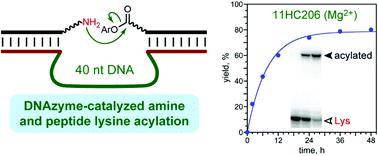
DNAzymes were previously identified by in vitro selection for a variety of chemical reactions, including several biologically relevant peptide modifications. However, finding DNAzymes for peptide lysine acylation is a substantial challenge. By using suitably reactive aryl ester acyl donors as the electrophiles, here we used in vitro selection to identify DNAzymes that acylate amines, including lysine side chains of DNA-anchored peptides. Some of the DNAzymes can transfer a small glutaryl group to an amino group. These results expand the scope of DNAzyme catalysis and suggest the future broader applicability of DNAzymes for sequence-selective lysine acylation of peptide and protein substrates.