (A) Schematic architecture of the BHJ solar cell with LDS layer. (B) UV-vis absorption and PL spectra of the CQDs filled polysiloxane composite (∼20 μm thick) coated on glass. (C) Wavelength dependences of external quantum efficiency of the P3HT:PCBM-based solar cell with and without the LDS layer. Adapted with permission.71 Copyright 2014, Elsevier. | ||
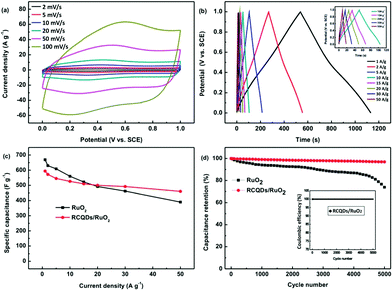 | ||
| Fig. 27 (a) Cyclic voltammograms of RCQD/RuO2 at different scan rates. (b) Charge–discharge curves of RCQD/RuO2 at different current densities, and the inset shows a magnification at current densities of 10–50 A g−1. (c) Comparison of the specific capacitance change of RuO2 and RCQD/RuO2 as a function of current density. (d) Comparison of the cycling stability of the RuO2 and RCQD/RuO2 at a current density of 5 A g−1, and the inset shows the coulombic efficiency of RCQD/RuO2. Adapted with permission.118 Copyright 2013, Royal Society of Chemistry. | ||
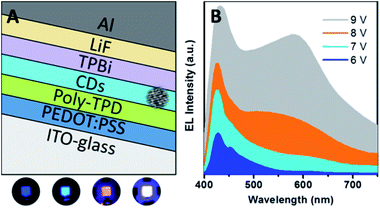 | ||
| Fig. 28 (A) Sandwiched structure of LEDs using CQDs as a single emitting layer. (B) Electroluminescence (EL) spectra and true colour photographs of the blue, cyan, magenta, and white emissions. Adapted with permission.121 Copyright 2013, American Chemical Society. | ||
4.3. Catalysis
It is expected to utilize the NIR region of sunlight for its large share of sun's energy. CQDs with the UCPL properties can improve the PEC properties of CQD/CdSe/TiO2 NR photoanodes at the NIR region (over 750 nm) (Fig. 29).126 The one-dimensional ordered TiO2/CdSe core/shell heterostructural network (Fig. 29A) not only provides a large surface area for efficient loading of up-conversion CQDs, but also allows an excellent interfacial chemical reaction between the CdSe and electrolyte. Subsequently, CQDs were electrodeposited on the surface of the TiO2/CdSe core/shell NRs to construct a CQD/CdSe/TiO2 composite photoanode. Fig. 29B is the schematic diagram illustrating the whole PEC process under near-IR illumination. The CQDs can absorb near-IR photons and emit visible photons through the up-conversion effect to excite CdSe. Subsequently, the excited electrons in the conduction band of CdSe would rapidly inject into the conduction band of TiO2, and then transfer along the axial direction of the TiO2 NRs to the FTO substrate. The electrons would finally be transferred to the Pt counter electrode under the assistance of a little external bias voltage, and then drive the hydrogen evolution reaction. The holes remaining in the valence band of CdSe would be consumed by the sacrificial agent.
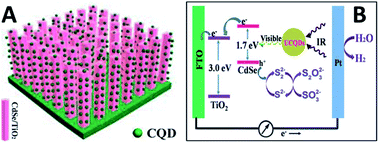 | ||
| Fig. 29 (A) A schematic diagram showing the CQD/CdSe/TiO2 NR photoanode. (B) A schematic diagram to illustrate how up-conversion of the carbon quantum dots (UCQDs) served as spectral converters for the CdSe/TiO2 NR photoanode. Adapted with permission.126 Copyright 2013, Royal Society of Chemistry. | ||
The oxygen reduction reaction (ORR) is another important photocatalytic reaction in many renewable-energy technologies, including fuel cells and water splitting.127 The aggregates of nitrogen-doped CQDs from the hydrothermal treatment of natural willow leaves exhibit excellent electrocatalytic activity for the ORR via a dominant four-electron oxygen reduction pathway in 0.1 M KOH aqueous solution, great stability (even after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), as well as methanol and CO tolerance superior to a commercial Pt/C catalyst.128 The heteroatom-doped carbon materials not only exhibit good electrochemical activity for the ORR, but also excellent stability and immunity towards methanol and CO in practical applications, which is one of the main challenges that metal-based catalysts are facing.
