Efficient protein–ligand interaction by guaranteeing mesospacing between immobilized biotins†
Young-Seo
Choi
,
Chang
Won Yoon
,
Hae
Dong Lee
,
Minyoung
Park
and
Joon
Won Park
*
Center for Integrated Molecular Systems, Department of Chemistry, Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang 790-784, Korea. E-mail: jwpark@postech.ac.kr; Fax: +82-54-279-0653; Tel: +82-54-279-2119
First published on 10th May 2004
Abstract
The streptavidin–biotin interaction on a monolayer of a conically shaped dendrimer was investigated by surface plasmon resonance (SPR) spectroscopy and the interaction on the mesospaced surface was found to be as efficient as the one on the mixed monolayers at a lower concentration of immobilized biotin.
The concentration of a ligand on the surface is a key factor that governs interactions between immobilized ligands and their corresponding proteins. In spite of several advantages, ligands immobilized at high densities frequently have chemical and biological properties that are substantially different from those of the same ligand presented in a natural environment.1,2 Moreover, non-inert ligands of a high density may promote nonspecific protein adsorption.2 Optimizing the density to relieve the surface materials from steric congestion while also maintaining signal intensities and an apparent binding capacity sufficient for applications such as biosensors and biochips, is a primary goal of new bioactive surfaces. Typically, the functional group densities of the thin film are commonly adjusted by co-deposition of both an inert adsorbate and a functionalized one.3,4 However, phase separation into microscopic or nanoscopic domains with distinct functional groups is difficult to prevent especially when strong inter-group interactions are present.5
To provide the optimal ligand density and avoid the deleterious phase separation, we have studied the self-assembly of a conically shaped dendrimer of which the termini are capable of binding to the surface and the apex is reactive for the immobilization of biomolecules.6 This approach is reminiscent of an earlier report by Whitesell and Chang in which enhanced α-helix formation of oligopeptides on the surface through the use of conically shaped thiol adsorbates was demonstrated.7 Our previous study showed that multiple ionic attraction between cations on the glass substrates and anionic carboxylates at the termini of the dendrimer was successful in generating a well-behaved monolayer and guaranteeing an inter-ligand spacing over 24 Å. It was realized that the molecular structure of the dendrimers needs to be optimized for further application. For more facile deprotection and enhanced amine reactivity, a 9-fluorenylmethoxycarbonyl (Fmoc) group and a spacer were employed (Fig. 1a).‡ Also, we found that covalent bond formation between the carboxylic acid group and surface amine group was as effective as the ionic attraction while also providing enhanced environmental stability.
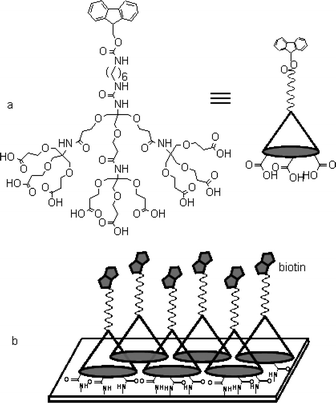 | ||
| Fig. 1 Molecular structure of the dendrimer used in this study (a) and a schematic drawing of the biotinylated dendrimer monolayer (b). | ||
As indicated schematically in Fig. 1b, a biotin immobilized dendrimer monolayer was prepared and its efficiency in streptavidin (SA) binding was compared with biotinylated mixed self-assembled monolayers (SAMs) via surface plasmon resonance (SPR) spectroscopy. First, we generated the dendrimer monolayer on a gold surface modified by 11-mercaptoundecylamine (MA). The dendrimer was dissolved in aqueous solution, dissolving 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC, 0.10 M) and N-hydroxysulfosuccinimide (sulfo-NHS, 0.10 M) in order to activate the terminal carboxylic acid group. The activated dendrimer was allowed to react with the amine group of the MA layer, and formation of multiple amide bonds resulted. In order to block the residual amine group, a capping process utilizing acetic anhydride was followed. After deprotection of the Fmoc group, the amine density of the dendrimer-modified monolayer was measured by the method established in this laboratory.6 Fluorescence intensity measurements showed that amine density, in other words, density of the dendrimer was 0.083 ea per 100 Å2. The observed density is close to that achieved in the previous study utilizing the ionic attraction and corresponds to an average spacing of 37 Å.§
For a comparison, we selected a representative set of mixed SAMs composed of 16-mercaptohexadecanoic acid (16-MHA) and 11-mercaptoundecanol (11-MUOH). The mixed SAMs were biotinylated afterward with a (+)-biotinyl-3,6,9-trioxaundecanediamine (biotin-LC-PEO-amine) to compare with properties of the mesospaced monolayer. Knoll and coworkers showed in their SPR study that these mixed SAMs consisting of biotin-terminated and hydroxyl-terminated alkylthiols could successfully capture SA with high specificity and coverage.3 We modified the dendrimer layer with 4.0 mg mL−1 of succinimidyl D-biotin (12 mM) and biotinylated the mixed SAMs with the same concentration of biotin-LC-PEO-amine solution. To analyze the streptavidin binding, the biotinylated surfaces were pre-rinsed with PBS for 10 min, and the streptavidin solution (0.50 μM, in PBS) was injected with a flow rate of 1.0 μLmin−1 for 30 min. After washing with PBS for 30 min, the increase in response (ΔRU) of each layer for streptavidin association was estimated. The observed ΔRU of each layer was summarized in Table 1. Considering the projected area of SA (∼2500 Å2),8 ΔRU in the dendrimer monolayer corresponds to approximately 67% surface coverage.¶ In the case of the 16-MHA/11-MUOH mixed SAMs, the surface coverage was 62% and 45% for 1 ∶ 12 and 1 ∶ 100 ratios, respectively. Assuming that all the functional groups of each SAM were biotinylated, the density of biotin in 1 ∶ 12 and 1 ∶ 100 mixed SAMs, respectively, was 0.37 and 0.047 ea per 100 Å2.|| Though the density of the biotin in the dendrimer-modified monolayer (0.083 ea per 100 Å2) is close to the one seen for the 1 ∶ 100 mixed SAM, interestingly, the binding level of SA on the dendrimer-modified layer is close to the 1 ∶ 12 mixed SAM case. This observation indicates that the proper ligand spacing realized by the particular dendrimer is very successful in retaining the binding capability of the biotin.
