Liquid-based cationic ligand engineering in one-dimensional bismuth bromide perovskites: A-site influence on scintillation properties†
Riki
Subagyo
 a,
Mohanad S.
Eid
a,
Mohanad S.
Eid
 b,
Nabila
Safitri
a,
Rifdah
Hanifah
a,
Afif Akmal
Afkauni
b,
Nabila
Safitri
a,
Rifdah
Hanifah
a,
Afif Akmal
Afkauni
 c,
Lei
Zhang
c,
Lei
Zhang
 d,
Michal
Makowski
d,
Michal
Makowski
 e,
Md Abdul Kuddus
Sheikh
e,
Md Abdul Kuddus
Sheikh
 e,
Muhammad Haris
Mahyuddin
e,
Muhammad Haris
Mahyuddin
 fg,
Dominik
Kowal
fg,
Dominik
Kowal
 e,
Marcin
Witkowski
e,
Marcin
Witkowski
 b,
Winicjusz
Drozdowski
b,
Winicjusz
Drozdowski
 b,
Muhammad Danang
Birowosuto
b,
Muhammad Danang
Birowosuto
 e,
Arramel
Arramel
e,
Arramel
Arramel
 *c and
Yuly
Kusumawati
*c and
Yuly
Kusumawati
 *a
*a
aDepartment of Chemistry, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember, Kampus ITS Keputih, Sukolilo, Surabaya 60111, Indonesia. E-mail: y_kusumawati@chem.its.ac.id
bInstitute of Physics, Faculty of Physics, Astronomy, and Informatics, Nicolaus Copernicus University in Toruń, ul. Grudziądzka 5, 87-100 Toruń, Poland
cCenter of Excellence Applied Physics and Chemistry, Nano Center Indonesia, Jl PUSPIPTEK, South Tangerang, Banten 15314, Indonesia. E-mail: arramel@nano.or.id
dDepartment of Physics, National University of Singapore, Singapore 117551, Singapore
eŁukasiewicz Research Network—PORT Polish Center for Technology Development, Stabłowicka 147, 54-066 Wrocław, Poland
fDoctoral Program of Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia
gQuantum and Nano Technology Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia
First published on 17th March 2025
Abstract
Low-dimensional bismuth-based hybrid organic–inorganic halide perovskites (Bi-HOIPs) are intriguing scintillating materials due to their anisotropic nature. However, a systematic investigation on Bi-HOIP frameworks for X-ray detection imaging remains lacking. In this work, we present the diverse X-ray detection responses in a series of Bi-HOIPs that are incorporated into a polydimethylsiloxane (PDMS) matrix utilizing ionic liquids (ILs) as organic ligands, namely, 1-butyl-1-methyl-pyrrolidinium (BMP), 1-(3-aminopropyl)imidazole (API), and 1-butyl-3-methyl-imidazolium (BMI). The as-synthesized Bi-HOIPs in this series exhibited a modest light yield of ∼1000 photons per keV at room temperature. Interestingly, the incorporation of tin (Sn), instead of bismuth, resulted in radioluminescence intensity enhancement at low temperatures. An abrupt thermal quenching occurred in the case of APISnBiBr5 and APISn2Br10, leading to an absence of light yield at RT. Interestingly, thermoluminescence (TL) measurements showed that the glow curve was absent in BMIBiBr4 and APIBiBr5, demonstrating the weak contribution of a defect within the crystal lattices. On the contrary, BMPBiBr4, APISnBiBr5 and APISn2Br10 displayed glow curves at temperatures above 100 K. We believe that controlling the number of defects promoted tunable modest distribution traps, manifesting their light yield profile. In terms of the decay profile, BMIBiBr4 exhibited a fast decay component of 4 ns, while APIBiBr5 and BMPBiBr4 yielded a fast decay of 6 ns. A distinct constituent within the ILs could serve as a tuning factor, thereby generating different optical responses and scintillation features. We postulate that this can be attributed to the band gap and polaron signature in BMIBiBr4, which manifested a faster quenching rate than BMPBiBr4 and APIBiBr5. This work sheds new insights on how the population of direct manipulation traps in IL-based bismuth halide perovskites can be driven by regulating the number of halogens at the X-sites of the targeted compounds.
1. Introduction
Scintillating materials have been extensively designed and used for radiation detection applications in various fields, including X-ray protection, medical imaging, harmful sensor detection, and nuclear cameras, as they possess the ability to transform ionizing radiation into visible or ultraviolet photons.1,2 In general, scintillators are constructed entirely from high-density heavy elements. Commercial scintillating materials with high light yields, including CsI:Tl (∼66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV),3 LaBr3:Ce (∼61
000 photons per MeV),3 LaBr3:Ce (∼61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV),4 and Gd2O2S:Tb (∼60
000 photons per MeV),4 and Gd2O2S:Tb (∼60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV),4,5 have recently been developed. Nonetheless, assembling scintillators in a high-temperature and vacuum environment is considered a time-consuming and expensive method. Thus, it is not a desirable approach for the environment.6,7 Consequently, an ideal scintillator should be directed towards a solution-processable, easy to use, and inexpensive route.
000 photons per MeV),4,5 have recently been developed. Nonetheless, assembling scintillators in a high-temperature and vacuum environment is considered a time-consuming and expensive method. Thus, it is not a desirable approach for the environment.6,7 Consequently, an ideal scintillator should be directed towards a solution-processable, easy to use, and inexpensive route.