000 cycles), as well as methanol and CO tolerance superior to a commercial Pt/C catalyst.128 The heteroatom-doped carbon materials not only exhibit good electrochemical activity for the ORR, but also excellent stability and immunity towards methanol and CO in practical applications, which is one of the main challenges that metal-based catalysts are facing.
Dey et al. showed the fabrication of CQDs from a bio-precursor and used them for the reduction of PdCl42− salts leading to the formation of Pd@CQD core–shell nanostructures.130 Although bare CQDs were not capable of preventing the agglomeration of the Pd NPs during the Suzuki and Heck reactions, addition of a co-stabilizer in the form of poly-(N-vinyl-2-pyrrolidone) (PVP) led to an efficient composite that showed high catalytic activity towards the formation of C–C bonds. The Pd@CQD-PVP catalysts were reused in subsequent reactions and the results showed efficient catalytic activity after the third cycle even with obvious structural changes.
4.4. Chemical sensors
By monitoring the changes in their fluorescence intensity under external physical or chemical stimuli, CQDs were used to detect substances and quantities such as DNA,56 PO43−,55 thrombin,131 nitrite,132 glucose,106 biothiol,133 Fe3+,134 pH,62 Ag+,135 Hg2+,133,136 and Cu2+.137,138Qu et al. reported a preparative route toward distinctive fluorescent CQDs from dopamine (DA). Such CQDs exhibit excellent PL properties, and they can be used for multicolour bioimaging. More importantly, these CQDs were used as a new type of sensor for label-free detection of Fe3+ and dopamine (DA) with high sensitivity and selectivity.61 The method relies on the fact that Fe3+ can oxidize the hydroquinone groups on the surfaces of CQDs to the quinone species, which can quench the fluorescence of the CQDs and DA can effectively shelter the fluorescence quenching due to their competition with CQDs to react with Fe3+ (Fig. 30). It offers a convenient “mix-and-detect” protocol for rapid detection of Fe3+ and DA and can be easily accomplished with a rapid one-step (within 10 min) operation. Moreover, this sensing platform exhibits high sensitivity and selectivity toward Fe3+ and DA versus other metal ions and the other DA analogues. Furthermore, no further chemical modification of the CQDs was required, which offers the advantages of simplicity and cost efficiency. More importantly, the new strategy eliminates the need of QDs, organic dyes, and/or organic solvents, showing much more environmentally friendliness.
