Phenylboronic acid-based glucose-responsive polymeric nanoparticles: synthesis and applications in drug delivery
Rujiang
Ma
and
Linqi
Shi
*
Key Laboratory of Functional Polymer Materials, Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin 300071, China. E-mail: shilinqi@nankai.edu.cn
First published on 11th October 2013
Abstract
Glucose-responsive materials have attracted great intention in recent years due to their potential application in drug delivery. Phenylboronic acid-containing materials have been most widely studied and used in construction of glucose-responsive system for insulin delivery. This review covers the recent advances in synthesis of phenylboronic acid-based glucose-responsive materials, especially in forms of nanogels (microgels), micelles, vesicles, and mesoporous silica nanoparticles. Applications of these nanomaterials in drug delivery are discussed.
1. Introduction
Diabetes mellitus has become one of the most serious threats to human health worldwide. Life-long treatment is needed for diabetic patients to maintain the glucose concentration at the normal level and prevent acute complications. Currently, daily injection of insulin is the most effective way to treat insulin-dependent diabetes mellitus. It is a long-suffering inconvenience for the diabetics. Therefore, it is desirable to find an alternative strategy for the treatment of diabetes, which can deliver and release insulin in a self-regulated way in response to the fluctuation of blood glucose concentration. In recent years, phenylboronic acid (PBA) based glucose-responsive materials have attracted great interest because of their potential applications in the construction of self-regulated insulin delivery systems for the treatment of diabetes.It is known that phenylboronic acid and its derivatives are a kind of Lewis acid with reported pKa in the range of 8.2–8.86.1–3 As shown in Fig. 1, PBA exists in an equilibrium between an uncharged form and a charged form.4 The uncharged form is hydrophobic but the charged form is hydrophilic. While both forms react reversibly with 1,2-cis-diols of glucose, the complexation of the uncharged form with glucose is unstable because it is highly susceptible to hydrolysis. The charged form of PBA can complex with cis-diol compounds, for example glucose, and form stable but hydrophilic phenylborates, which shifts the equilibrium in the direction of increasing the hydrophilic form but decreasing the hydrophobic form.5–7 This shift increases the swelling degree of the crosslinked nanogels or disintegrates self-assembled micelles, causing the entrapped payload to be released into solution. This provides a way for designing polymeric carriers for self-regulated delivery of insulin.
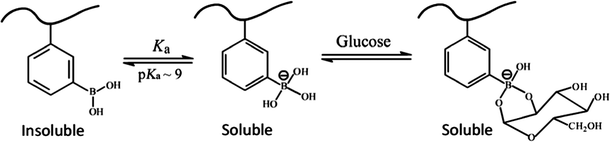 | ||
| Fig. 1 Equilibria between different forms of phenylboronic acid. (Adapted with permission from ref. 3. Copyright 2003 American Chemical Society.) | ||
However, PBA-based glucose-responsive materials could only work in alkaline media but not at physiological pH, due to the high pKa value of the PBA moiety.4,8–11 This was because only a small fraction of the phenylboronic acid moieties were ionized under physiological conditions (pH 7.4), resulting in poor water solubility of the PBA-containing copolymer and thus lower binding affinity to glucose. Two methods were proposed for reducing the pKa of the PBA group and thus increasing glucose-sensitivity at physiological pH. One method was introduction of electron-withdrawing groups such as nitro, fluoro, and carbonyl groups on the phenylboronic acid at a meta-position with respect to boronic acid.3,12–14 For example, 4-(1,6-dioxo-2,5-diaza-7-oxamyl)phenylboronic acid (DDOPBA), with a pKa of 7.8, was synthesized and used for the synthesis of glucose-sensitive hydrogels operating at physiological pH.3,15 Another method was introduction of amino groups into a PBA-containing polymer2,16 or onto the phenyl ring in the vicinity of boronic acid17 to decrease the apparent pKa value of PBA because of the possible coordination between nitrogen and boron.
Recently, several new methods were developed to either decrease the pKa of PBA or increase glucose-sensitivity at physiological conditions. The first one was partial modification of pendent carboxyls on a polymer chain leading to a PBA/carboxyl interaction in the polymer and thus decreasing its apparent pKa.18,19 The second method was complexation of PBA-containing polymer with a glycopolymer resulting in PBA/polyol complexes with lowered pKa.20,21 The third method was using benzoboroxoles, a new kind of PBA analogue which possessed high water solubility and a low pKa compared with that of simple PBA.22
Until now, PBA-based glucose-responsive materials in the forms of bulk gels, nanogels (microgels), micelles, vesicles, and silica NPs have been synthesized and used for insulin delivery. Several excellent reviews have summarized boronic acid-containing glucose-responsive systems from different views.6,23–29 This review will cover the recent advances in PBA-containing glucose-responsive materials especially in the form of nanoparticles including nanogels (microgels), micelles, vesicles, and mesoporous silica NPs. Synthesis and application in drug (insulin) delivery are reviewed.
2. Chemically cross-linked PBA-based nanogels
Kataoka and coworkers made outstanding contributions to the synthesis and application of PBA-based glucose-responsive materials. Their pioneering work was the synthesis of a complex gel based on the reversible complexation between PBA of P(NVP-co-PBA) and diols or PVA.30 Glucose-sensitive release of insulin using this complex gel was successfully achieved.31 Later, chemically crosslinked gels composed of alkylacrylamide and 3-acrylamidophenylboronic acid (AAPBA) were synthesized and used for glucose-regulated on–off release of insulin.4,32 Though PBA-containing bulk gels could be synthesized and used as an implantable carrier for insulin delivery,33 more desirable PBA-based glucose-responsive materials were suggested to be in the nanoscale forms such as nanogels (microgels), which may meet different needs, such as prompt drug release, oral administration, and intravenous injection.Typical glucose-responsive nanogels based on PBA were synthesized through modification of poly(N-isopropylacrylamide-co-acrylic acid) P(NIPAM-co-AA) nanogels with 3-aminophenylboronic acid (APBA) (Fig. 2).8,10,34 The introduction of the hydrophobic PBA group significantly decreased the volume phase transition temperature of the resultant microgels. As a result, the P(NIPAM–PBA) nanogels with a 10.0 mol% PBA content were in a collapsed state at room temperature. However, the addition of glucose makes the microgels swell dramatically at pH 9.0.8 The swelling degree was proportional to the PBA content, glucose concentration, and pH value. Theoretically, insulin could be absorbed into the nanogels and released in response to glucose. But a higher pH value than 7.4 was needed. In another case, Zhang and coworkers35 reported temperature-controlled release of diols from poly(N-isopropylacrylamide-co-3-acrylamidophenylboronic acid) P(NIPAM–AAPBA) microgels. Glucose and Alizarin Red S (ARS) were absorbed into microgels via binding with PBA at lower temperature. The diols were released as the temperature increased and the microgels shrunk.
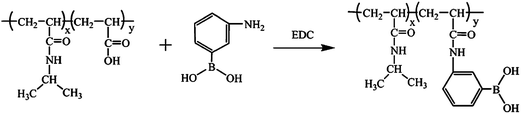 | ||
| Fig. 2 Synthesis of P(NIPAM–AAPBA) nanogels by modification of P(NIPAM–AA) nanogels with 3-aminophenyboronic acid. (Reprinted with permission from ref. 8. Copyright 2006 American Chemical Society.) | ||
Ravaine and coworkers36 reported the synthesis of monodispersed glucose-responsive P(NIPAM–AAPBA) microgels by direct copolymerization of N-isopropylacrylamide (NIPAM) and 3-acrylamidophenylboronic acid (AAPBA) in aqueous solution. Microgels with 10 mol% PBA displayed remarkable glucose-responsiveness at pH 8.5. They also synthesized glucose-responsive microgels with a core–shell structure.37 The PNIPAM core microgels were first prepared by an aqueous free radical precipitation polymerization. Then the shell was synthesized by the polymerization of a mixture of NIPAM–BIS–AAPBA in the presence of the core as seeds. Fluorescein isothiocyanate (FITC)-labelled insulin was loaded into the core–shell microgels and released in the presence of 100 mM glucose at 25 °C and physiological pH.
Phenylboronic acid based glucose-sensitive microgels with 4-vinylpyridine (4VP) for working at physiological pH and temperature were reported by Siddiq and coworkers.38 The microgels with different 4VP content were synthesized by the functionalization of P(NIPAM–4VP–AA) microgels with 3-aminophenylboronic acid. The incorporation of the 4VP moiety into the PBA-based copolymer microgels could significantly reduce the pKa of the PBA moiety by Lewis acid/base interaction, increase the volume phase transition temperature (VPTT), and thus enhance the glucose sensitivity at physiological pH and temperature conditions (Fig. 3).
