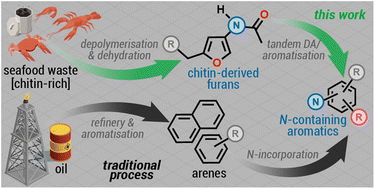
Herein, we addressed the challenge of using two chitin-derived furans, namely 3-acetamido-5-ethylfuran (3A5EF) and 3-acetamido-5-(1-hydroxyethyl)furan (3A5HF), for the formation of 4-acetylaminophthalimides and other N-containing aromatic compounds. The transformation is carried out sequentially and involves the formation of the Diels–Alder adduct, forming a 7-oxa-norbornene backbone, which, in an acidic/acetylating medium, undergoes a dehydration/aromatisation process providing nitrogenated aromatic compounds in yields ranging from 16–80%. The preparation of 4-acetylaminophthalimides from a chitin derivative is the first step towards expanding the toolbox of chitin-derived furans in the synthesis of unique nitrogenated aromatic compounds with the nitrogen atom directly attached to the benzene ring.