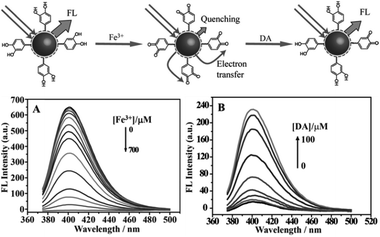 | ||
| Fig. 30 (Up) Schematic representation of fluorescent CQDs for the detection of Fe3+ ions and dopamine. (A) Representative fluorescence emission spectra of CQDs in the presence of increasing Fe3+ concentration (0–700 μM) in a HEPES buffer (10 mM) at pH 7.0. (B) Fluorescence emission spectra of CQDs containing Fe3+ (600 μM) with increase in DA concentration (0–100 μM). Adapted with permission.61 Copyright 2013, Wiley-VCH. | ||
A FRET ratiometric fluorescent sensor was developed for detecting H2S in aqueous media and serum over a wide pH range of 4.0–9.0, as well as inside live cells. In the sensing system, upon being reduced by H2S, the probe turns into an energy acceptor (naphthalimide–amine), and accordingly exhibits an absorption at around 425 nm and emission at about 526 nm.139 The fluorescence change of the sensor upon addition of H2S was measured and established a working curve by plotting the emission intensity ratio (I526/I425) versus H2S concentration (Fig. 31A). It can be seen that, in the absence of H2S, the excitation of CQDs at 340 nm led to emission of CQDs at 425 nm; moreover, with the addition of H2S, the CQD emission at 425 nm gradually decreased, and a new emission band at 526 nm appeared, corresponding to the fluorescence of naphthalimide–amine. In the presence of H2S, the probe (naphthalimide–azide) changed into an energy acceptor (naphthalimide–amine) due to its reduction by H2S. From Fig. 31B, it is clear that the fluorescence ratio (I526/I425) increases gradually with an increase in H2S concentration. Furthermore, the fluorescence of the suspension also became bright green upon addition of H2S. By adjusting the amount of the probe moieties coupled onto the CQD, the detection limit can reach 10 nM, which is the lowest among fluorescent sensors for H2S.
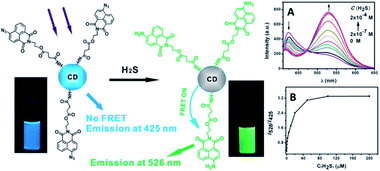 | ||
Fig. 31 (Left) Schematic illustration for the structure of the CQD-based sensor and its ratiometric detection of H2S. Photographs were obtained under a hand-held UV lamp. (Right) (A) Fluorescence spectra of the CQD-based sensor (concentration: 0.45 mg mL−1) in the presence of different amounts of H2S; (B) fluorescence intensity ratio of the CQD-based sensor as a function of H2S concentration in HEPES buffered (pH 7.4) water–ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (λexc = 340 nm). Adapted with permission.139 Copyright 2013, Royal Society of Chemistry. 1, v/v) (λexc = 340 nm). Adapted with permission.139 Copyright 2013, Royal Society of Chemistry. | ||
5. Summary and outlook
In this feature article, we have described the recent progress in the field of CQDs, focusing on their synthetic methods, size control, modification strategies, PL properties, luminescent mechanism, and applications in biomedicine, energy conversion and storage, catalysis, and sensor issues.Though several methods have been proposed towards the synthesis of CQDs, well-defined structure and precise sizes are hardly available yet. It is critical to synthesize CQDs in a facile and green manner with designed structure and size for property studies and selected applications.
The explored properties of CQDs and their regulations are fascinating for extensive applications in science, which have been demonstrated. New properties and how to subtly tune these properties, such as fresh phosphorescence and debatable UCPL, are also challengeable for its nubilous luminescent mechanism. The amorphous to nearly crystalline internal structure, nonquantitative surface structure and virtual size polydispersion may block the clarification of the luminescence mechanism. This problem will be solved through the accurate synthesis, careful analysis, and intelligent consideration.
Many studies have demonstrated the CQDs' versatility in biomedicine: (i) multimodal bioimaging for its flexibility in surface modification to combine other imaging agents for its high biocompatibility, (ii) biosensors for its multi stimulus responses, (iii) delivery carrier for its various combination with biomolecules or drugs via multi reaction and stimulus responses.
It will arouse research interest in using CQDs in optronics, including photovoltaic conversion, photochemical transformation and energy storage, for the optical properties of CQDs such as electroluminescence, down- and up-conversion, as well as its dual role as electron donor and acceptor with good conductivity and distribution.
Acknowledgements
The support of the National Natural Science Foundation of China (20874026, 91023008, 21274042), the Ph.D. Programs Foundation of Ministry of Education of China (20100074110002), the Fundamental Research Funds for the Central Universities, and the Shanghai Leading Academic Discipline Project (B502) is gratefully acknowledged.Notes and references
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang and H. Wang, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686 RSC.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J.-J. Zhu, Nanoscale, 2013, 5, 4015 RSC.