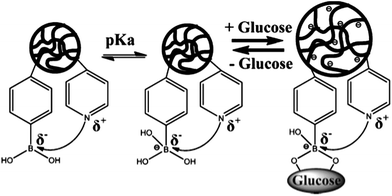 | ||
| Fig. 3 Illustration for Lewis acid/base interaction in the microgels and glucose-induced swelling. (Reprinted with permission from ref. 38. Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
For general PBA-containing nanogels, determination of glucose-responsiveness could be achieved by monitoring the change of particle size. Recently, sugar-responsive fluorescent nanospheres were reported by Zenkl and coworkers,39 which offered high sensitivity for measuring changes in small dimensions on the nanoscale. Except NIPAM and AAPBA, fluorescence donor and acceptor were also polymerized into the nanospheres. The nanospheres were in their shrunken state without sugar and the fluorescent donor and acceptor were in close proximity for effective fluorescence resonance energy transfer (FRET) effect. After the addition of sugar, the nanospheres began to swell, and the distance between the fluorescent donor and acceptor increased. Consequently, the FRET effect decreased (Fig. 4).
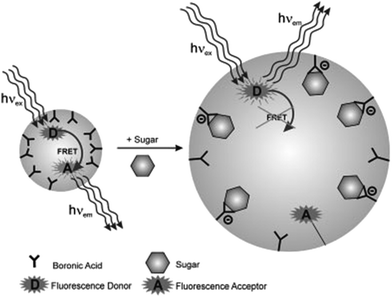 | ||
| Fig. 4 Schematic illustration for the sugar-responsive FRET effect in the nanospheres. (Reprinted with permission from ref. 39. Copyright © 2008 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
The above nanogels are non-degradable and cannot be easily cleared from blood circulation or metabolized in cells. Recently, Chen and coworkers40 reported a facile one-pot synthesis of a glucose-sensitive nanogel via thiol-ene click chemistry for self-regulated drug delivery. Copolymerization of pentaerythritol tetra(3-mercaptopropionate) (QT), poly(ethylene glycol) diacrylate (PEGDA), methoxyl poly(ethylene glycol) acrylate (mPEGA) and N-acryloyl-3-aminophenylboronic acid (AAPBA) resulted in core–shell nanogels with disulfide cross-linked core and PEG shell (Fig. 5). Owing to the presence of PBA, the nanogel exhibited high glucose sensitivity in phosphate-buffered saline. ARS and insulin were loaded into this glucose-sensitive nanogel. In vitro release profiles revealed that the drug release from the nanogel could be triggered by the presence of glucose. The more glucose in the release medium, the more drugs were released and the faster the release rate. At the later period of drug delivery, the disulfide linkage could be reduced and thus favored the clearance of the drug carriers.
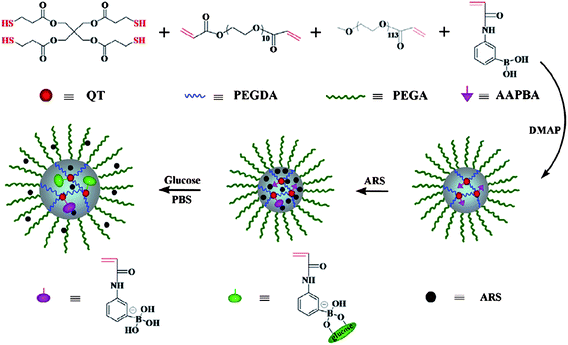 | ||
| Fig. 5 Structures of the nanogel and glucose-sensitive behavior of ARS-loaded nanogel in PBS. (Reprinted with permission from ref. 40. Copyright © 2013, Elsevier.) | ||
Another kind of degradable multifunctional PBA-containing microgel for self-regulated insulin delivery under physiological conditions was reported by Liu and coworkers.41 Such multifunctional microgels were fabricated with N-isopropylacrylamide (NIPAM), (2-dimethylamino)ethyl methacrylate (DMAEMA) and 3-acrylamidophenylboronic acid (AAPBA) through a precipitation emulsion method and cross-linked by reductive degradable N,N′-bis(arcyloyl)cystamine (BAC) (Fig. 6). This kind of microgel with an average size of about 250 nm was suitable for diabetes treatment because it can adapt to the surrounding medium of different glucose concentrations over a clinically relevant range (0–20 mM), control the release of preloaded insulin and was highly stable under physiological conditions (pH 7.4, 0.15 M NaCl, 37 °C). During drug delivery, the microgels gradually degraded as time passed and showed enhanced biocompatibility.
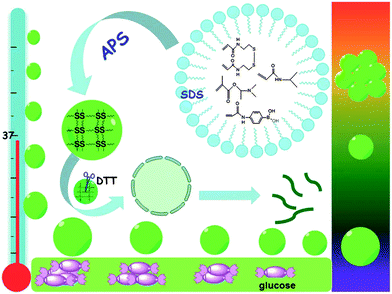 | ||
| Fig. 6 Schematic illustration of synthesis, multi-response, and degradation of the multifunctional microgels at physiological conditions. (Reproduced from ref. 41 with permission from The Royal Society of Chemistry.) | ||
An injectable and glucose-sensitive nanogel for controlled insulin release was synthesized by Wu and coworkers.42 The nanogels were composed of three interpenetrating polymer networks of poly(N-isopropylacrylamide), dextran, and poly(3-acrylamidophenylboronic acid) (P(NIPAM–Dex–AAPBA)). The nanogels swelled with increased glucose concentration. Insulin was loaded into the nanogels and released in response to glucose. In vivo study revealed that insulin-loaded nanogels decreased the blood glucose levels in diabetic rats and maintained 51% of the baseline level for almost 2 hours. Importantly, the drug-loaded nanogels could keep blood glucose levels stable and avoided blood sugar fluctuations compared with free insulin.
3. Self-assembled PBA-based micelles
Glucose-responsive nanogels of several hundred nanometers in diameter offer structural integrity during drug delivery but may encounter the plights of quick clearance in blood circulation, because the nanogels lack the protection by a corona layer just like self-assembled micelles protected by a PEG corona and the difficulty of renal excretion because of their non-biodegradability. Recently, glucose-responsive micelles self-assembled by PBA-containing amphiphilic block polymers have been reported as potential and excellent carriers for insulin delivery due to their faster response to glucose at neutral pH, longer circulation stability as a result of the protection by PEG, and easier drug loading upon self-assembly.For the preparation of PBA-based glucose-responsive micelles by self-assembly, the first and most important is the synthesis of PBA-containing block copolymers. Jäkle and coworkers43 synthesized well-defined organoboron block copolymers via atom transfer radical polymerization (ATRP) by using two different routes as shown in Fig. 7. In the direct polymerization method, route A, well-defined organoboron polymer was synthesized by ATRP of pinacol protected styreneboronic acid and used as a macroinitiator in the chain extension with styrene or other monomers to obtain organoboron block copolymers. In route B, postpolymerization modification of silylated polymers was used as an alternative. Silicon-functionalized monomer was first polymerized by ATRP44 and then followed by chain extension with styrene. Subsequent borylation of the silylated polymer resulted in well-defined organoboron block copolymers.
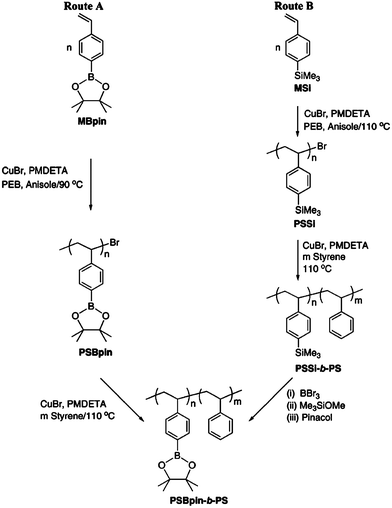 | ||
| Fig. 7 Synthesis of organoboron block copolymer via ATRP through two different routes of direct polymerization and postpolymerization modification. (Reprinted with permission from ref. 43. Copyright © 2005, American Chemical Society.) | ||
Recently, Sumerlin et al.45 reported a facile strategy to synthesize well-defined water-soluble boronic acid-based (co)polymers by reversible addition-fragmentation chain transfer (RAFT) polymerization (Fig. 8). They polymerized a pinacol ester of 4-vinylphenylboronic acid and subsequently deprotected utilizing a mild and convenient strategy to obtain the boronic acid (co)polymers. This was the first example of boronic acid/ester block copolymers being prepared by RAFT polymerization. The amphiphilic block copolymer could self-assemble into polymeric micelles in water. After transesterification with another boronic acid, the pinacol was removed from PBA, and the block copolymer became glucose responsive. But an alkaline condition was needed for glucose-triggered dissociation of the self-assembled micelles because of the higher pKa of PBA. In another case, the same group synthesized sugar-responsive block copolymers by direct RAFT polymerization of unprotected and less hydrophobic boronic acid monomers.46 This block copolymer self-assembled into micelles in aqueous solution and dissociated in response to glucose at lower alkaline pH.
