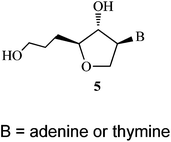
Antisense oligonucleotides and siRNAs are potential therapeutic agents and their chemical modifications play an important role to improve the properties and activities of oligonucleotides. Isonucleoside is a type of nucleoside analogue, in which the nucleobase is moved from C-1 to other positions of ribose. In this report, a novel isonucleoside 5 containing a 5′-CH2-extended chain at the sugar moiety was synthesized, thus isoadenosine 5a and isothymidine 5b were incorporated into a DNA single strand and siRNA. It was found that isonucleoside 5 modified oligonucleotides can form stable double helical structures with their complementary DNA and RNA and the stability towards nuclease and ability to activate RNase H are more promising compared with the unmodified, natural analogues. In siRNA, passenger strand modified with isonucleoside (5a/b) at 3′ or 5′ terminal can retain the silencing activity and minimize the passenger strand specific off-target effect.