| Layer | ΔRU (in unit of kRU) | Density of derivatized biotin/100 Å2 | Surface coverage (%) |
|---|---|---|---|
| Mixed SAM (1 ∶ 100) | 1.8 ± 0.3 | 0.047 | 45 ± 7 |
| Mixed SAM (1 ∶ 12) | 2.5 ± 0.2 | 0.37 | 62 ± 5 |
| Dendrimer layer | 2.7 ± 0.2 | 0.083 ± 0.001 | 67 ± 5 |
To scrutinize the binding event on the biotinylated dendrimer monolayer in more detail, the monolayer was functionalized with various concentrations of biotin and the SA association level was quantitatively investigated. As a result, it was observed that a concentration of succinimidyl D-biotin as low as 1.0 mg mL−1 was sufficient to reach the maximum binding of SA (Fig. 2). In the case of mixed SAMs reacted at the same concentration of biotin-LC-PEO-amine (1.2 mg mL−1), the surface coverage was 42% and 26% for 1 ∶ 12 and 1 ∶ 100 respectively. These values are far short from the saturation coverages (62% and 45%). This difference clearly indicates that the immobilization of a ligand on the mesospaced surface is also efficient.
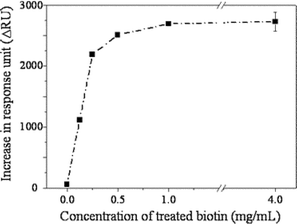 | ||
| Fig. 2 Binding level of strepavidin on the dendrimer monolayer that had been biotinylated with various concentrations of succinimidyl D-biotin. | ||
We examined the specificity of the interaction between SA and biotin on the dendrimer monolayer. The mesospaced monolayer modified with 1.0 mg mL−1 biotin was reacted with a solution of SA that had been pre-saturated with biotin, and ΔRU was recorded. It was found that the binding level of SA was less than 2% of that seen without the pre-saturation. Secondly, a SA solution was added to a pristine dendrimer monolayer (not biotinylated), and the binding level was found to be as low as the previous control experiment. These results demonstrate that the dendrimer monolayer is resistant to the nonspecific adsorption of SA, and the above-recorded ΔRU originated solely from the specific binding.
In summary, we have demonstrated that a focally functionalized dendrimeric monolayer is efficient in achieving specific streptavidin–biotin interaction. As compared with the mixed SAMs, the comparable binding level of SA was observed with the biotinylated dendrimer layer even at lower ligand density. The results demonstrate that appropriate spacing control is very important in enhancing the interaction on the surface. It is believed that this approach is general enough to be applied for study of other protein–ligand interactions on the solid surface.
This work was supported by R&D Program for Fusion Strategy of Advanced Technologies (MOST) and Student fellowships of the Brain Korea 21. PCT Application No. PCT/ KR03/02261.
Notes and references
- D. J. Revell, J. R. Knight, D. J. Blyth, A. H. Haines and D. A. Russell, Langmuir, 1998, 14, 4517 CrossRef CAS; J. K. Whitesell, H. K. Chang and C. S. Whitesell, Angew. Chem., Int. Ed. Engl., 1994, 33, 871 CrossRef; B. T. Houseman and M. Mrksich, Angew. Chem., Int. Ed., 1999, 38, 782 CrossRef CAS; J. Lahiri, L. Isaacs, J. Tien and G. M. Whitesides, Anal. Chem., 1999, 71, 777 CrossRef CAS; J. Lahiri, L. Isaacs, B. Grzybowski, J. D. Carbeck and G. M. Whitesides, Langmuir, 1999, 15, 7186 CrossRef CAS.