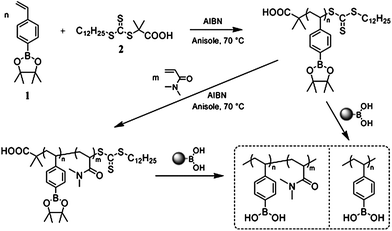 | ||
| Fig. 8 Synthesis of boronic ester and boronic acid homopolymers and block copolymers by RAFT polymerization. (Reprinted with permission from ref. 45. Copyright © 2007, American Chemical Society.) | ||
To further decrease the critical pH value for apparent response to glucose, Sumerlin and coworkers reported a similar PBA-containing block copolymer with a lower boronic acid pKa = 8.2. This block copolymer was synthesized by RAFT copolymerization of N,N-dimethylacrylamide and a boronic acid-containing acrylamide monomer with an electron-withdrawing substituent on the pendant phenylboronic acid moiety.47 Below pH 8.2, the block copolymer self-assembled into aggregates. Addition of base to yield a pH > pKa or addition of glucose at pH 7.4 resulted in aggregate dissociation (Fig. 9). This may prove promising for controlled delivery applications under physiological relevant conditions.
 | ||
| Fig. 9 Structure of the PBA-containing block copolymer and schematic illustration for glucose-triggered dissociation of the micelles. (Reprinted with permission from ref. 47. Copyright © 2012, American Chemical Society.) | ||
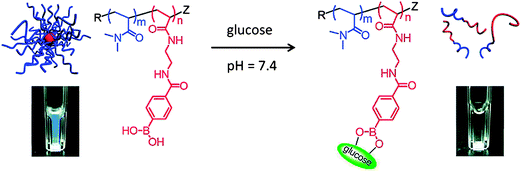
The above block copolymer micelles were formed by hydrophobic association of PBA-containing blocks and could be disintegrated by sugar binding to PBA moieties on the polymer chains, which transformed PBA-containing blocks from hydrophobe to hydrophile. Recently, Yang and coworkers48,49 synthesized an amphiphilic block copolymer poly(ethylene glycol)-block-poly[(2-phenylboronic ester-1,3-dioxane-5-ethyl) methylacrylate] (PEG-b-PPBDEMA) with phenylborate ester as a leaving group in response to glucose in the hydrophobic block (Fig. 10). Acrylate monomer containing pinacol phenylboronate was prepared and used for polymerization. The block copolymer self-assembled into core–shell micelles in aqueous solution with PPBDEMA as the core and PEG as the shell. Due to saccharide molecules having a predominant association force with organoboron, the presented pinacol phenylboronate moieties on the polymer are prone to combine with sugar molecules and detach from the polymer architecture as a phenylboronate–saccharide complex. The disengagement of phenylboronate groups will trigger the polarity transfer of the polymer from amphiphile to double hydrophile, thus leading to disruption of the initial nanoaggregates formed via self-assembly of the amphiphilic polymer. The micelles were used as glucose-sensitive drug carriers and glucose-responsive release of insulin was successfully realized at neutral pH.
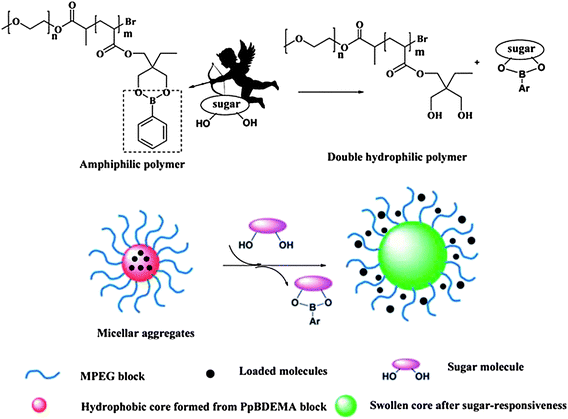 | ||
| Fig. 10 Schematic illustration for sugar-responsive behavior of PEG-b-PPBDEMA micelles. (Reproduced from ref. 48 with permission from The Royal Society of Chemistry.) | ||
Complete synthesis of new block copolymers always involves complicated work. Shi and coworkers18,19 reported a simple but effective method to synthesize a PBA-containing block copolymer PEG-b-P(AA-co-AAPBA) that could respond to glucose at pH 7.4. The block copolymer PEG-b-P(AA-co-AAPBA) was synthesized by partial modification of an existing poly(ethylene glycol)-block-poly(acrylic acid) (PEG-b-PAA) with 3-aminophenylboronic acid (APBA) (Fig. 11). A modification degree of 63% AA units transformed into AAPBA units resulted in an amphiphilic block copolymer that self-assembled into core–shell micelles but dissociated in response to glucose at pH 7.4. Solid 11B MAS NMR analysis indicated that coordination between carboxyl and PBA induced the transform of PBA from the trigonal planar to the tetrahedral form, resulting in the decrease of apparent pKa and glucose-responsiveness of the micelles at pH 7.4. Insulin was loaded into the micelles through hydrophobic interaction during self-assembly and released in the presence of glucose at neutral pH (Fig. 11). Higher glucose concentration led to faster and complete dissociation of the micelles and also faster release of insulin.
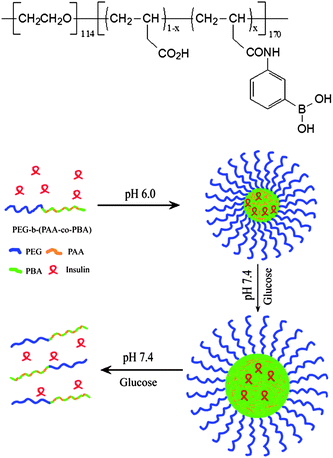 | ||
| Fig. 11 Structure of PEG-b-P(AA-co-AAPBA) and schematic illustration of the formation, swelling and dissociation of insulin-loaded micelles. (Reprinted with permission from ref. 18. Copyright © 2009, American Chemical Society.) | ||
The block copolymer PEG-b-P(AA-co-AAPBA) can also be considered as one obtained by introduction of hydrophilic comonomer AA into a PBA-based polymer. Sumerlin and coworkers50 found that the incorporation of hydrophilic comonomers within the responsive PBA-containing block was a possible way to tune the glucose sensitivity of the block copolymers. A hydrophilic comonomer N,N-dimethylacrylamide (DMA) was used in this case and block copolymers PAAPBA-b-PDMA with different compositions were studied. Their data suggested that micelles self-assembled of the block copolymer with relatively higher content of PDMA had a lower transition pH value to dissociate. This indicates a decrease of apparent pKa of PBA and thus an increase of glucose sensitivity of PBA-containing block copolymers by incorporating DMA.
Poly(acrylic acid) based PBA-containing polymers are non-degradable, which is unfavorable for blood clearance after drug delivery. Recently, glucose-sensitive polypeptide micelles with better biocompatibility for self-regulated insulin release at physiological pH were reported by Chen and coworkers.51 PBA functionalized block copolymers, monomethoxy poly(ethylene glycol)-b-poly(L-glutamic acid-co-N-3-L-glutamylamidophenylboronic acid) (mPEG-b-P(GA-co-GPBA)), were synthesized by modifying mPEG-b-PGA with 3-aminophenylboronic acid (APBA). The resultant diblock copolymers self-assembled into micelles in phosphate buffer at physiological pH (pH 7.4) and insulin-loaded micelles were successfully prepared. The in vitro release profiles revealed that the release of insulin from the micelles could be triggered by glucose, i.e. less insulin was released at healthy blood glucose level (1 g L−1), while quick release occurred under diabetic blood glucose level conditions (above 2 g L−1).
Though PBA-based block copolymer micelles were successfully used to encapsulate insulin and glucose-responsive release profiles were obtained, the simple core–shell structure was hardly able to achieve repeated on–off release due to its disassembly in response to glucose. Shi and coworkers52 developed a glucose-responsive complex polymeric micelle through the self-assembly of two amphiphilic diblock copolymers, poly(ethylene glycol)-b-poly(aspartic acid-co-aspartamidophenylboronic acid) (PEG-b-P(Asp-co-AspPBA)) and poly(N-isopropylacrylamide)-b-poly(aspartic acid-co-aspartamidophenylboronic acid) (PNIPAM-b-P(Asp-co-AspPBA)) (Fig. 12). The complex micelles had a common P(Asp-co-AspPBA) core and a mixed PEG/PNIPAM shell. Upon increasing temperature, PNIPAM chains collapsed on the glucose-responsive P(Asp-co-AspPBA) core, forming a novel core–shell–corona structure. The complex micelles exhibited a reversible swelling in response to changes in the glucose concentration, enabling the repeated on–off release of insulin regulated by the glucose level. Furthermore, the continuous PNIPAM membrane of the complex micelles could effectively protect the encapsulated insulin against protease degradation. This kind of glucose-responsive complex micelles provided a simple and powerful strategy to construct a self-regulated insulin delivery system for diabetes treatment.