Hybrid organic–inorganic halide perovskites (HOIPs) have been at the center stage as an alternative scintillating material owing to their good luminescent properties, high absorption coefficients for ionizing radiation, and solution-processability.8–11 Birowosuto et al. reported various lead-based halide perovskites as scintillating materials, including methylammonium lead iodide (MAPbI3), methylammonium lead bromide (MAPbBr3), and 2,2′-(ethylenedioxy)bis(ethylammonium) lead chloride ((EBDE)PbCl4).12 At low temperatures, all of these perovskite single crystals displayed strong X-ray stimulated luminescence yields of more than 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV. Additionally, at room temperature, (EBDE)PbCl4 generated a modest light yield of 9
000 photons per MeV. Additionally, at room temperature, (EBDE)PbCl4 generated a modest light yield of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per meV. The influence of organic chain length on lead-based HOIPs was also investigated by Maulida et al.13 Benzylammonium as the organic cation-based HOIPs exhibited the highest light yield value at RT. This was reflected in the alteration of the trap states, as the longer organic chain ligands in lead-based HOIPs were inclined to hinder the exciton recombination and yield a longer decay time. Sheikh et al. implemented a dual organic cation (phenethylammonium and benzylammonium) in a lead bromide perovskite structure, which resulted in >80% rapid component scintillation decay time and light yields of over 149
000 photons per meV. The influence of organic chain length on lead-based HOIPs was also investigated by Maulida et al.13 Benzylammonium as the organic cation-based HOIPs exhibited the highest light yield value at RT. This was reflected in the alteration of the trap states, as the longer organic chain ligands in lead-based HOIPs were inclined to hinder the exciton recombination and yield a longer decay time. Sheikh et al. implemented a dual organic cation (phenethylammonium and benzylammonium) in a lead bromide perovskite structure, which resulted in >80% rapid component scintillation decay time and light yields of over 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per eV at RT.14 The encapsulation of MAPbBr3 nanocrystals (NCs) in a metal–organic framework has been designed to generate high exciton binding energy, bright and efficient luminescence, and improve stability.15 Consequently, MOF⊃MAPbBr3 NC scintillator exhibits a linear response to X-ray with a detection limit determined to be 170 nGyair s−1. It can be observed that lead-based HOIPs display good scintillation properties. However, owing to non-toxicity challenges and stability requirements, there are challenges that remain to be addressed in subsequent years.
000 photons per eV at RT.14 The encapsulation of MAPbBr3 nanocrystals (NCs) in a metal–organic framework has been designed to generate high exciton binding energy, bright and efficient luminescence, and improve stability.15 Consequently, MOF⊃MAPbBr3 NC scintillator exhibits a linear response to X-ray with a detection limit determined to be 170 nGyair s−1. It can be observed that lead-based HOIPs display good scintillation properties. However, owing to non-toxicity challenges and stability requirements, there are challenges that remain to be addressed in subsequent years.
Because of their structural similarities, bismuth-based HOIPs have been proposed as replacements for lead-based HOIPs. Bismuth has a comparable mass to lead so that its compounds have the same radiation stopping power as lead compounds although it is much better for the environment.16,17 Moreover, bismuth-based HOIPs create various configurations of [Bi-halide] connection,18,19 which has potential to be as a game changer driven by their dimensional control, hence influencing the scintillation characteristics. However, many studies on bismuth perovskite compounds have focused on direct X-ray detectors. Zhuang et al. previously synthesized a 2D (NH4)2Bi2I9 layered perovskite that performed well for X-ray detection, with a limit detection of 55 nGyair s−1.20 Tao et al. employed top-seed solution growth to produce 1D (H2MDAP)BiI5, which can convert X-rays to electrical impulses with a sensitivity of roughly one μCGyair−1 cm−2.21 Yao et al. applied delayed solvent evaporation to synthesis 1D (DMEDA)BiI5.22 The perovskite has an X-ray detection sensitivity of 72.5 μCGyair−1 cm−2 at 300 V. Furthermore, the employment of ionic liquid as cationic or anionic sites established a potential strategy for changing the electronic characteristics of bismuth-based HOIPs.23,24 For scintillators, Jin et al. reported the hydrothermal synthesis of [Emim]BiCl4(bp2do), which displayed X-ray excited luminescence spectrum spanning from 425 to 750 nm, peaking at about 485 nm.25 The use of ILs improves the compactness of supramolecular packing, resulting in longer lifetimes up to the second level time and larger quantum yields of 25.05%. It should be mentioned that the modification of organic cations impacts the resultant characteristics of HOIPs, which affect the scintillation outcomes. Thus, our motivation lies in the absence of a structure–property relationship in recent years to modulate the A-site in one-dimensional lead-free hybrid perovskites by rational design.26
Previously, we investigated the structural, vibrational, and electronic properties of bismuth-based HOIPs with various ILs, including 1-butyl-1-methyl-pyrrolidinium (BMP), 1-(3-aminopropyl)imidazole (API), and 1-butyl-3-methyl-imidazolium (BMI), resulting in BMPBiBr4, APIBiBr5, and BMIBiBr4 perovskite.26 However, the scintillation characteristics of such perovskites have not yet been studied. In this study, we focused on the following five compounds: BMPBiBr4, BMIBiBr4, APIBiBr5, APISnBiBr5, and APISn2Br10 perovskites. The monoclinic structure of APIBiBr5 facilitated the observation of a fast decay of 6 ns (15%), with the domination of slow decay (709 ns, 85%). We find that the slow component is dominant in the case of APIBiBr5 with a monoclinic structure, while BMIBiBr4 and BMPBiBr4 share a different number of nitrogen atoms within their skeleton backbone, yielding slow scintillation decay behaviour. The slow component of BMPBiBr4 is slower than that of BMIBiBr4 even though both perovskite crystal systems share a triclinic structure. This finding indicated that BMPBiBr4 had a high number of self-trapped electrons and defects, as evidenced by the presence of a glow curve above 100 K in the thermoluminescence (TL) measurement. Furthermore, the deconvolution of the BMPBiBr4 curvature indicated that three peaks are observed, highlighting shallow and depth trap variation. In particular, a sharp peak in APISn2Br10 suggests the occurrence of first order kinetics, while a broadened line shape exists for APISnBiBr5 in terms of distribution traps at 312, 473, and 501 meV, which can be further improved by regulating the defect number in their lattice. This effort further extends the attempt to manipulate the trap number of the IL-based Bi-HOIPs by providing an excellent scintillating hybrid material.
