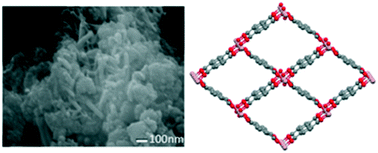
The effects of using low molecular weight alcohols, methanol (MeOH) and ethanol (EtOH), for the synthesis of MIL-53(Al) are investigated and the results are directly compared with analogous synthesis in water and N,N-dimethylformamide (DMF). We have successfully synthesised MIL-53(Al), termed MIL-53(MeOH), using MeOH as the solvent and employing a reaction temperature of 150 °C, lower than that typically used for analogous water or DMF-based reactions. Several unique properties are observed for MIL-53(MeOH). The breathing phenomenon which is known for MIL-53(Al) derivatives, prepared using water or DMF as the reaction solvent, is not observed for samples prepared from MeOH and the framework adopts and remains in the large-pore form. Thus, measurement of N2-isotherms and calculation of internal surface areas have verified that the synthesis of MIL-53(MeOH) leads to a product which is highly porous with only minimal or no activation required. Furthermore, X-ray diffraction measurements and scanning electron microscopy at different humidity levels reveal a reversible loss of crystallinity at high humidity levels for MIL-53(MeOH) which was not observed previously for other MIL-53 derivatives. In contrast, the synthesis of MIL-53(Al) in ethanol leads to a product with low crystallinity.