Hiromitsu Maeda, Ayaka Fukui, Ryohei Yamakado and Nobuhiro Yasuda
Chem. Commun., 2015,51, 17572-17575
DOI:
10.1039/C5CC07493B,
Communication
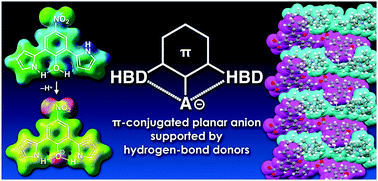
A nitro-substituted dipyrrolylphenol was synthesized as a precursor of a π-electronic anion, whose phenolate (phenoxide) moiety upon deprotonation was stabilized by the hydrogen-bond-donating pyrrole NH, thus forming solid-state ion pairs with various cationic species.