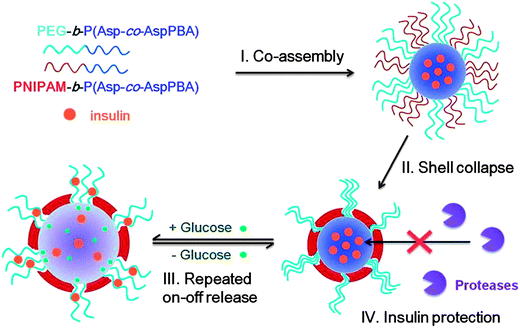 | ||
| Fig. 12 Schematic illustration of glucose-responsive complex polymeric micelle (CPM) self-assembled from two diblock copolymers for repeated on–off release and insulin protection under physiological conditions. (Reproduced from ref. 52 with permission from The Royal Society of Chemistry.) | ||
All of the above micelles were obtained by self-assembly of PBA-containing block copolymers driven by the hydrophobic association of PBA-rich blocks under appropriate conditions. Dissociation of the micelles could be simply achieved by addition of glucose which combined PBA and transformed it into a hydrophilic state. Recently, Sumerlin and coworkers53 reported the preparation of dynamic-covalent nanoaggregates based on reversible boronic ester linkages (Fig. 13). They demonstrated that well-defined PBA-containing block copolymers were highly versatile building blocks for the construction of nanoaggregates via PBA–diol interaction. The addition of multifunctional 1,2/1,3-diols to block copolymers that contain one segment with pendent boronic acid moieties led to nanoaggregates that were composed of covalent boronic ester linkages. The dynamic-covalent nature of the boronic ester cross-links allowed the nanoaggregates to reconfigure their covalent structure in the presence of monofunctional diols that competed for bonding with the boronic acid component. Therefore, the nanoaggregates could be induced to dissociate via competitive exchange of the multi-diol cross-linkers with monodiols.
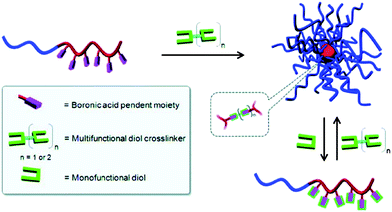 | ||
| Fig. 13 Formation and disintegration of dynamic-covalent nanoaggregates via boronic ester formation and transesterification. (Reprinted with permission from ref. 53. Copyright © 2011, American Chemical Society.) | ||
By amplifying small multifunctional diols into glycopolymers with many more diols, Shi and coworkers20,21 reported the fabrication of glucose-responsive complex micelles by self-assembly of a phenylboronic acid-containing block copolymer PEG-b-P(AA-co-AAPBA) and a glycopolymer poly(acrylic acid-co-acrylglucosamine) (P(AA-co-AGA)) based on the covalent complexation between phenylboronic acid and glycopolymer (Fig. 14). This kind of complex micelles combined a variety of advantages such as stability against aggregation due to PEG shell, fast response to glucose due to nanoscale size and hydrophilic micelle core, and more importantly better glucose sensitivity ascribed to the decrease of apparent pKa of PBA as a result of complexation with glycopolymer. The complex micelles displayed an enhanced glucose-responsiveness compared to the simple PEG-b-P(AA-co-AAPBA) micelles and the sensitivity of the complex micelles to glucose increased with the decrease of the amount of P(AA-co-AGA) in the compositions. Glucose-triggered on–off release of insulin was obtained under physiological pH 7.4 with 2 g L−1 glucose (hyperglycemia), which provided an effective strategy for self-regulated insulin delivery in response to physiological glucose level. The enhanced glucose-responsiveness of the complexes was ascribed to free boronic acids having a lower Lewis acidity than the corresponding neutral esters.23,54 For example, the pKa of phenylboronic acid decreases from 8.8 to 6.8 upon esterification with glucose leading to the formation of boronate.55
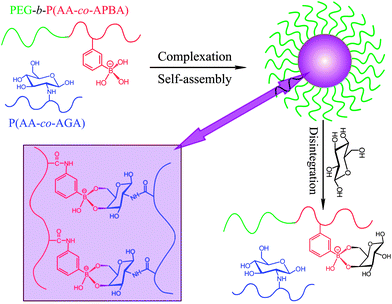 | ||
| Fig. 14 Schematic illustration for the formation and glucose responsive disintegration of the PEG-b-P(AA-co-AAPBA)/P(AA-co-AGA) complex micelles. (Adapted with permission from ref. 20. Copyright © 2012, American Chemical Society.) | ||
Based on the reversible interaction between PBA and glycopolymer, Zhang and coworkers reported a series of self-assembled glucose-responsive nanoparticles.56–62 They synthesized a PBA-containing random amphiphilic glycopolymer P(AAPBA-r-MAGA) by copolymerization of 3-acrylamidophenylboronic acid (AAPBA) and maleimide-glucosamine (MAGA) (Fig. 15).56 Self-assembled NPs with a narrow size distribution were successfully prepared using this copolymer. These nanoparticles were glucose-sensitive and were used as carriers for the nasal delivery of insulin.57 The plasma glucose level of rats was significantly decreased by insulin-loaded P(AAPBA-r-MAGA) nanoparticles across the nasal respiratory.
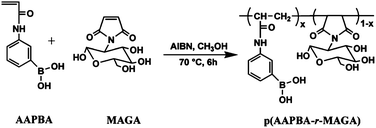 | ||
| Fig. 15 Synthesis of the PBA-containing random amphiphilic glycopolymer P(AAPBA-r-MAGA). (Reprinted with permission from ref. 56. Copyright © 2009, American Chemical Society.) | ||
Block and random copolymers composed of AAPBA and another sugar unit 2-lactobionamidoethyl methacrylate (LAMA) were also reported by Zhang and coworkers.58,59 The block copolymer P(LAMA-b-AAPBA) was synthesized by RAFT polymerization and self-assembled into spherical nanoparticles due to the interaction between lactose moieties and PBA (Fig. 16). Insulin was encapsulated into the nanoparticles and a sustained release of insulin was obtained. The random copolymer P(LAMA-r-AAPBA) could also self-assemble into nanoparticles and was used as a vehicle for nasal delivery of peptides and proteins. There was a significant decrease in the blood glucose levels after the nasal administration of insulin-loaded P(LAMA-r-AAPBA) nanoparticles to diabetic rats.
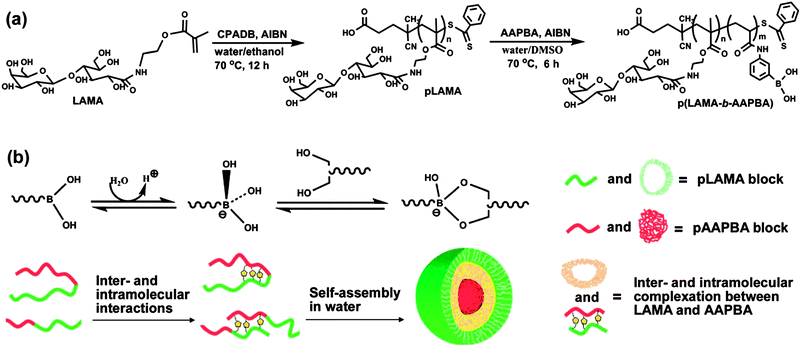 | ||
| Fig. 16 Synthesis of PLAMA and P(LAMA-b-AAPBA) by RAFT polymerization and self-assembly diagram of P(LAMA-b-AAPBA) in aqueous solution. (Reproduced from ref. 58 with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry.) | ||
In addition to copolymers containing PBA and sugars, Zhang and coworkers60,61 reported the synthesis of boronate crosslinked nanoparticles that were composed of a PBA-containing polymer and another sugar-containing polymer (Fig. 17) This method was more convenient than the synthesis of PBA-containing glycopolymer for the fabrication of glucose-responsive nanoparticles. For example, homopolymers PLAMA and PAAPBA were simply mixed and nanoparticles were obtained due to the LAMA/PBA complexation and the stabilization by uncomplexed PLAMA chains. The insulin-loaded nanoparticles by intranasal administration led to a significant decrease in the plasma glucose levels. In another case, they synthesized poly(3-methacrylamido phenylboronic acid) (PMAPBA) homopolymer and poly(ethylene glycol)-block-poly(2-acryloxyethyl-galactose) (PEG-b-PAEG) glycopolymer via RAFT and ATRP, respectively. Boronate cross-linked nanoparticles were prepared based on the complexation of PMAPBA and PEG-b-PAEG. The nanoparticles showed obvious sensitivity to glucose, galactose, mannose, and sucrose at pH 10.
