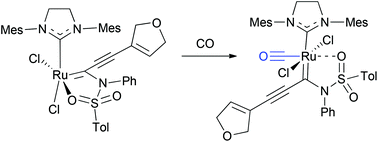
Novel ruthenium alkylidene complexes are prepared via enyne ring-closing metathesis, and the structural features of acetate- and sulfonamide-chelates are explored by single crystal X-ray diffraction analyses. All 5-membered sulfonamide chelates and 6-membered acetate chelates have the chelated oxygen and the NHC ligand in cis relationship, which force the two chloride ligands into cis orientation. Upon exposure to carbon monoxide, while acetate chelates decomposed to generate vinyl acetates, the sulfonamide chelate formed an unprecedented CO-bound octahedral ruthenium alkylidene complex. In this complex, the alkylidene carbene moiety and the NHC ligand have a trans relationship, which is the first example of Grubbs-type ruthenium alkylidene to have this unique trans geometry. This complex is devoid of metathesis activity because of the coordinatively saturated nature of the ruthenium center with a completely locked ligand environment, which does not allow either associative or dissociative ligand–substrate exchange with alkene substrates.