2. Experimental
2.1. Synthetic procedures
The preparation of bismuth-based HOIPs was reported in our previous work.26 Because pristine perovskite samples are very sensitive and too small for further optical characterization, the prepared bismuth-halide perovskites do not have transparent features in nature, leading to an undesired scattering process during scintillation measurement. We therefore grounded the as-prepared perovskites into fine powders and subsequently introduced them into the PDMS matrix. The samples were incorporated with polymer adopted from the previous studies by Maddalena et al.,27 Haposan et al.,28 and recent work by Makowski et al.29 The perovskite samples were mixed with polydimethylsiloxane (PDMS) at a weight ratio of 20% and subsequently cured for 3 h on a hot plate in aluminum foil cuvette at 120 °C. The resulting perovskite microcrystal had a diameter and thickness of 1.0 cm and 0.5 cm, respectively.2.2. Radioluminescence (RL) and TL measurement
We utilized a single integrated setup to perform all these measurements. It is composed of an Inel XRG3500 X-ray generator Cu-anode tube (45 kV/10 mA), an Acton Research Corporation SpectraPro-500i monochromator, a Hamamatsu R928 photomultiplier tube (PMT), and an APD Cryogenic Inc. closed-cycle helium chiller. First, we evaluated RL at temperatures ranging from 10 to 350 K, beginning with the highest and progressing to the lowest, to prevent any interference through the thermal release of charge carriers to the emission yield. Following the RL measurements, we obtained low temperature afterglow and TL glow curves. Prior to TL evaluations, the crystals were irradiated to X-rays for 10 minutes at 10 K, with the monochromator adjusted to zeroth order. The glow curves were obtained at temperatures ranging from 10 to 350 K, with a heating rate of around 0.14 K s−1. For imaging, the X-ray source was a PHYWE XR 4.0 expert unit (Mo source, 17.5 keV) operated at 35 kV voltage and 1 mA current, while the camera was a commercial colour camera with a 1-second exposure duration. The card with the chip was simply placed in front of the scintillator, while the back side was placed at the aperture of the X-ray tube, where the uncollimated X-ray emerged. They were put together as closely as possible to minimize light scattering.2.3. Scintillation decay measurements
The scintillation time profile was performed using the delayed coincidence single photon counting method, which involves detecting correlated photon events to measure decay times accurately. In this method, a 137Cs radioactive source was used for gamma radiation. The detection setup includes two Hamamatsu photomultiplier tubes, the R1104 for detecting the initial scintillation event (“start”) and the R928 for detecting subsequent events (“stop”). To operate the PMT, we applied a voltage of 1.19 kV. The timing of these events was precisely measured using a Canberra 2145 time-to-amplitude converter. These signals were then recorded and analysed by applying a TUKAN-8K-USB multichannel analyzer. Multi-exponential decay function fitting was then applied to the obtained spectra to obtain decay constants and their relative contributions, which are essential for calculating effective decay times.3. Results and discussion
3.1. Characterization of ionic liquid bismuth hybrid perovskites
The traditional synthetic pathway of perovskites can be grown using slow solvent evaporation crystallization.26 Because the chemical and mechanical stabilities of perovskites are very sensitive to humidity and thermal response, we therefore incorporated several targeted Bi-HOIPs into PDMS resin, as dictated in the photographs (Fig. S1, ESI†). PDMS was chosen because PDMS possesses the traits of the high elasticity, low temperature flexibility and full transparency and serves as a double protective matrix.30,31 The incorporation of perovskites with PDMS can improve the stabilization of perovskites and produce samples of appropriate size for analysis.32,33 Physically, the crystal has a sphere-like shape with diameter and thickness size of ±1.5 cm and 0.5 cm, respectively. In detail, SEM images of the crystal after grinding are depicted in Fig. S2 (ESI†), showing that the crystal is micrometer in size, which indicates that the incorporation of perovskite with PDMS yielded microcrystals of various sizes. The resulting perovskite crystals were subsequently used to analyse the scintillation features, which are discussed in the next section. The crystal structure analyses of the perovskites using Rietveld refinements are outlined in Fig. S3 (ESI†). The chemical states of APISn2Br10 were examined by applying an X-ray photoelectron spectrophotometer, as presented in Fig. S4 (ESI†), whereas other compounds can be found in ref. 26. The absorption, photoluminescence and time-resolved photoluminescence spectroscopies are outlined in Fig. S5 (ESI†). A detailed discussion is provided in the ESI.†3.2. Structural order of one-dimensional bismuth-based HOIPs
Prior to discussing the scintillation properties, we revisit the impact of ionic liquid variations on their perovskite crystal arrangements, as depicted in Fig. 1. In agreement with the previous work,26 the overall inorganic networks of Bi-HOIPs share a one axis arrangement formed by the 1D corner-sharing of zigzag chains, displaying a similar trend apart from the IL-type. In addition, we found that the crystal structures of BMPBiBr4 and BMIBiBr4 exhibit a triclinic crystal structure. In contrast, APIBiBr5, APIBiSnBr5 and APISn2Br10 share a monoclinic system (see Table S1, ESI†). Each of the bismuth atoms is coordinated with six bromine anions to form BiBr6 octahedrons. Here, four bromine anionic sites are situated in the terminals, and the other two moieties serve as bridges between two neighbouring BiBr6 octahedrons. In terms of Bi–Br connectivity and lattice parameters, we found that the three crystallographic axes of APIBiBr5 and APIBiSnBr5 were moderately extended. For instance, the lattice along the a-axis is changed upon Sn-introduction (from 15.7941 Å to 19.8608 Å, see Table S1, ESI†). Interestingly, the incorporation of Sn into the APIBiBr5 crystal lattice did not generate significant geometric distortion (see Table S2, ESI†). For instance, we elaborate a fair geometric analysis by extracting Sn–Br–Sn bond angles between APIBiSnBr5 and APISn2Br10 (see Computational details, ESI†). Here, a noticeable change upon fully substituting Sn atoms into the perovskite was observed. The reduction bond length is 0.19 Å. The Sn–Br–Sn of APISnBiBr5 is 3° narrower than that of APISn2Br10. The wider Sn–Br–Sn in APISnBiBr5 is fair because of the existence of Bi–Br–Sn and Bi–Br–Bi connectivity, which affects the bond angle for the stabilization of octahedral tilting.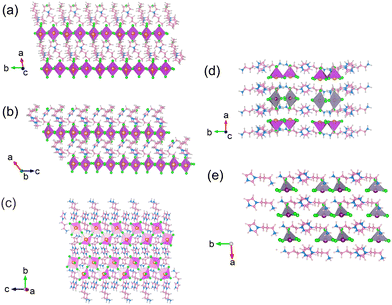 | ||