 | ||
| Fig. 17 Schematic illustration for the formation of PAAPBA/PLAMA NPs. (Reproduced from ref. 60 with permission from The Royal Society of Chemistry.) | ||
As discussed above, PBA and its derivatives are generally hydrophobic and have much lower binding affinity toward glucose at neutral pH, which limits their application in the delivery of insulin. In recent years, a new kind of PBA analogue, o-hydroxymethyl phenylboronic acid (benzoboroxole), has been emerging as an interesting and useful scaffold in drug discovery. The benzoboroxoles were found to possess better water solubility, higher affinity toward saccharides under physiologically relevant conditions, and a lower pKa compared with that of simple phenylboronic acid.22,63,64 The binding constant of benzoboroxoles to glucose was determined to be 17, which was much larger than that of PBA at neutral pH.22 This indicates a remarkable enhancement of binding affinity toward glucose by benzoboroxoles. When hexopyranosides were used, benzoboroxole had similar binding constants for α-D-glucose and different methyl α-D-glycopyranoside. The exceptional carbohydrate-binding behavior of benzoboroxoles may be attributed to their relatively high Lewis acidity along with subtle factors such as intramolecular hydrogen bonding with other pyranoside hydroxyl groups in the resulting anionic complex.63
Based on the unique properties of benzoboroxoles, glucose-responsive nanogels via simple mixing of benzoboroxole- and glyco-based polymers were reported by Narain and coworkers (Fig. 18).65 Benzoboroxole-containing copolymer poly(N-isopropylacrylamide-st-5-methacrylamido-1,2-benzoboroxole) P(NIPAM-st-MAAmBO) and glycopolymer were synthesized by RAFT polymerization. The strong interaction between benzoboroxole and hydroxyl groups on the glycopolymer allowed cross-linking of the polymer chains and resulted in complex gels or nanogels. This process was found to be fully reversible as a function of temperature and the presence of free glucose. Variation of MAAmBO in P(NIPAM-st-MAAmBO) was used to control the stimuli-responsive properties of the nanogels. This responsive gelation system was suggested to be very useful for the encapsulation of therapeutic drugs and their slow release could be triggered by temperature, pH, and glucose concentration.
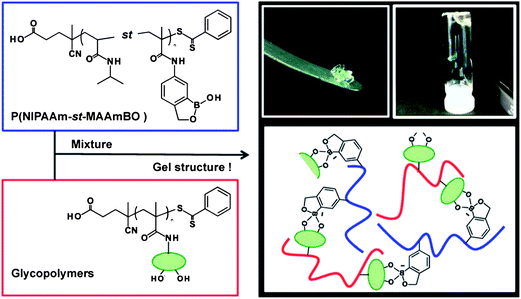 | ||
| Fig. 18 Schematic illustration for the formation of glucose-responsive nanogels by mixing of benzoboroxole- and glyco-based polymers. (Reprinted with permission from ref. 65. Copyright © 2013, American Chemical Society.) | ||
Most of the PBA-based micelles involved unitary or binary components. Very recently, Scherman and coworkers66 synthesized a ternary supramolecular double hydrophilic glucose responsive block copolymer (DHBC) held together by cucurbit[8]uril (CB[8]) complexation (Fig. 19). The supramolecular block copolymer assembly consists of poly(N-isopropylacrylamide) (PNIPAM) and poly(acrylamidophenylboronic acid) (PAAPBA) as temperature and glucose responsive blocks, respectively, and poly(dimethylacrylamide) (PDMAA) as a hydrophilic block. This supramolecular DHBC self-assembled into micelles upon increasing temperature to 37 °C at pH 7.4 and insulin was successfully loaded. Drug release could be triggered by changing temperature, glucose concentration or by adding a competitive guest for CB[8].
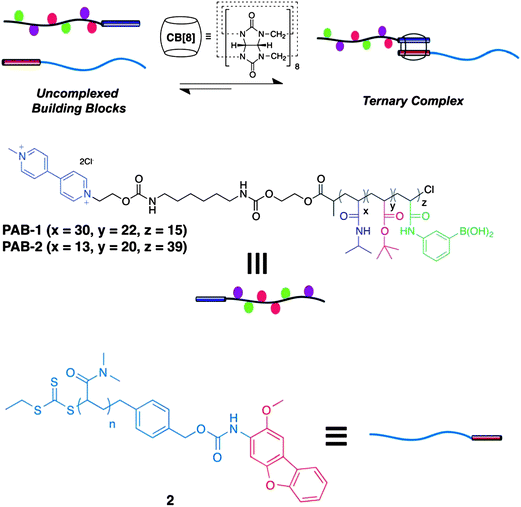 | ||
| Fig. 19 Schematic illustration for the formation of polymeric cucurbit[8]uril ternary complex. (Reproduced from ref. 66 with permission from The Royal Society of Chemistry.) | ||
Except for potential application in insulin delivery, PBA-based micelles were also used for the delivery of other drugs. Recently, PBA-functionalized polyion complex (PIC) micelles for ATP-triggered release of siRNA were reported by Kataoka and coworkers (Fig. 20).67 The polyplex micelles were constructed from 3-fluoro-4-carboxyphenylboronic acid (FPBA) modified PEG-b-PLys and siRNAs, in which reversible covalent esters were formed from phenylboronic acid and 1,2- or 1,3-cis-diols on a ribose ring, a structure which is present at the 3′ end of RNAs and several kinds of ribonucleotides. This kind of polyplex micelle incorporated three representative approaches to stabilize PIC-based siRNA carriers while maintaining a wide window of control for environmental sensitivity. The polyplex micelles could be tailored to exhibit dramatic structural disruption, accompanied by the release of siRNAs in response to a change of ATP concentration. Preliminary studies of the polyplex micelle-based siRNA delivery system demonstrated dose-dependent silencing of polo-like kinase 1 (PLK-1) gene, a well-known proto-oncogene, in a human renal carcinoma cell line (OSRC-2). In another study, Raines and coworkers68 demonstrated the successful delivery of a protein (RNase A) into the cytosol of mammalian cells mediated by pendant boronic acids.
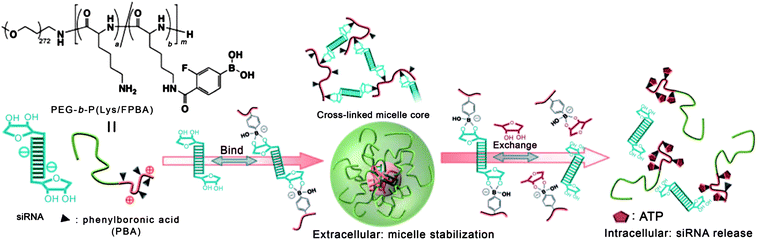 | ||
| Fig. 20 Schematic illustration of the phenylboronic acid modified block copolymer assemblies for ATP-responsive siRNA delivery. (Reprinted with permission from ref. 67. Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
4. Self-assembled PBA-based vesicles
PBA-based glucose-responsive nanogels and micelles have been mostly studied in past decades. In recent years, self-assembled PBA-based vesicles were also reported with an important superiority of large loading capacity. A pioneering work by van Hest and coworkers69 has shown a polymersome nanoreactor with controllable permeability induced by sugar-responsive block copolymers. They devised a simple method to generate membrane permeability of polymersomes consisting of a conventional amphiphilic block copolymer, polyethylene glycol-block-polystyrene (PEG-b-PS), as shown in Fig. 21. The key concept of their method was to use stimuli-responsive block copolymers as pore-generating components. They synthesized a series of sugar-responsive block copolymers, poly(ethylene glycol)-b-poly(styrene boronic acid) (PEG-b-PSBA, 2b–4b, Fig. 21A) via ATRP of a pinacol-protected styrene boronic acid initiated by a PEG macroinitiator followed by removal of pinacol. PEG-b-PSBA self-assembled into vesicular objects (orange in Fig. 21B) which could disassemble into molecularly dissolved block copolymers upon increase of pH or addition of sugars. When a mixture of PEG-b-PS and PEG-b-PSBA were used, polymersomes with controllable permeability were obtained. This was ascribed to the immiscibility between PS and PSBA, which resulted in a stimuli-responsive PSBA domain in the phase-separated vesicular membrane. This kind of polymersome was successfully used as a nanoreactor by encapsulating an enzyme, where PSBA domains became voids that permitted transmembrane diffusion of small reagents.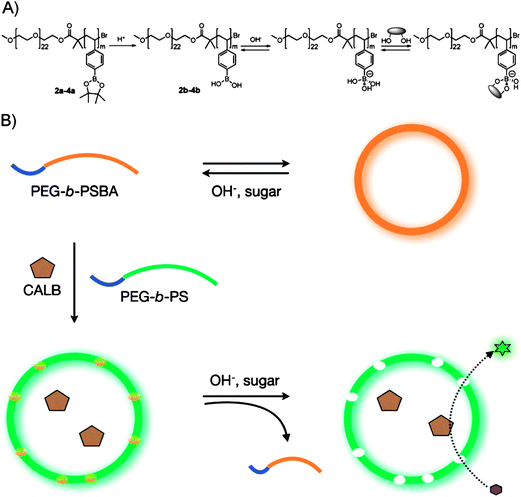 | ||
| Fig. 21 (A) Molecular structure of block copolymers, PEG-b-PSBA, and their equilibrium with sugar molecules in a basic aqueous phase. (B) Schematic representation of the formation of bioreactors with a permeable membrane utilizing the sugar responsiveness of the block copolymers. (Reprinted with permission from ref. 69. Copyright © 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
Monosaccharide-responsive release of Insulin from polymersomes of polyboroxole block copolymers at neutral pH was reported by Kim et al.70 A boroxole-containing styrenic monomer with increased Lewis acidity was synthesized and polymerized via RAFT method with end-functionalized PEG as a chain transfer agent. The resultant block copolymer poly(ethylene glycol)-b-poly(styreneboroxole) (PEG-b-PBOx) self-assembled into a variety of nanostructures including spherical and cylindrical micelles and polymer vesicles (polymersomes) (Fig. 22). Polymersomes of these block copolymers exhibited monosaccharide-responsive disassembly in a neutral pH medium. Encapsulated insulin could be released from the polymersomes only in the presence of sugars under physiologically relevant pH conditions.