- Z. Zhang, J. Zhang, N. Chen and L. Qu, Energy Environ. Sci., 2012, 5, 8869 CAS.
- B. Zhu, S. Sun, Y. Wang, S. Deng, G. Qian, M. Wang and A. Hu, J. Mater. Chem. C, 2013, 1, 580 RSC.
- S. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546 CAS.
- L. Tian, D. Ghosh, W. Chen, S. Pradhan, X. Chang and S. Chen, Chem. Mater., 2009, 21, 2803 CrossRef CAS.
- Z.-A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812 RSC.
- Y. Dong, N. Zhou, X. Lin, J. Lin, Y. Chi and G. Chen, Chem. Mater., 2010, 22, 5895 CrossRef CAS.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563 CrossRef CAS.
- L. Shen, L. Zhang, M. Chen, X. Chen and J. Wang, Carbon, 2013, 55, 343 CrossRef CAS PubMed.
- J. Zhou, C. Booker, R. Li, X. Zhou, T.-K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744 CrossRef CAS PubMed.
- D. B. Shinde and V. K. Pillai, Chem.–Eur. J., 2012, 18, 12522 CrossRef CAS PubMed.
- L. Bao, Z. L. Zhang, Z. Q. Tian, L. Zhang, C. Liu, Y. Lin, B. Qi and D. W. Pang, Adv. Mater., 2011, 23, 5801 CrossRef CAS PubMed.
- L. Zheng, Y. Chi, Y. Dong, J. Lin and B. Wang, J. Am. Chem. Soc., 2009, 131, 4564 CrossRef CAS PubMed.
- Q.-L. Zhao, Z.-L. Zhang, B.-H. Huang, J. Peng, M. Zhang and D.-W. Pang, Chem. Commun., 2008, 5116, 10.1039/b812420e.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526 RSC.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430 CrossRef CAS PubMed.
- J. Deng, Q. Lu, N. Mi, H. Li, M. Liu, M. Xu, L. Tan, Q. Xie, Y. Zhang and S. Yao, Chem.–Eur. J., 2014, 20, 4993 CrossRef CAS PubMed.
- S.-L. Hu, K.-Y. Niu, J. Sun, J. Yang, N.-Q. Zhao and X.-W. Du, J. Mater. Chem., 2009, 19, 484 RSC.
- X. Li, H. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2011, 47, 932 RSC.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca and D. Murray, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS PubMed.
- S.-T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y.-P. Sun, J. Am. Chem. Soc., 2009, 131, 11308 CrossRef CAS PubMed.
- S.-T. Yang, X. Wang, H. Wang, F. Lu, P. G. Luo, L. Cao, M. J. Meziani, J.-H. Liu, Y. Liu and M. Chen, J. Phys. Chem. C, 2009, 113, 18110 CAS.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC.
- X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Chem. Commun., 2012, 48, 7955 RSC.
- Y. Liu, N. Xiao, N. Gong, H. Wang, X. Shi, W. Gu and L. Ye, Carbon, 2014, 68, 258 CrossRef CAS PubMed.
- A. Jaiswal, S. S. Ghosh and A. Chattopadhyay, Chem. Commun., 2012, 48, 407 RSC.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813 CrossRef CAS PubMed.
- M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103 RSC.
- Z.-C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X.-H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615 RSC.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 125, 4045 CrossRef.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380 RSC.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286 RSC.
- Z. Zhang, J. Hao, J. Zhang, B. Zhang and J. Tang, RSC Adv., 2012, 2, 8599 RSC.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835 RSC.
- S. K. Bhunia, A. Saha, A. R. Maity, S. C. Ray and N. R. Jana, Sci. Rep., 2013, 3, 1473 CAS.
- Y. Xu, M. Wu, Y. Liu, X. Z. Feng, X. B. Yin, X. W. He and Y. K. Zhang, Chem.–Eur. J., 2013, 19, 2276 CrossRef CAS PubMed.