| Fig. 1 Calculated structures of perovskites, which were extracted from CIF files, including (a) BMPBiBr4, (b) BMIBiBr4, (c) APIBiBr5, (d) APISnBiBr5, and (e) APISn2Br10. | ||
3.3 Radioluminescence measurements of Bi-HOIPs and PDMS matrix
The RL spectra of the admixture Bi-HOIPs and PDMS matrix are presented in Fig. 2. We note that two spectral areas are found in BMPBiBr4, APIBiBr5, and BMIBiBr4. The first region is covered in the range of 325–520 nm, while the second region appears in the 525–560 nm spectral range. According to the earlier study, we found a resemblance feature in the recorded PL spectra (Fig. S5, ESI†) of the three aforementioned Bi-HOIPs with a maximum centered at 540 nm.26 This peak is shifted approximately 20 nm towards a higher wavelength compared to the PL results because the different excitation sources of RL and PL were used.14 We indirectly relate that the second region in the RL mapping originated from the light-mater interaction dominantly governed by the excitonic contribution. Such findings can be addressed in the first area as a direct consequence of the chemical integration of perovskite into PDMS. As previously noted by Kim et al.,34 the inclusion of perovskite using a PDMS matrix inhibited the deprotonation of organic cations owing to the interaction of the oxygen element with the metal via coordination bonds. A contrasting RL spectral behavior is observed in the case of BMPPBiBr4 (Fig. 2(a)) and BMIBiBr4 (Fig. 2(b)) centering at 550 nm. In addition, the A-site substitution of Bi-HOIPs with the API cation (Fig. 2(c)) led to a slight broadening and became higher in terms of intensity close to 200 K.To investigate the impact of synergetic substitution, we introduce Sn and simultaneously increase the number of Br atoms into APIBiBr5 structure and then APISn2Br10. In contrast, here, we observed that by partially replacing Bi, the metallic signature (close to 550 nm) is largely suppressed in APISnBiBr5 (Fig. 2(d)) and smeared out for APISn2Br10 (Fig. 2(e)). The collected RL spectra of APIBiSnBr5 at 10 K in the second area are significantly reduced although the RL spectra in the first region are greater than those in other perovskites. This result reveals that the substitution of Sn in APIBiBr5 creates a new recombination center that contributes to the improvement of RL intensity. The RL intensity of APISnBiBr5 decreases with increasing temperature, indicating the presence of thermal quenching (TQ), which is explored further below. APISn2Br10 exhibits the highest RL intensity in the first region compared to the others. This result further demonstrates that the existence of a small fraction of Sn in APIBiBr5 influences the enhancement of RL intensity owing to the formation of a recombination center. Similarly, the RL profile of APISn2Br10 changes with temperature, resulting in the creation of broad-band spectra. The broad-band spectra show several recombination pathways of luminescence that are generated using high energetic X-ray irradiation.
3.4. Thermal effects on the radioluminescence profile
We argue that thermal interaction contributes to the RL spectral variation to some extent, as shown in Fig. 3. Here, we successfully unravel the peculiar observation as we break the RL into four distinct temperature profiles in the first region (see Fig. 3). To the best of our knowledge, there is no specific argument concerning the unusual spectra reported in the literature. We propose that the unusual RL spectra possibly originated from the multiple exciton recombination, bulk and surface defect, re-absorption and self-absorption, and photon replica.35 Additionally, the formation of unusual shapes in RL spectra could be related to the presence of a vibrational structure in the spectrum.36 The weak interfacial interaction between PDMS and perovskite can also trigger the formation of four RL regimes.37 A high energy excitation promotes unconventional emission.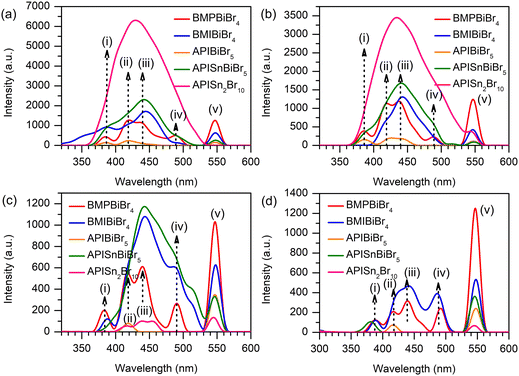 | ||
| Fig. 3 Representation of RL spectra of perovskites at temperatures of (a) 10 K, (b) 100 K, (c) 200 K, and (d) 300 K. | ||
Upon further investigation, the broad RL peaks of BMPBiBr4 (Fig. 3(a)) preserve their profile throughout temperature-dependent RL analysis. This discovery shows that the electronic structure of BMPBiBr4/PDMS (Fig. 3(b)) does not change as a result of the X-ray temperature effect. For the sake of clarity, we labelled each of the prominent deconvoluted RL maxima (i–v) within the first region to monitor the thermal effect at different temperatures. Although the overall trend seems to be random, we found that a certain peak of APISnBiBr5 (green) exhibited a gradually reduced intensity. In particular, peak (iii) across 200 K (Fig. 3(c)) and subsequent heating to 300 K (Fig. 3(d)) led to a nearly depleted signal. For comparison, we also present the photoluminescence (PL) profile and charge dynamic behaviour of these compounds using time-resolved photoluminescence (TRPL), as depicted in Fig. S5 (ESI†). Here, we attribute that octahedral tilting contributes significantly to tailor the defect states of the 1D ionic liquid perovskites.