 | ||
| Fig. 22 Self-assembly of PEG-b-PBOx and its disassembly in the presence of monosaccharides. (Reprinted with permission from ref. 70. Copyright © 2012, American Chemical Society.) | ||
A new class of sugar-responsive polymers that were based on a sequence-specific copolymer of styreneboroxole and N-functionalized maleimide were reported by Kim and coworkers.71 The RAFT polymerization of this pair of monomers ensured that a glucose receptor alternates with a nonresponsive solubilizing group throughout the sugar-responsive polymer chain. Amphiphilic block copolymers constructed using this sequence-specific sugar-responsive polymer block self-assembled into polymersomes in water, which could encapsulate water-soluble molecules, such as fluorescein-labeled insulin, within their inner compartment (Fig. 23). Due to the presence of hydrophilic solubilizing groups between the solubility-switching boroxole moieties in the membrane-forming block, the polymersomes of the block copolymers responded to a lower level of glucose in the medium, resulting in disassembly of the bilayer membrane under a physiologically relevant pH and glucose level. The encapsulated insulin was released via the glucose-triggered disassembly of the polymersomes.
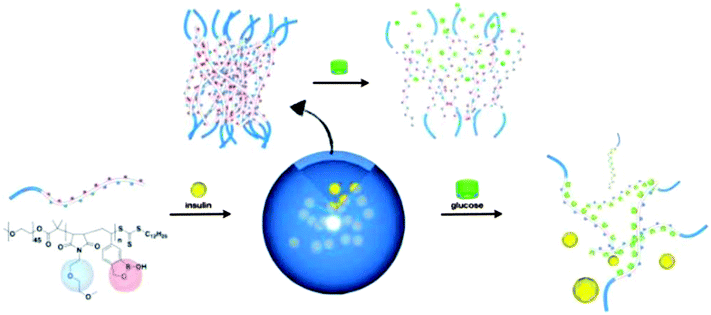 | ||
| Fig. 23 Schematic illustration of glucose-triggered disassembly of polymersomes of sugar-responsive block copolymers. (Reprinted with permission from ref. 71. Copyright © 2012, American Chemical Society.) | ||
PBA-based glucose-responsive polyelectrolyte capsules were also fabricated by layer-by-layer self-assembly of a phenylboronic acid-containing polycation poly(3-acrylamidophenylboronic acid-co-dimethylaminoethyl acrylate) (P(AAPBA-co-DMAEA)) and a polyanion poly(sodium-p-styrenesulfonate) (PSS).72 Addition of glucose induced a rather fast dissolution of the capsules. This concept was based on a glucose-induced change in electrostatic interactions between a polyanion and a phenylboronic acid containing polycation. The mechanism was thought to be that the addition of glucose increased the amount of negatively charged PBA groups, leading to repulsions between P(AAPBA-co-DMAEA) and PSS (Fig. 24). These polyelectrolyte capsules were suggested as promising vehicles for application in the biomedical field for the controlled delivery of insulin.
 | ||
| Fig. 24 Polyelectrolytes used for the fabrication of the hollow nanocapsules and proposed mechanism of the glucose-induced decomplexation of the polyelectrolyte bilayer. (Reprinted with permission from ref. 72. Copyright © 2006, American Chemical Society.) | ||
Another kind of templated polymer microcapsule with sugar-sensitive properties was reported by Levy et al.,73 where polysaccharide mannan and phenylboronic acid moieties grafted PAA were assembled onto colloidal CaCO3 particles based on the interaction between PBA groups on PAA and diols on polysaccharide, forming sugar-sensitive multilayer films. The resulting microcapsules are sensitive to several carbohydrates, and show the highest sensitivity to fructose. This kind of microcapsule was an attractive candidate for the design of carbohydrate-sensing systems and sugar-sensitive delivery systems with the capsule content as a chemical amplifier.
Glucose and temperature dual-responsive monodispersed hollow nanospheres were synthesized via a facile one-pot two-step “self-removing” approach based on the consecutive radical (co)polymerization (Fig. 25).74 PNIPAM oligomers were synthesized and precipitated as a template for the P(NIPAM-co-AAPBA) hollow nanospheres. Increase of the feeding content of the glucose-responsive monomer AAPBA led to smaller nanospheres with porous shells. The hollow nanospheres were glucose responsive at a pH value near the pKa (8.86) of the PBA derivative, with a swelling degree proportional to the concentration of glucose.
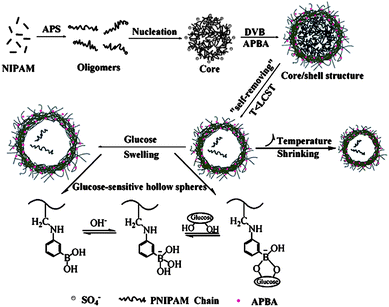 | ||
| Fig. 25 Preparation process of the dual-responsive hollow nanospheres. (Reprinted with permission from ref. 74. Copyright © 2012, Elsevier.) | ||
In addition to macromolecules, small amphiphiles were also used to construct PBA-based vesicles. Karpichev and coworkers75 reported a new approach for creating water-soluble functionalized vesicles employing N-alkyl-3-boronopyridinium triflates (alkyl = Me, C12H25, C16H33) as sensors for monosaccharides (Fig. 26). They synthesized N-alkyl-3-boronopyridinium salts with a single chain which spontaneously form vesicular structures in water. The vesicle size (from 30 to 200 nm) was controllable by adjusting the surfactant chain length. This system was suggested to find application in recognizing biologically important polyols.
 | ||
| Fig. 26 A mechanism of diol sensing by boronic acid functionalized vesicles. (Reprinted with permission from ref. 75. Copyright © 2013, American Chemical Society.) | ||
5. PBA-Functionalized silica nanoparticles
On–off regulated drug release was always pursued by scientists but was difficult to achieve on the basis of soft polymeric nanoparticles. Mesoporous silica nanoparticles (MSNPs) offer us an opportunity to achieve this target because of their hard structure, large capacity for drug loading, and the surface that can easily be modified. Zhao et al.76 developed a glucose-responsive MSNPs-based double delivery system for both insulin and cyclic adenosine monophosphate (cAMP) with precise control over the sequence of release (Fig. 27). cAMPs gluconic acid-modified insulin (G-Ins) proteins were immobilized on the outermost surface of phenylboronic acid-functionalized MSNPs via reversible covalent bonding. G-Ins also served as caps to encapsulate cAMP molecules inside the mesoporous channels. The performance of this nanogated system relies on the fact that phenylboronic acid forms much more stable cyclic esters with the adjacent diols of saccharides than with acyclic diols. Therefore, the presence of saccharides, such as glucose, would trigger the release of both G-Ins and cAMP from MSNPs.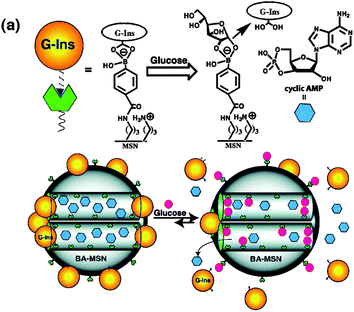 | ||
| Fig. 27 Schematic representation of phenylboronic acid-functionalized MSN for controlled release of bioactive G-Ins and cAMP. (Reprinted with permission from ref. 76. Copyright © 2009, American Chemical Society.) | ||
Martínez-Mañez and coworkers77 proposed a nanoscopic molecular movable gate-like functional hybrid system by modifying the pore outlets of MSNPs with a saccharide derivative capable of interacting with boronic acid functionalized gold nanoparticles (AuNPs) acting as nanoscopic caps (Fig. 28). The AuNPs were linked to the surface of saccharide-functionalized MSNPs through the formation of boronate ester bonds and thus blocked the mesopores. The boronate ester bonds could be hydrolyzed under acid conditions (pH 3) or broken by laser irradiation caused surface plasmon resonance of AuNPs at 1064 nm. The nanosystem exhibited an “on–off” release of entrapped guest because of the reversible reaction between the saccharide derivative anchored on the external surface of the mesoporous silica and boronic acids anchored on AuNPs.