- Y. Wang, L. Dong, R. Xiong and A. Hu, J. Mater. Chem. C, 2013, 1, 7731 RSC.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539 CrossRef CAS.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598 CrossRef CAS PubMed.
- J. Zong, Y. Zhu, X. Yang, J. Shen and C. Li, Chem. Commun., 2011, 47, 764 RSC.
- C. Tang, K. Qi, K. L. Wooley, K. Matyjaszewski and T. Kowalewski, Angew. Chem., Int. Ed., 2004, 116, 2843 CrossRef.
- X. Guo, C.-F. Wang, Z.-Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692 RSC.
- M. E. Mackay, A. Tuteja, P. M. Duxbury, C. J. Hawker, B. Van Horn, Z. Guan, G. Chen and R. Krishnan, Science, 2006, 311, 1740 CrossRef CAS PubMed.
- Y. Xiao and A. Hu, Macromol. Rapid Commun., 2011, 32, 1688 CrossRef CAS PubMed.
- B. Zhu, J. Ma, Z. Li, J. Hou, X. Cheng, G. Qian, P. Liu and A. Hu, J. Mater. Chem., 2011, 21, 2679 RSC.
- B. Zhu, G. Qian, Y. Xiao, S. Deng, M. Wang and A. Hu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5330 CrossRef CAS.
- J.-Y. Yin, H.-J. Liu, S. Jiang, Y. Chen and Y. Yao, ACS Macro Lett., 2013, 2, 1033 CrossRef CAS.
- M. Zheng, S. Liu, J. Li, D. Qu, H. Zhao, X. Guan, X. Hu, Z. Xie, X. Jing and Z. Sun, Adv. Mater., 2014, 26, 3554 CrossRef CAS PubMed.
- Y. Dong, R. Wang, H. Li, J. Shao, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 2810 CrossRef CAS PubMed.
- H. X. Zhao, L. Q. Liu, Z. De Liu, Y. Wang, X. J. Zhao and C. Z. Huang, Chem. Commun., 2011, 47, 2604 RSC.
- H. Li, Y. Zhang, L. Wang, J. Tian and X. Sun, Chem. Commun., 2011, 47, 961 RSC.
- F. Wang, Z. Xie, H. Zhang, C. y. Liu and Y. g. Zhang, Adv. Funct. Mater., 2011, 21, 1027 CrossRef CAS.
- Y. Mao, Y. Bao, D. Han, F. Li and L. Niu, Biosens. Bioelectron., 2012, 38, 55 CrossRef CAS PubMed.
- B. Liao, P. Long, B. He, S. Yi, B. Ou, S. Shen and J. Chen, J. Mater. Chem. C, 2013, 1, 3716 RSC.
- J. Zhou, Y. Yang and C.-y. Zhang, Chem. Commun., 2013, 49, 8605 RSC.
- K. Qu, J. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2013, 19, 7243 CrossRef CAS PubMed.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2011, 134, 747 CrossRef PubMed.
- D. Sun, R. Ban, P.-H. Zhang, G.-H. Wu, J.-R. Zhang and J.-J. Zhu, Carbon, 2013, 64, 424 CrossRef CAS PubMed.
- S. Chandra, P. Patra, S. H. Pathan, S. Roy, S. Mitra, A. Layek, R. Bhar, P. Pramanik and A. Goswami, J. Mater. Chem. B, 2013, 1, 2375 RSC.
- K. S. Prasad, R. Pallela, D. M. Kim and Y. B. Shim, Part. Part. Syst. Charact., 2013, 30, 557 CrossRef CAS.
- P. Ayala, R. Arenal, A. Loiseau, A. Rubio and T. Pichler, Rev. Mod. Phys., 2010, 82, 1843 CrossRef CAS.
- F. Li, C. Liu, J. Yang, Z. Wang, W. Liu and F. Tian, RSC Adv., 2014, 4, 3201 RSC.
- H. Zhang, H. Ming, S. Lian, H. Huang, H. Li, L. Zhang, Y. Liu, Z. Kang and S.-T. Lee, Dalton Trans., 2011, 40, 10822 RSC.