To further quantify the finding, we exploited temperature-dependent RL analysis (see Fig. 4) by carefully monitoring the intensity variation from low to high temperatures. As previously outlined by Thirumal et al. and Li et al., such a TQ process is triggered by the suppression of phono-assisted nonradiative recombination.38,39 Temperature-dependent intensities are further examined to evaluate and compare the intrinsic differences of hybrid halide perovskites. The graphs in Fig. 4(a)–(e) are fitted using the Arrhenius fitting equation (eqn (1)),40 and the parameters are summarized in Table 1.
 | (1) |
 | ||
| Fig. 4 Arrhenius fitting of thermal quenching behaviour of perovskites, including (a) BMPBiBr4, (b) BMIBiBr4, (c) APIBiBr5, (d) APISnBiBr5, and (e) APISn2Br10. | ||
| Parameters | Perovskites | ||||
|---|---|---|---|---|---|
| BMPBiBr4 | BMIBiBr4 | APIBiBr5 | APISnBiBr5 | APISn2Br10 | |
| Γ 0/Γv (s−1) | 4.9 ± 1.3 | 1418.5 ± 530.5 | 6.5 ± 0.4 | 9298.2 ± 252.8 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755 ± 4159 755 ± 4159 |
| ΔEq (meV) | 28 ± 6 | 185 ± 97 | 39 ± 2 | 81 ± 5 | 185 ± 59 |
We notice that this type of IL significantly affects the TQ outcome. Let us revisit the XRD data from the prior research, which shows that BMIBiBr4 and BMPBiBr4 compounds exhibit triclinic crystallography, but APIBiBr5 has a monoclinic crystal structure. Such structural variation caused by the various ILs in the A-site could influence the quenching behaviour of bismuth-based HOIPs. To shed more light, we propose that the observation is related to the band gap energy implication of halide perovskite upon interchanging the number of nitrogen atoms and the skeletal backbone. Based on our previous investigation,26 geometric consideration exhibited small displacement in terms of Bi–Br bond distance and angles of specific type ILs, hosting the minute band gap variation. In a similar triclinic structure, the apical Bi–Br bond of BMPBiBr4 is longer than that of BMIBiBr4, resulting in a narrow band gap of BMPBiBr4 (3.04 eV) compared with BMIBiBr4 (3.16 eV). Consequently, a faster quenching rate (1418.5 ± 530.5 s−1, see Table 1) is obtained for BMIBiBr4. However, we note that the band gap of APIBiBr5 (3.01 eV) has a fast quenching rate (6.5 s−1). We believe that the occurrence of double bonds in the API internal chemical structure governs the increasing number of small polarons. Because the photoexcited free electrons are feasible to capture by a small polaron, such a condition suppresses the radiative recombination, leading to a fast-quenching rate. In a similar approach, a BMI structure containing imidazole rings causes a significant outcome of a faster quenching rate compared to BMPBiBr4 and APIBiBr5. For instance, Sn substitution in the APIBiBr5 structure elevates the Γ0/Γv value by approximately 1500 times compared to APIBiBr5. These data suggest that replacing partial bismuth with tin atoms promotes a rapid TQ succession rate. These findings show that the RL intensity of APISnBiBr5 is reduced by 50% and approaches zero at 200 K. The findings are related to the mismatch of atomic size, leading to structural distortion and creating high numbers of defect states.
The occurrence of TQ behaviour in APISnBiBr5 (Fig. 4(d)) and APISn2Br10 (Fig. 4(e)) is consistent with a previous work by Xie et al.,40 showing the presence of TQ in lead-based HOIPs. A comparable TQ process was also observed in the case of (C6H5CH2NH3)2SnBr4.42 APISn2Br10 has a comparable ΔEq to (C6H5CH2NH3)2SnBr4 but has a greater Γ0/Γv value than the latter. This finding shows that the TQ process in APISn2Br10 is faster than the TQ process in (C6H5CH2NH3)2SnBr4, which occurs at lower temperatures. The presence of TQ in bismuth-based HOIPs alters the light yield of the perovskite. Based on the RL measurements, three candidates such as BMPBiBr4, APIBiBr5, and BMPBiBr4 had a low light yield at room temperature of approximately 1000 ph PER keV, indicating that they might be used as scintillating materials, particularly at low temperatures, owing to their great sensitivity at low temperatures.
3.5. High-energetic γ-ray scintillation decay time of Bi-HOIPs
The scintillation decays of BMPBiBr4, BMIBiBr4, and APIBiBr4 were analysed by exposing gamma (γ) irradiation to the perovskite samples. The γ-ray scintillation decay curves of BMPBiBr4, BMIBiBr4, and APIBiBr4 are presented in Fig. 5. All samples exhibit bi-exponential features, showing the fast and slow decay components. The Bi-HOIPs, which contain BMP (Fig. 5(a)) and API (Fig. 5(c)) as the ILs, exhibit a considerably fast decay component of 6 ns, while BMI exhibits a fast component of 4 ns. We propose that such a feature might be correlated to the type of IL structure used as the molecular backbone in the bismuth-based HOIP configuration. Let us consider that the different positions of nitrogen cations affect the resulting final geometry structure of bismuth-halide networks, which influences the charge carrier decay pathway. We note that the entire compound shows a dominant slow component rather than a fast counterpart. This result indicates that a high number of self-trapped electrons and defects heavily contribute to the secondary decay event, which is in line with the existence of the TQ process in the RL measurements. The undesired slow component of Bi-HOIPs can be mitigated under two approaches by suppressing the number of defect states within the crystals. First, the chemical composition optimalization during the synthesis of targeted bismuth-based halide perovskite promoted a high crystallinity order, thus lowering the chance of defect formation. Second, microstructure engineering is suggested as an applicable route to retaining the integrity of high-quality bismuth-based hybrid perovskites.43,44 We revisit the fundamental mechanism of scintillation decay to illustrate the formation of fast and slow components. Initially, a free electron is created owing to the material exposure to gamma radiation, followed by a thermalization process into the conduction band. Thus, the electron is recombined at an ultrafast time.45 Alternatively, electrons could also be transferred into the deep and shallow traps, subsequently emitting and generating a slow emission phenomenon.46 This finding is further explained in the next section to elucidate the occurrence of trap states in the bismuth-based halide perovskites. | ||
| Fig. 5 Scintillation decay of (a) BMPBiBr4, (b) BMIBiBr4, and (c) APIBiBr4 collected at RT with biexponential features of the respective fast and slow decay component. | ||
3.6. Afterglow, thermoluminescence, and radiation hardness
For further scintillation analysis of the samples, afterglow and TL experiments were used to analyse the energy trap profile, as represented in Fig. 6. Upon termination of X-ray excitation at 10 K, the emission yields up to thousands of seconds. There is no afterglow during the temperature increase in BMIBiBr4 (Fig. 6(b)) and APIBiBr5 (Fig. 6(c)), revealing the absence of energy traps. This result is similar to previous work by Xie et al.40 and Diguna et al.,42 as reported in lead-based and tin-based HOIPs, respectively. Meanwhile, BMPBiBr4 (Fig. 6(a)) shows the existence of afterglow (see Fig. S6, ESI†), indicating the presence of the energy trap. Similarly, the substitution of Sn in APIBiBr5 and the replacement of Bi with Sn indicate the presence of afterglow. The glow curve of APISnBiBr5 (Fig. 6(d)) is extremely broad, implying the existence of a trap distribution in APISnBiBr5. We revisit previous work that highlights that Sn substitution increases the length of the axial Bi–Br bonds from 3.41 Å to 3.8 Å and consequently introduces a geometric distortion within the system, introducing defect sites in the crystal lattice of APISnBiBr5. This in turn influences the scintillation performance of APISnBiBr5.In addition, we believe that the distortion can trigger the existence of self-trapped excitons owing to the enhanced electron–phonon coupling,47 which is mutually related to the current finding. To determine the trap, we analyse the experiments after heating the samples (at 3580 seconds, red dashed lines in Fig. 6 and heat profile in ESI,† Fig. S5), and we present the TL spectra, as shown in Fig. 7.