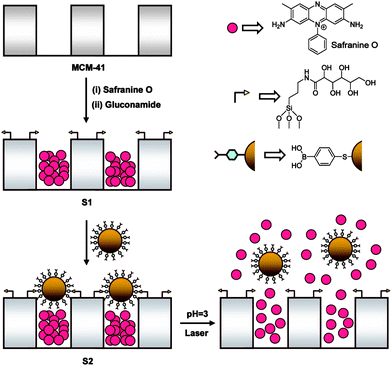 | ||
| Fig. 28 Schematic preparation of mesoporous silica with boronic acid functionalized AuNPs as nanocaps and the pH/laser light triggered release of the entrapped guest. (Reprinted with permission from ref. 77. Copyright © 2009, American Chemical Society.) | ||
6. Conclusion and outlook
Because of their versatility for different designs, phenylboronic acid and its derivatives have been widely studied for the construction of glucose-responsive nanomaterials, such as nanogels (microgels), micelles, vesicles, and mesoporous silica nanoparticles. Most drug delivery studies based on PBA-containing nanoparticles, until now, were focused on insulin aiming at treatment of diabetes mellitus. A series of progresses have been made in these years including synthesis of new polymers, fabrication of nanomaterials operating under physiological conditions, successful in vivo delivery of insulin. Crosslinked PBA-based nanogels offer structure integrity during drug delivery but may encounter the plight of quick clearance in blood circulation because the nanogels lack the protection by a corona layer just like self-assembled micelles protected by a PEG corona and the difficulty of renal excretion because of their non-biodegradability. Self-assembled PBA-based micelles have been most frequently studied in recent years as potential and excellent carriers for insulin delivery due to their faster response to glucose at neutral pH, longer circulation stability as a result of the protection by PEG, and easier drug loading upon self-assembly. Self-assembled PBA-based vesicles have an important superiority of large loading capacity. PBA-Functionalized silica nanoparticles offer large capacity for drug loading and on–off regulated glucose-responsive drug release due to the PBA moieties modified on the surface. By making full use of their advantages and minimizing their disadvantages, PBA-based nanoparticles may find more exciting advances in the future.However, more attention should be paid to some problems. The first one is that effective encapsulation of insulin is still difficult for PBA-based nanomaterials because of the lack of strong interaction between them. Possible ways to increase the loading efficiency of insulin may include encapsulation by polymeric nanocapsules78,79 and/or self-assembly between glycosylated insulin and PBA-containing polymers. The second problem is on–off regulated or quantitative release of insulin on-demand by PBA-based nanocarriers. This is vitally important for practical application of them in the treatment of diabetes mellitus. But until now successful examples are still very rare. The third problem is to develop nanocarriers that can be effectively absorbed by intestinal mucosa. Though a lot of polymeric nanocarriers such as chitosan-based nanoparticles80,81 have been reported aiming at oral administration and effective absorption of insulin by intestinal mucosa, it is still very challenging for PBA-containing nanoparticles to be used in oral administration of protein drugs. Last but not least, PBA-based nanocarriers should find more applications in delivery of drugs other than insulin.
Acknowledgements
We thank the National Natural Science Foundation of China (no. 21274001, 91127045), the National Basic Research Program of China (973 Program, no. 2011CB932503), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), and PCSIRT (IRT 1257) for financial support.References
- S. Kitano, I. Hisamitsu, Y. Koyama, K. Kataoka, T. Okano and Y. Sakurai, Polym. Adv. Technol., 1991, 2, 261–264 CrossRef CAS.
- D. Shiino, Y. Murata, A. Kubo, Y. J. Kim, K. Kataoka, Y. Koyama, A. Kikuchi, M. Yokoyama, Y. Sakurai and T. Okano, J. Controlled Release, 1995, 37, 269–276 CrossRef CAS.
- A. Matsumoto, S. Ikeda, A. Harada and K. Kataoka, Biomacromolecules, 2003, 4, 1410–1416 CrossRef CAS PubMed.
- K. Kataoka, H. Miyazaki, M. Bunya, T. Okano and Y. Sakurai, J. Am. Chem. Soc., 1998, 120, 12694–12695 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed., 1996, 35, 1910–1922 CrossRef.
- F. Cheng and F. Jakle, Polym. Chem., 2011, 2, 2122–2132 RSC.
- A. P. Vogt, V. Trouillet, A. M. Greiner, M. Kaupp, U. Geckle, L. Barner, T. Hofe and C. Barner-Kowollik, Macromol. Rapid Commun., 2012, 33, 1108–1113 CrossRef CAS PubMed.
- Y. J. Zhang, Y. Guan and S. Q. Zhou, Biomacromolecules, 2006, 7, 3196–3201 CrossRef CAS PubMed.
- T. Hoare and R. Pelton, Macromolecules, 2007, 40, 670–678 CrossRef CAS.
- T. Hoare and R. Pelton, Biomacromolecules, 2008, 9, 733–740 CrossRef CAS PubMed.
- A. Matsumoto, T. Kurata, D. Shiino and K. Kataoka, Macromolecules, 2004, 37, 1502–1510 CrossRef CAS.
- H. R. Mulla, N. J. Agard and A. Basu, Bioorg. Med. Chem. Lett., 2004, 14, 25–27 CrossRef CAS PubMed.
- A. Matsumoto, T. Ishii, J. Nishida, H. Matsumoto, K. Kataoka and Y. Miyahara, Angew. Chem., Int. Ed., 2012, 51, 2124–2128 CrossRef CAS PubMed.
- Y. P. Li, W. W. Xiao, K. Xiao, L. Berti, J. T. Luo, H. P. Tseng, G. Fung and K. S. Lam, Angew. Chem., Int. Ed., 2012, 51, 2864–2869 CrossRef CAS PubMed.
- A. Matsumoto, R. Yoshida and K. Kataoka, Biomacromolecules, 2004, 5, 1038–1045 CrossRef CAS PubMed.
- W. T. Wu, N. Mitra, E. Yan and S. Q. Zhou, ACS Nano, 2010, 4, 4831–4839 CrossRef CAS PubMed.
- K. T. Kim, J. Cornelissen, R. Nolte and J. van Hest, J. Am. Chem. Soc., 2009, 131, 13908 CrossRef CAS PubMed.
- B. L. Wang, R. J. Ma, G. Liu, Y. Li, X. J. Liu, Y. L. An and L. Q. Shi, Langmuir, 2009, 25, 12522–12528 CrossRef CAS PubMed.
- B. L. Wang, R. J. Ma, G. Liu, X. J. Liu, Y. H. Gao, J. Y. Shen, Y. L. An and L. Q. Shi, Macromol. Rapid Commun., 2010, 31, 1628–1634 CrossRef CAS PubMed.
- R. J. Ma, H. Yang, Z. Li, G. Liu, X. C. Sun, X. J. Liu, Y. L. An and L. Q. Shi, Biomacromolecules, 2012, 13, 3409–3417 CrossRef CAS PubMed.
- H. Yang, X. Sun, G. Liu, R. Ma, Z. Li, Y. An and L. Shi, Soft Matter, 2013, 9, 8589–8599 RSC.
- M. Dowlut and D. G. Hall, J. Am. Chem. Soc., 2006, 128, 4226–4227 CrossRef CAS PubMed.
- A. R. Martin, J. Vasseur and M. Smietana, Chem. Soc. Rev., 2013, 42, 5684–5713 RSC.
- Y. Guan and Y. Zhang, Chem. Soc. Rev., 2013, 42, 8106–8121 RSC.
- Q. Wu, L. Wang, H. J. Yu, J. J. Wang and Z. F. Chen, Chem. Rev., 2011, 111, 7855–7875 CrossRef CAS PubMed.
- M. Colilla, B. Gonzalez and M. Vallet-Regi, Biomater. Sci., 2013, 1, 114–134 RSC.
- J. N. Cambre and B. S. Sumerlin, Polymer, 2011, 52, 4631–4643 CrossRef CAS PubMed.
- N. Fujita, S. Shinkai and T. D. James, Chem.–Asian J., 2008, 3, 1076–1091 CrossRef CAS PubMed.
- W. T. Wu and S. Q. Zhou, Macromol. Biosci., 2013 DOI:10.1002/mabi.201300120.
- S. Kitano, K. Kataoka, Y. Koyama, T. Okano and Y. Sakurai, Macromol. Rapid Commun., 1991, 12, 227–233 CrossRef CAS.
- S. Kitano, Y. Koyama, K. Kataoka, T. Okano and Y. Sakurai, J. Controlled Release, 1992, 19, 161–170 CrossRef CAS.
