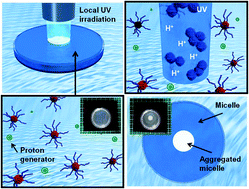
The effects caused by photo-induced proton generation on the assembly formation of dual-temperature/pH-responsive block copolymers are investigated. The block copolymer of P(N-isopropylacrylamide (NIPAAm)-co-N-(isobutoxymethyl)acrylamide (BMAAm))-b-P(NIPAAm-co-2-carboxyisopropylacrylamide (CIPAAm)) was synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization. The block copolymers underwent self-assembly and formed a spherical micellar structure because the P(NIPAAm-co-BMAAm) block turned hydrophobic above its lower critical solution temperature (LCST), while the electrostatic repulsion of the P(NIPAAm-co-CIPAAm) block stabilized the micelle at pH levels above the pKa of the CIPAAm units. To achieve a local and remote control of micelle aggregation, the micelle solution was exposed to UV radiation in the presence of a photoacid generator of o-nitrobenzaldehyde (NBA). When UV irradiation was limited to a specific region, we were able to restrict the proton generation to only the exposed region. Interestingly, the opaque area created by micelle aggregation was stable even when UV radiation was turned off. Finally, the photo-induced proton transfer from a micelle core was demonstrated using an NBA-encapsulated micelle. This proposed system of micelle aggregation via photo-induced proton diffusion may be beneficial not only for prompt pH changes in the nanoparticle solution, but also for the design of predictive and programmable nanocontainers for drug delivery applications.