 | ||
| Fig. 7 Classic Randall–Wilkins fitting of TL glow curve. (a), (b), and (c) are BMPBiBr4, APISn2Br10, and APISnBiBr5, respectively. | ||
The classic Randall–Wilkins approach (eqn (2)) was used to calculate the parameter of trap arising in TL spectra of BMIBiBr4, APISn2Br10, and APISnBiBr5, as shown in Fig. 7(a)–(c), including concentration, depth and frequency factors (presented in Table 2).
 | (2) |
| Parameter | Perovskites | ||||
|---|---|---|---|---|---|
| BMPBiBr5 | APISnBiBr2 | APISn2Br10 | |||
| Peak | Peak 1 | Peak 2 | Peak 3 | Peak 1 | Peak 1 |
| n 0 (cm−3) | 3.6 × 103 | 8.8 × 103 | 6.9 × 103 | 3.7 × 105 | 8.1 × 103 |
| E (meV) | 349 | 692 | 910 | 672 | 1022 |
| s (s−1) | 3.8 × 105 | 4.4 × 1011 | 5.3 × 109 | 7.1 × 1010 | 2.3 × 1023 |
| Distribution | Distribution 1 | Distribution 2 | Distribution 1 |
|---|---|---|---|
| A (cm−3) | 3.5 × 103 | 7.2 × 103 | 2.2 × 102 |
| E (meV) | 312 | 473 | 501 |
| σ (meV) | 101 | 104 | 102 |
According to the fitting, three deconvoluted peaks associated with the shallow and depth traps are extracted. Here, we consider the lowest energy (peak 1) corresponding to the shallow traps, while peak 2 and peak 3 are associated with the depth traps. The initial trap concentration is comparable with several hybrid perovskite systems. In APISn2Br10, the glow curve corresponds to the first order kinetic. The sharp first order peak is correlated with the depth traps; meanwhile, the broad deconvoluted peak is related to the distribution traps. Similarly, the substitution of Sn in APIBiBr5 shows a sharp first order peak, which is attributed to the depth traps. Two broad deconvoluted peaks are yielded, indicating the occurrence of distribution traps in APISnBiBr5. This results in evidence that the substitution of Sn induces the formation of several defect sites, which act as the distribution traps in the lattice.
To investigate the structure endurance upon high-energy irradiation, we present the radiative hardness experiments, as depicted in Fig. S7 (ESI†). The results exhibit a linear profile between the normalized integrated intensity and dose in the log-scale plot. We note the absence of strong saturation in all the under-study perovskites, which is comparable to the previous work in lead-based halide perovskites.40 We noticed that BMPBiBr4 and BMIBiBr4 shared similar patterns, while APIBiBr5 exhibited a ramp profile. This finding implies that the latter has a low radiation hardness compared to the BMPBiBr4 and BMIBiBr4. Based on this test, we believe that the Bi-HOIPs with monoclinic crystal structure are more stable towards radiation compared to the triclinic counterparts. The substitution of Bi with Sn atoms in the APIBiBr5 system decreases the radiation hardness, suggesting the consequence of an atomic size mismatch between Bi3+ and Sn4+. The full conversion of Bi with Sn atoms in the B-site of the API system improves radiation hardness. This finding infers that a reduction in defect density within the crystal lattice of APISn2Br10 is achieved.