- J. Wang, M. Gao and G. W. Ho, J. Mater. Chem. A, 2014, 2, 5703 CAS.
- J. Zong, X. Yang, A. Trinchi, S. Hardin, I. Cole, Y. Zhu, C. Li, T. Muster and G. Wei, Nanoscale, 2013, 5, 11200 RSC.
- J. J. Huang, Z. F. Zhong, M. Z. Rong, X. Zhou, X. D. Chen and M. Q. Zhang, Carbon, 2014, 70, 190 CrossRef CAS PubMed.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751 RSC.
- Z. Lin, W. Xue, H. Chen and J.-M. Lin, Chem. Commun., 2012, 48, 1051 RSC.
- P. Teng, J. Xie, Y. Long, X. Huang, R. Zhu, X. Wang, L. Liang, Y. Huang and H. Zheng, J. Lumin., 2014, 146, 464 CrossRef CAS PubMed.
- X. Dou, Z. Lin, H. Chen, Y. Zheng, C. Lu and J.-M. Lin, Chem. Commun., 2013, 49, 5871 RSC.
- L. Zhao, F. Di, D. Wang, L.-H. Guo, Y. Yang, B. Wan and H. Zhang, Nanoscale, 2013, 5, 2655 RSC.
- J. Shi, C. Lu, D. Yan and L. Ma, Biosens. Bioelectron., 2013, 45, 58 CrossRef CAS PubMed.
- Y. Xu, M. Wu, X. Z. Feng, X. B. Yin, X. W. He and Y. K. Zhang, Chem.–Eur. J., 2013, 19, 6282 CrossRef CAS PubMed.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572 RSC.
- X. Wen, P. Yu, Y.-R. Toh, X. Ma and J. Tang, Chem. Commun., 2014, 50, 4703 RSC.
- X. Wang, L. Cao, F. Lu, M. J. Meziani, H. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarrec and Y.-P. Sun, Chem. Commun., 2009, 3774 RSC.
- J. Xu, S. Sahu, L. Cao, C. E. Bunker, G. Peng, Y. Liu, K. S. Fernando, P. Wang, E. A. Guliants and M. J. Meziani, Langmuir, 2012, 28, 16141 CrossRef CAS PubMed.
- P. Yu, X. Wen, Y.-R. Toh, Y.-C. Lee, K.-Y. Huang, S. Huang, S. Shrestha, G. Conibeer and J. Tang, J. Mater. Chem. C, 2014, 2, 2894 RSC.
- Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong and E. Wang, ACS Nano, 2011, 6, 400 CrossRef PubMed.
- X.-J. Mao, H.-Z. Zheng, Y.-J. Long, J. Du, J.-Y. Hao, L.-L. Wang and D.-B. Zhou, Spectrochim. Acta, Part A, 2009, 75, 553 CrossRef PubMed.
- A. B. Bourlinos, R. Zbořil, J. Petr, A. Bakandritsos, M. Krysmann and E. P. Giannelis, Chem. Mater., 2011, 24, 6 CrossRef.
- S. Srivastava and N. S. Gajbhiye, ChemPhysChem, 2011, 12, 2624 CrossRef CAS PubMed.
- S. K. Das, Y. Liu, S. Yeom, D. Y. Kim and C. I. Richards, Nano Lett., 2014, 14, 620 CrossRef CAS PubMed.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650 RSC.
- L. Wang, S.-J. Zhu, H.-Y. Wang, S.-N. Qu, Y.-L. Zhang, J.-H. Zhang, Q.-D. Chen, H.-L. Xu, W. Han and B. Yang, ACS Nano, 2014, 8, 2541 CrossRef CAS PubMed.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546 CAS.
- Y. Wang, P. Anilkumar, L. Cao, J.-H. Liu, P. G. Luo, K. N. Tackett, S. Sahu, P. Wang, X. Wang and Y.-P. Sun, Exp. Biol. Med., 2011, 236, 1231 CrossRef CAS PubMed.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 6220 CrossRef CAS PubMed.
