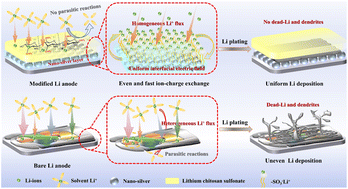
Lithium metal is promising anode material for next-generation ultra-high energy batteries due to its unparalleled theoretical capacity. Nonetheless, its practical application is largely hindered by interfacial instability. Herein, we propose an interfacial engineering strategy employing a sandwich-structured interface comprising a nano-silver (Ag) inner layer and a lithium chitosan sulfonate (LCS) outer layer. The lithophilic nano-silver layer, with its uniformly distributed three-dimensional structure, ensures a consistent interfacial electric field and robustly anchors the LCS, mitigating delamination or decoupling from the Li metal surface during Li plating/stripping. Simultaneously, the LCS coating, characterized by its polysaccharide glycosidic structure, not only delivers exceptional elasticity and mechanical strength but also serves as a robust artificial solid-electrolyte interphase (SEI) layer, preserving the interface's structural integrity. Additionally, the LCS's sulfonic acid groups (–SO3Li) further promote uniform Li-ion flux and maintain high Li+ ionic conductivity. These synergistic effects significantly improve the specific discharge capacity and cycling stability of a C–AgLi‖LiCoO2 full cell, achieving a capacity retention of 83.8% after 350 cycles. These findings elucidate a pathway towards the practical utilization of Li metal anodes by enhancing Li-ion flux, electric field uniformity, and interface adhesion, thus effectively inhibiting Li dendrites.