4. Conclusions
In summary, the utilization of ILs as organic cations in bismuth-based HOIPs exhibits modest scintillation features. All perovskites exhibit high RL spectra at low temperatures, and an opposite trend at elevated temperatures, indicating the existence of the TQ process. By rational design, we demonstrate that A-site tuning is used to tailor the optical response and scintillation properties of Bi-HOIPs, particularly their impact on the thermal quenching rate. We rationalize that the finding originates from the IL internal structure composition, which is regulated by the nitrogen atom and skeletal backbone, yielding a minute change within the band gap and polaron contribution. Consequently, BMPBiBr4, BMIBiBr4 and APIBiBr5 show a light yield value of approximately 1000 ph per keV. A fast TQ process is observed at APISnBiBr5 and APISn2Br10, leading to the absence of RL spectra at RT. In TL analysis, BMIBiBr4 and APIBiBr5 show no trap in their crystal lattice. Meanwhile, BMPBiBr4 generates shallow and depth traps according to glow curve fitting. We found that the imidazole ring provided fewer defect sites than the pyrroline ring in BMP. The depth traps and distribution traps are observed in APISnBiBr5 and APISn2Br10. The presence of distribution traps induced by X-rays reveals that the perovskites have a high defect level in their crystal lattice. Several Bi-HOIPs show modest scintillation results, which could be improved via subsequent treatments.Author contributions
R. Subagyo: writing – original draft, visualization, resources, methodology, formal analysis. M. S. Eid: data curation, formal analysis, investigation, resources. N. Safitri: methodology, visualization. R. Hanifah: methodology, visualization. A. A. Afkauni: visualization, formal analysis, investigation. L. Zhang: data curation, formal analysis. M. Makowski: data curation, formal analysis. M. A. K. Sheikh: data curation, writing – review & editing. M. H. Mahyuddin: formal analysis, methodology, resources, software. D. Kowal: data curation, writing – review & editing. M. Witkowski: formal analysis, resources. W. Drozdowski: formal analysis, resources, validation. M. D. Birowosuto: conceptualization, data curation, supervision, methodology, validation, investigation, funding acquisition, writing – review & editing. A. Arramel: conceptualization, formal analysis, investigation, supervision, validation, writing – review & editing. Y. Kusumawati: conceptualization, supervision, resources, investigation, project administration, funding acquisition, validation. All authors have given their approval to the final manuscript version.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors from Institut Teknologi Sepuluh Nopember acknowledge funding of this research from Asian Development Bank (ADB) through the Partnership Research HETI (Penelitian Kemitraan HETI, Higher Education for Technology and Innovation) with grant number 0005/01.PKS/PPK-HETI/2023. Authors from Nano Center Indonesia express their gratitude to PT. Nanotech Indonesia Global Tbk for the start-up research grant. The funder was not involved in the study design, collection, analysis, data interpretation, article writing, or the decision to submit it for publication. M. D. B. and D. K. acknowledge funding from the National Science Center, Poland under grant OPUS-24 no. 2022/47/B/ST5/01966. M. H. M. acknowledges the research fund from Institut Teknologi Bandung under the “Penelitian, Pengabdian Masyarakat, dan Inovasi (PPMI) 2023” research scheme (grant no. 3AJ/IT1.07/SK-KP/2023).References
- L. Lu, M. Sun, Q. Lu, T. Wu and B. Huang, Nano Energy, 2021, 79, 105437 CrossRef CAS.
- L. Lu, M. Sun, T. Wu, Q. Lu, B. Chen and B. Huang, Nanoscale Adv., 2022, 4, 680–696 RSC.
- P. Schotanus, IEEE Trans. Nucl. Sci., 1990, 37, 177 CAS.
- C. W. E. v Eijk, Phys. Med. Bio., 2002, 47, R85 CrossRef PubMed.
- E. V. D. van Loef, P. Dorenbos, C. W. E. van Eijk, K. Krämer and H. U. Güdel, Appl. Phys. Lett., 2001, 79, 1573–1575 CrossRef CAS.
- T. He, Y. Zhou, X. Wang, J. Yin, L. Gutiérrez-Arzaluz, J.-X. Wang, Y. Zhang, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2022, 7, 2753–2760 CrossRef CAS.
- F. Zhou, Z. Li, W. Lan, Q. Wang, L. Ding and Z. Jin, Small Methods, 2020, 4, 1–17 Search PubMed.
- M. Xia, G. Niu, L. Liu, R. Gao, T. Jin, P. Wan, W. Pan, X. Zhang, Z. Xie, S. Teale, Z. Cai, J. Luo, S. Zhao, H. Wu, S. Chen, Z. Zheng, Q. Xie, X. Ouyang, E. H. Sargent and J. Tang, InfoMat, 2022, 1–12, DOI:10.1002/inf2.12325.
- A. Wibowo, M. A. K. Sheikh, L. J. Diguna, M. B. Ananda, M. A. Marsudi, A. Arramel, S. Zeng, L. J. Wong and M. D. Birowosuto, Commun. Mater., 2023, 4, 1–10 CrossRef.
- Y. Zhou, J. Chen, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2021, 6, 739–768 CrossRef CAS.
- F. Maddalena, L. Tjahjana, A. Xie, A. Arramel, S. Zeng, H. Wang, P. Coquet, W. Drozdowski, C. Dujardin, C. Dang and M. D. Birowosuto, Crystals, 2019, 9, 88 CrossRef.
- M. D. Birowosuto, D. Cortecchia, W. Drozdowski, K. Brylew, W. Lachmanski, A. Bruno and C. Soci, Sci. Rep., 2016, 6, 1–10 CrossRef PubMed.
- P. Y. D. Maulida, S. Hartati, D. Kowal, L. J. Diguna, M. A. Kuddus Sheikh, M. H. Mahyuddin, I. Mulyani, D. Onggo, F. Maddalena, A. Bachiri, M. E. Witkowski, M. Makowski, W. Drozdowski, A. Arramel and M. D. Birowosuto, ACS Appl. Energy Mater., 2023, 6, 5912–5922 CrossRef CAS.
- M. A. K. Sheikh, F. Maddalena, D. Kowal, M. Makowski, S. Mahato, R. Jedrzejewski, R. Bhattarai, M. E. Witkowski, K. J. Drozdowski, W. Drozdowski, C. Dang, T. D. Rhone and M. D. Birowosuto, ACS Appl. Mater. Interfaces, 2024, 16, 25529–25539 CrossRef PubMed.
- H. Wu, P. Ran, L. Yao, H. Cai, W. Cao, Y. Cui, M. Yang, D. Yang and G. Qian, Chem. Eng. J., 2024, 491, 152098 CrossRef CAS.
- M. Q. Li, Y. Q. Hu, L. Y. Bi, H. L. Zhang, Y. Wang and Y. Z. Zheng, Chem. Mater., 2017, 29, 5463–5467 CrossRef CAS.
- L. Zhang, K. Wang and B. Zou, ChemSusChem, 2019, 12, 1612–1630 CAS.
- J. K. Pious, M. G. Basavarajappa, C. Muthu, N. Krishna, R. Nishikubo, A. Saeki, S. Chakraborty and V. C. Nair, J. Phys. Chem. Lett., 2020, 11, 6757–6762 CAS.
- J. K. Pious, C. Muthu and C. Vijayakumar, Acc. Chem. Res., 2022, 55, 275–285 CAS.
- R. Zhuang, X. Wang, W. Ma, Y. Wu, X. Chen, L. Tang, H. Zhu, J. Liu, L. Wu, W. Zhou, X. Liu and Y. M. Yang, Nat. Photonics, 2019, 13, 602–608 CAS.