- K. Kataoka, H. Miyazaki, T. Okano and Y. Sakurai, Macromolecules, 1994, 27, 1061–1062 CrossRef CAS.
- V. Ravaine, C. Ancla and B. Catargi, J. Controlled Release, 2008, 132, 2–11 CrossRef CAS PubMed.
- S. Y. Xing, Y. Guan and Y. J. Zhang, Macromolecules, 2011, 44, 4479–4486 CrossRef CAS.
- H. Ge, Y. W. Ding, C. C. Ma and G. Z. Zhang, J. Phys. Chem. B, 2006, 110, 20635–20639 CrossRef CAS PubMed.
- V. Lapeyre, I. Gosse, S. Chevreux and V. Ravaine, Biomacromolecules, 2006, 7, 3356–3363 CrossRef CAS PubMed.
- V. Lapeyre, C. Ancla, B. Catargi and V. Ravaine, J. Colloid Interface Sci., 2008, 327, 316–323 CrossRef CAS PubMed.
- Z. H. Farooqi, W. T. Wu, S. Q. Zhou and M. Siddiq, Macromol. Chem. Phys., 2011, 212, 1510–1514 CrossRef CAS.
- G. Zenkl, T. Mayr and I. Khmant, Macromol. Biosci., 2008, 8, 146–152 CrossRef CAS PubMed.
- L. Zhao, C. S. Xiao, J. X. Ding, P. He, Z. H. Tang, X. Pang, X. L. Zhuang and X. S. Chen, Acta Biomater., 2013, 9, 6535–6543 CrossRef CAS PubMed.
- X. Zhang, S. Lu, C. Gao, C. Chen, X. Zhang and M. Liu, Nanoscale, 2013, 5, 6498–6506 RSC.
- Z. M. Wu, X. G. Zhang, H. L. Guo, C. X. Li and D. M. Yu, J. Mater. Chem., 2012, 22, 22788–22796 RSC.
- Y. Qin, V. Sukul, D. Pagakos, C. Z. Cui and F. Jäkle, Macromolecules, 2005, 38, 8987–8990 CrossRef CAS.
- Y. Qin, G. L. Cheng, A. Sundararaman and F. Jäkle, J. Am. Chem. Soc., 2002, 124, 12672–12673 CrossRef CAS PubMed.
- J. N. Cambre, D. Roy, S. R. Gondi and B. S. Sumerlin, J. Am. Chem. Soc., 2007, 129, 10348–10349 CrossRef CAS PubMed.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Chem. Commun., 2008, 2477–2479 RSC.
- D. Roy and B. S. Sumerlin, ACS Macro Lett., 2012, 1, 529–532 CrossRef CAS.
- Y. Yao, X. M. Wang, T. W. Tan and J. Yang, Soft Matter, 2011, 7, 7948–7951 RSC.
- Y. Yao, L. Y. Zhao, J. J. Yang and J. Yang, Biomacromolecules, 2012, 13, 1837–1844 CrossRef CAS PubMed.
- J. N. Cambre, D. Roy and B. S. Sumerlin, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3373–3382 CrossRef CAS.
- L. Zhao, J. X. Ding, C. S. Xiao, P. He, Z. H. Tang, X. Pang, X. L. Zhuang and X. S. Chen, J. Mater. Chem., 2012, 22, 12319–12328 RSC.
- G. Liu, R. J. Ma, J. Ren, Z. Li, H. X. Zhang, Z. K. Zhang, Y. L. An and L. Q. Shi, Soft Matter, 2013, 9, 1636–1644 RSC.
- A. P. Bapat, D. Roy, J. G. Ray, D. A. Savin and B. S. Sumerlin, J. Am. Chem. Soc., 2011, 133, 19832–19838 CrossRef CAS PubMed.
- J. Yoon and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 5874–5875 CrossRef CAS.
- G. Springsteen and B. H. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- X. J. Jin, X. G. Zhang, Z. M. Wu, D. Y. Teng, X. J. Zhang, Y. X. Wang, Z. Wang and C. X. Li, Biomacromolecules, 2009, 10, 1337–1345 CrossRef CAS PubMed.
- X. G. Zhang, Y. X. Wang, C. Zheng and C. X. Li, Eur. J. Pharm. Biopharm., 2012, 82, 76–84 CrossRef CAS PubMed.
- C. Cheng, X. Zhang, Y. Wang, L. Sun and C. Li, New J. Chem., 2012, 36, 1413–1421 RSC.
- C. Zheng, Q. Guo, Z. Wu, L. Sun, Z. Zhang, C. Li and X. Zhang, Eur. J. Pharm. Sci., 2013, 49, 474–482 CrossRef CAS PubMed.
- C. Cheng, X. G. Zhang, J. X. Xiang, Y. X. Wang, C. Zheng, Z. T. Lu and C. X. Li, Soft Matter, 2012, 8, 765–773 RSC.
- Y. X. Wang, X. G. Zhang, J. Mu and C. X. Li, New J. Chem., 2013, 37, 796–803 RSC.
- Y. X. Wang, X. G. Zhang, Y. C. Han, C. Cheng and C. X. Li, Carbohydr. Polym., 2012, 89, 124–131 CrossRef CAS PubMed.
- M. Berube, M. Dowlut and D. G. Hall, J. Org. Chem., 2008, 73, 6471–6479 CrossRef CAS PubMed.
- J. W. Tomsho and S. J. Benkovic, J. Org. Chem., 2012, 77, 11200–11209 CrossRef CAS PubMed.
- Y. Kotsuchibashi, R. Agustin, J. Y. Lu, D. G. Hall and R. Narain, ACS Macro Lett., 2013, 2, 260–264 CrossRef CAS.
- X. J. Loh, M. H. Tsai, J. Del Barrio, E. A. Appel, T. C. Lee and O. A. Scherman, Polym. Chem., 2012, 3, 3180–3188 RSC.
- M. Naito, T. Ishii, A. Matsumoto, K. Miyata, Y. Miyahara and K. Kataoka, Angew. Chem., Int. Ed., 2012, 51, 10751–10755 CrossRef CAS PubMed.
- G. A. Ellis, M. J. Palte and R. T. Raines, J. Am. Chem. Soc., 2012, 134, 3631–3634 CrossRef CAS PubMed.
- K. T. Kim, J. Cornelissen, R. Nolte and J. van Hest, Adv. Mater., 2009, 21, 2787 CrossRef CAS.
- H. Kim, Y. J. Kang, S. Kang and K. T. Kim, J. Am. Chem. Soc., 2012, 134, 4030–4033 CrossRef CAS PubMed.
- H. Kim, Y. J. Kong, E. S. Jeong, S. Kong and K. T. Kim, ACS Macro Lett., 2012, 1, 1194–1198 CrossRef CAS.
- B. G. De Geest, A. M. Jonas, J. Demeester and S. C. De Smedt, Langmuir, 2006, 22, 5070–5074 CrossRef CAS PubMed.
- T. Levy, C. Dejugnat and G. B. Sukhorukov, Adv. Funct. Mater., 2008, 18, 1586–1594 CrossRef CAS.
- P. C. Du, B. Mu, Y. J. Wang and P. Liu, Mater. Lett., 2012, 75, 77–79 CrossRef CAS PubMed.
- O. Savsunenko, H. Matondo, S. Franceschi-Messant, E. Perez, A. F. Popov, I. Rico-Lattes, A. Lattes and Y. Karpichev, Langmuir, 2013, 29, 3207–3213 CrossRef CAS PubMed.
- Y. N. Zhao, B. G. Trewyn, I. I. Slowing and V. Lin, J. Am. Chem. Soc., 2009, 131, 8398 CrossRef CAS PubMed.
- E. Aznar, M. D. Marcos, R. Martínez-Mañez, F. Sancenon, J. Soto, P. Amoros and C. Guillem, J. Am. Chem. Soc., 2009, 131, 6833–6843 CrossRef CAS PubMed.
- M. Yan, J. Du, Z. Gu, M. Liang, Y. Hu, W. Zhang, S. Priceman, L. Wu, Z. H. Zhou, Z. Liu, T. Segura, Y. Tang and Y. Lu, Nat. Nanotechnol., 2010, 5, 48–53 CrossRef CAS PubMed.
- Y. Liu, J. J. Du, M. Yan, M. Y. Lau, J. Hu, H. Han, O. O. Yang, S. Liang, W. Wei, H. Wang, J. M. Li, X. Y. Zhu, L. Q. Shi, W. Chen, C. Ji and Y. F. Lu, Nat. Nanotechnol., 2013, 8, 187–192 CrossRef CAS PubMed.
- H. W. Sung, K. Sonaje, Z. X. Liao, L. W. Hsu and E. Y. Chuang, Acc. Chem. Res., 2012, 45, 619–629 CrossRef CAS PubMed.
- M. C. Chen, K. Sonaje, K. J. Chen and H. W. Sung, Biomaterials, 2011, 32, 9826–9838 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