- Y. Wang, L. Bao, Z. Liu and D.-W. Pang, Anal. Chem., 2011, 83, 8130 CrossRef CAS PubMed.
- X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y. P. Sun, Angew. Chem., Int. Ed., 2010, 122, 5438 CrossRef.
- B. Chen, F. Li, S. Li, W. Weng, H. Guo, T. Guo, X. Zhang, Y. Chen, T. Huang and X. Hong, Nanoscale, 2013, 5, 1967 RSC.
- A. B. Bourlinos, A. Bakandritsos, A. Kouloumpis, D. Gournis, M. Krysmann, E. P. Giannelis, K. Polakova, K. Safarova, K. Hola and R. Zboril, J. Mater. Chem., 2012, 22, 23327 RSC.
- P. G. Luo, S. Sahu, S.-T. Yang, S. K. Sonkar, J. Wang, H. Wang, G. E. LeCroy, L. Cao and Y.-P. Sun, J. Mater. Chem. B, 2013, 1, 2116 RSC.
- P. G. Luo, F. Yang, S.-T. Yang, S. K. Sonkar, L. Yang, J. J. Broglie, Y. Liu and Y.-P. Sun, RSC Adv., 2014, 4, 10791 RSC.
- C. Ding, A. Zhu and Y. Tian, Acc. Chem. Res., 2013, 47, 20 CrossRef PubMed.
- H. Tao, K. Yang, Z. Ma, J. Wan, Y. Zhang, Z. Kang and Z. Liu, Small, 2012, 8, 281 CrossRef CAS PubMed.
- D.-E. Lee, H. Koo, I.-C. Sun, J. H. Ryu, K. Kim and I. C. Kwon, Chem. Soc. Rev., 2009, 41, 2656 RSC.
- P. Gong, Z. Chen, Y. Chen, W. Wang, X. Wang and A. Hu, Chem. Commun., 2011, 47, 4240 RSC.
- Y. Chen, H. Yang, W. Tang, X. Cui, W. Wang, X. Chen, Y. Yuan and A. Hu, J. Mater. Chem. B, 2013, 1, 5443 RSC.
- S. Srivastava, R. Awasthi, D. Tripathi, M. K. Rai, V. Agarwal, V. Agrawal, N. S. Gajbhiye and R. K. Gupta, Small, 2012, 8, 1099 CrossRef CAS PubMed.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695 RSC.
- A. Zhu, Q. Qu, X. Shao, B. Kong and Y. Tian, Angew. Chem., Int. Ed., 2012, 124, 7297 CrossRef.
- W. Wei, C. Xu, J. Ren, B. Xu and X. Qu, Chem. Commun., 2012, 48, 1284 RSC.
- W. Shi, X. Li and H. Ma, Angew. Chem., Int. Ed., 2012, 51, 6432 CrossRef CAS PubMed.
- F. Du, Y. Min, F. Zeng, C. Yu and S. Wu, Small, 2014, 10, 964 CrossRef CAS PubMed.
- Z. Cheng, A. Al Zaki, J. Z. Hui, V. R. Muzykantov and A. Tsourkas, Science, 2012, 338, 903 CrossRef CAS PubMed.
- J. Kim, J. Park, H. Kim, K. Singha and W. J. Kim, Biomaterials, 2013, 34, 7168 CrossRef CAS PubMed.
- S. Pandey, M. Thakur, A. Mewada, D. Anjarlekar, N. Mishra and M. Sharon, J. Mater. Chem. B, 2013, 1, 4972 RSC.
- S. Pandey, A. Mewada, M. Thakur, A. Tank and M. Sharon, RSC Adv., 2013, 3, 26290 RSC.
- M. Thakur, S. Pandey, A. Mewada, V. Patil, M. Khade, E. Goshi and M. Sharon, J. Drug Delivery, 2014, 2014, 282193 CrossRef PubMed.
