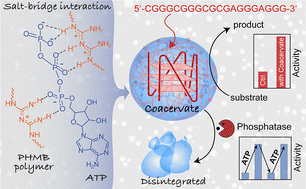
Biomolecular condensates formed via liquid–liquid phase separation (LLPS) are essential to cellular organization, catalysis, and regulation of biochemical pathways. Inspired by such natural systems, we present a new adaptive coacervate formed by multivalent salt-bridge interactions of polyhexamethylene biguanide (PHMB) polymer and adenosine triphosphate (ATP). These phase separated compartments efficiently sequester guanine-rich DNA sequences that adopt G-quadruplex (GQ) conformations in the presence of potassium ions. Hemin intercalates into these GQ structures to produce a catalytically active DNAzyme with amplified peroxidase-like activity. Within the coacervate, reduced molecular diffusion and increased local substrate concentrations synergistically augment the catalytic efficiency of the DNAzyme by 10-fold compared to that in the unconfined state. Integrating an enzymatic degradation cycle by alkaline phosphatase allows ATP-fueled dissipative behavior of the coacervates. By integrating self-assembling catalytic motifs within a dissipative host environment, this system demonstrates key principles of spatially and temporally regulated catalysis, mimicking features of cellular microreactors. Our work highlights the potential of synthetic LLPS-based platforms as tunable and compartmentalized catalytic systems, with implications for biomimetic reactor design and the development of advanced functional materials.