- A. Murza, R. Fernández-Lafuente and J. M. Guisán, J. Chromatogr. B, 2000, 740, 211 CrossRef CAS.
- L. Haussling, H. Ringsdorf, F.-J. Schmitt and W. Knoll, Langmuir, 1991, 7, 1837 CrossRef; W. Knoll, L. Angermaier, G. Batz, T. Fritz, S. Fujisawa, T. Furuno, H. J. Guder, M. Hara, M. Liley, K. Niki and J. Spinke, Synth. Met., 1993, 61, 5 CrossRef CAS; J. Spinke, M. Liley, F.-J. Schmitt, H. J. Guder, L. Angermaier and W. Knoll, J. Chem. Phys., 1993, 99, 7012 CrossRef CAS; J. Spinke, M. Liley, H. J. Guder, L. Angermaier and W. Knoll, Langmuir, 1993, 9, 1821 CrossRef CAS.
- V. H. Perez-Luna, M. J. O'Brien, K. A. Opperman, P. D. Hampton, P. S. Stayton, L. Klumb and G. P. Lopez, J. Am. Chem. Soc., 1999, 121, 6469 CrossRef CAS; L. S. Jung, K. E. Nelson, P. S. Stayton and C. T. Campbell, Langmuir, 2000, 16, 9421 CrossRef CAS; L. A. Ruiz-Taylor, T. L. Martin and P. Wagner, Langmuir, 2001, 17, 7313 CrossRef CAS.
- A. Heise, M. Stamm, M. Rauscher, H. Duschner and H. Menzel, Thin Solid Films, 1998, 329, 199 CrossRef; P. A. Lewis, Z. J. Donhauser, B. A. Mantooth, R. K. Smith, L. A. Bumm, K. F. Kelly and P. S. Weiss, Nanotechnology, 2001, 12, 231 CrossRef CAS; P. A. Lewis, R. K. Smith, K. F. Kelly, L. A. Bumm, S. M. Reed, R. S. Clegg, J. D. Gunderson, J. E. Hutchison and P. S. Weiss, J. Phys. Chem. B, 2001, 105, 10630 CrossRef CAS; T. Sawaguchi, Y. Sato and F. Mizutani, J. Electroanal. Chem., 2001, 496, 50 CrossRef CAS; R. K. Smith, S. M. Reed, P. A. Lewis, J. D. Monnell, R. S. Clegg, K. F. Kelly, L. A. Bumm, J. E. Hutchison and P. S. Weiss, J. Phys. Chem. B., 2001, 105, 1119 CrossRef CAS; J. F. Kang, S. Liao, R. Jordan and A. Ulman, J. Am. Chem. Soc., 1998, 120, 9662 CrossRef CAS.
- B. J. Hong, J. Y. Shim, S. J. Oh and J. W. Park, Langmuir, 2003, 19, 2357 CrossRef CAS.
- J. K. Whitesell and H. K. Chang, Science, 1993, 261, 73 CrossRef CAS.
- P. C. Weber, D. H. Ohlendorf, J. J. Wendoloski and F. R. Salemme, Science, 1989, 243, 85 CrossRef CAS.
- L. G. Fägerstam, Å. Frostell-Karlsson, R. Karlsson, B. Persson and I. Rönnberg, J. Chromatogr., B, 1992, 597, 397 CAS.
- I. Chaiken, S. Rose and R. Karlsson, Anal. Biochem., 1991, 201, 197.
- E. Stenberg, B. Persson, H. Roos and C. Urbaniczky, J. Colloid Interface Sci., 1991, 143, 513 CrossRef CAS.
- M. Mrksich, J. R. Grunwell and G. M. Whitesides, J. Am. Chem. Soc., 1995, 117, 12009 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental details for preparation of substrates for SPR spectroscopy and synthetic procedure for the dendrimer. See http://www.rsc.org/suppdata/cc/b4/b403797a/ |
| ‡ Deprotection of the Cbz group in the previous dendrimer was not efficient once the dendrimer was immobilized on the surface, while catalytic hydrogenation with Pd/C cleaved easily the protecting group in solution. In addition, use of trimethylsilyl iodide in chloroform requires strictly anhydrous conditions. |
| § The spacing between the dendrimers was calculated with a hexagonal closed packing model. |
| ¶ Within a family of similar compounds (e.g., proteins), SPR angle shift (Δdeg) correlates linearly with the mass per unit area of protein adsorbed.9,10 The BIAcore instrument reports Δdeg in response units (or RU; 10,000 RU = 1°). For most proteins, Δdeg of 1,000 RU corresponds to a change of 1 ng mm−2 in the quantity of protein adsorbed at the surface.11 |
| || The ligand density of the mixed SAMs was calculated with the occupancy of 21 Å2 per alkanethiolate reported by Whitesides et al.12 |
| This journal is © The Royal Society of Chemistry 2004 |