- P. Mirtchev, E. J. Henderson, N. Soheilnia, C. M. Yip and G. A. Ozin, J. Mater. Chem., 2012, 22, 1265 RSC.
- Z. Ma, Y.-L. Zhang, L. Wang, H. Ming, H. Li, X. Zhang, F. Wang, Y. Liu, Z. Kang and S.-T. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 5080 CAS.
- Y. Zhu, X. Ji, C. Pan, Q. Sun, W. Song, L. Fang, Q. Chen and C. E. Banks, Energy Environ. Sci., 2013, 6, 3665 CAS.
- Y. Wei, H. Liu, Y. Jin, K. Cai, H. Li, Y. Liu, Z. Kang and Q. Zhang, New J. Chem., 2013, 37, 886 RSC.
- W. Kwon, S. Do, D. C. Won and S.-W. Rhee, ACS Appl. Mater. Interfaces, 2013, 5, 822 CAS.
- X. Zhang, Y. Zhang, Y. Wang, S. Kalytchuk, S. V. Kershaw, Y. Wang, P. Wang, T. Zhang, Y. Zhao and H. Zhang, ACS Nano, 2013, 7, 11234 CrossRef CAS PubMed.
- L. Mao, W.-Q. Tang, Z.-Y. Deng, S.-S. Liu, C.-F. Wang and S. Chen, Ind. Eng. Chem. Res., 2014, 53, 6417 CrossRef CAS.
- W. Kwon, S. Do, J. Lee, S. Hwang, J. K. Kim and S.-W. Rhee, Chem. Mater., 2013, 25, 1893 CrossRef CAS.
- F. Wang, Y.-h. Chen, C.-y. Liu and D.-g. Ma, Chem. Commun., 2011, 47, 3502 RSC.
- H. Yu, Y. Zhao, C. Zhou, L. Shang, Y. Peng, Y. Cao, L.-Z. Wu, C.-H. Tung and T. Zhang, J. Mater. Chem. A, 2014, 2, 3344 CAS.
- X. Zhang, H. Huang, J. Liu, Y. Liu and Z. Kang, J. Mater. Chem. A, 2013, 1, 11529 CAS.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- S. Gao, Y. Chen, H. Fan, X. Wei, C. Hu, L. Wang and L. Qu, J. Mater. Chem. A, 2014, 2, 6320 CAS.
- C. Zhu, J. Zhai and S. Dong, Chem. Commun., 2012, 48, 9367 RSC.
- D. Dey, T. Bhattacharya, B. Majumdar, S. Mandani, B. Sharma and T. K. Sarma, Dalton Trans., 2013, 42, 13821 RSC.
- J. Liu, J. Li, Y. Jiang, S. Yang, W. Tan and R. Yang, Chem. Commun., 2011, 47, 11321 RSC.
- Z. Lin, W. Xue, H. Chen and J.-M. Lin, Anal. Chem., 2011, 83, 8245 CrossRef CAS PubMed.
- L. Zhou, Y. Lin, Z. Huang, J. Ren and X. Qu, Chem. Commun., 2012, 48, 1147 RSC.
- S. Qu, H. Chen, X. Zheng, J. Cao and X. Liu, Nanoscale, 2013, 5, 5514 RSC.
- H. Li, J. Zhai and X. Sun, Langmuir, 2011, 27, 4305 CrossRef CAS PubMed.
- H. Li, J. Zhai, J. Tian, Y. Luo and X. Sun, Biosens. Bioelectron., 2011, 26, 4656 CrossRef CAS PubMed.
- S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037 CrossRef CAS PubMed.
- Q. Qu, A. Zhu, X. Shao, G. Shi and Y. Tian, Chem. Commun., 2012, 48, 5473 RSC.
- C. Yu, X. Li, F. Zeng, F. Zheng and S. Wu, Chem. Commun., 2013, 49, 403 RSC.
| This journal is © The Royal Society of Chemistry 2014 |