Closed-loop chemical recycling of poly(ε-caprolactone) by tuning reaction parameters†
Abstract
Chemical upcycling of commercial plastic materials to their starting monomers is of great significance to address the end-of-use issues of plastic materials and offers a closed-loop pathway for plastic sustainability. Poly(ε-caprolactone) (PCL) materials, synthesized by ring-opening polymerization of ε-caprolactone (ε-CL), have been applied commercially in the fields of medical equipment and 3D printing materials. Here, we demonstrate the first “ε-CL to PCL to ε-CL” and “PCL to ε-CL to PCL” closed-loop processes under the catalysis of magnesium bis[bis(trimethylsilyl)amide] [Mg(HMDS)2]. The thermodynamic parameters 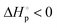 and
and 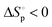 for ε-CL indicate that ε-CL showed a ceiling temperature. After bulk polymerization of ε-CL, the direct bulk depolymerization of the resultant PCL to ε-CL was achieved under high temperature (180 °C) and vacuum (0.07 mbar) conditions via a domino-type unzipping depolymerization mode. The depolymerizations of PCL commodities were also achieved, providing monomeric ε-CL with high yield (up to 98%), selectivity and purity. The resultant ε-CL could smoothly repolymerize into new virgin-quality PCL materials, thus establishing its circular life cycle.
for ε-CL indicate that ε-CL showed a ceiling temperature. After bulk polymerization of ε-CL, the direct bulk depolymerization of the resultant PCL to ε-CL was achieved under high temperature (180 °C) and vacuum (0.07 mbar) conditions via a domino-type unzipping depolymerization mode. The depolymerizations of PCL commodities were also achieved, providing monomeric ε-CL with high yield (up to 98%), selectivity and purity. The resultant ε-CL could smoothly repolymerize into new virgin-quality PCL materials, thus establishing its circular life cycle.

- This article is part of the themed collection: Plastic Conversion


 Please wait while we load your content...
Please wait while we load your content...