- K. Tao, Y. Li, C. Ji, X. Liu, Z. Wu, S. Han, Z. Sun and J. Luo, Chem. Mater., 2019, 31, 5927–5932 CAS.
- L. Yao, G. Niu, L. Yin, X. Du, Y. Lin, X. Den, J. Zhang and J. Tang, J. Mater. Chem. C, 2020, 8, 1239–1243 CAS.
- N.-N. Shen, M.-L. Cai, Y. Song, Z.-P. Wang, F.-Q. Huang, J.-R. Li and X.-Y. Huang, Inorg. Chem., 2018, 57, 5282–5291 CAS.
- J. C. Jin, N. N. Shen, Z. P. Wang, Y. C. Peng and X. Y. Huang, Coord. Chem. Rev., 2021, 448, 214185 CAS.
- J. C. Jin, Y. P. Lin, Y. H. Wu, L. K. Gong, N. N. Shen, Y. Song, W. Ma, Z. Z. Zhang, K. Z. Du and X. Y. Huang, J. Mater. Chem. C, 2021, 9, 1814–1821 CAS.
- R. Subagyo, P. Y. D. Maulida, D. Kowal, S. Hartati, R. M. Muslimawati, Y. Zetra, L. J. Diguna, S. Akhlus, M. H. Mahyuddin, L. Zhang, C. S. Tang, C. Diao, A. T. S. Wee, M. D. Birowosuto, A. Arramel, A. Rusydi and Y. Kusumawati, ACS Appl. Mater. Interfaces, 2023, 15, 54677–54691 CAS.
- F. Maddalena, M. E. Witkowski, M. Makowski, A. Bachiri, B. Mahler, Y.-C. Wong, C. Y. E. Chua, J. X. Lee, W. Drozdowski, S. V. Springham, C. Dujardin, M. D. Birowosuto and C. Dang, ACS Appl. Mater. Interfaces, 2021, 13, 59450–59459 CrossRef CAS PubMed.
- T. Haposan, M. Makowski, D. Kowal, L. J. Diguna, M. E. Witkowski, S. Mahato, W. Drozdowski, A. Arramel and M. D. Birowosuto, Phys. Status Solidi RRL, 2024, 2400298 Search PubMed.
- M. Makowski, Y. Wenzheng, D. Kowal, F. Maddalena, S. Mahato, Y. T. Amrillah, W. Zajac, M. E. Witkowski, K. J. Drozdowski, N. Nathaniel, C. Dang, J. Cybinska, W. Drozdowski, F. A. A. Nugroho, C. Dujardin, L. J. Wong and M. D. Birowosuto, Arxiv, 2024, https://arxiv.org/abs/2411.18477.
- L. J. Xu, X. Lin, Q. He, M. Worku and B. Ma, Nat. Commun., 2020, 11, 1–7 CrossRef PubMed.
- J. Ding, Y. Zhou, Z. He, Q. He, X. Liang, W. Xia and W. Xiang, Appl. Surf. Sci., 2022, 596, 153568 CrossRef CAS.
- F. Jiang and P. S. Lee, Nanoscale Horiz., 2022, 7, 1029–1046 RSC.
- J. Kang, R. Huang, S. Guo, G. Han, X. Sun, I. Ismail, C. Ding, F. Li, Q. Luo, Y. Li and C. Q. Ma, Sol. Energy, 2021, 217, 105–112 CrossRef CAS.
- W. Kim, J. B. Park, H. Kim, K. Kim, J. Park, S. Cho, H. Lee, Y. Pak and G. Y. Jung, J. Mater. Chem. A, 2019, 7, 20832–20839 RSC.
- C. Rodà, M. Fasoli, M. L. Zaffalon, F. Cova, V. Pinchetti, J. Shamsi, A. L. Abdelhady, M. Imran, F. Meinardi, L. Manna, A. Vedda and S. Brovelli, Adv. Funct. Mater., 2021, 31, 2104879 CrossRef.
- K. E. Lukyashin and A. V. Ishchenko, Russ. J. Inorg. Chem., 2021, 66, 1203–1211 CrossRef CAS.
- M. Sygletou, M. E. Kyriazi, A. G. Kanaras and E. Stratakis, Chem. Sci., 2018, 9, 8121–8126 RSC.
- K. Thirumal, W. K. Chong, W. Xie, R. Ganguly, S. K. Muduli, M. Sherburne, M. Asta, S. Mhaisalkar, T. C. Sum, H. S. Soo and N. Mathews, Chem. Mater., 2017, 29, 3947–3953 CrossRef CAS.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435–2445 CrossRef CAS.
- A. Xie, T. H. Nguyen, C. Hettiarachchi, M. E. Witkowski, W. Drozdowski, M. D. Birowosuto, H. Wang and C. Dang, J. Phys. Chem. C, 2018, 122, 16265–16273 CrossRef CAS.
- R. Subagyo, A. Arramel, L. J. Diguna, A. L. Ivansyah, M. E. Witkowski, M. Makowski, D. Kowal, W. Drozdowski, M. D. Birowosuto and Y. Kusumawati, Mater. Res. Express, 2022, 9, 096202 CrossRef CAS.
- L. J. Diguna, S. Kaffah, M. H. Mahyuddin, A. Arramel, F. Maddalena, S. A. Bakar, M. Aminah, D. Onggo, M. E. Witkowski, M. Makowski, W. Drozdowski and M. D. Birowosuto, RSC Adv., 2021, 11, 20635–20640 RSC.
- C. Dujardin, E. Auffray, E. Bourret-Courchesne, P. Dorenbos, P. Lecoq, M. Nikl, A. N. Vasil’ev, A. Yoshikawa and R. Y. Zhu, IEEE Trans. Nucl. Sci., 2018, 65, 1977–1997 CAS.
- A. Erroi, S. Mecca, M. L. Zaffalon, I. Frank, F. Carulli, A. Cemmi, I. Di Sarcina, D. Debellis, F. Rossi, F. Cova, K. Pauwels, M. Mauri, J. Perego, V. Pinchetti, A. Comotti, F. Meinardi, A. Vedda, E. Auffray, L. Beverina and S. Brovelli, ACS Energy Lett., 2023, 8, 3883–3894 CrossRef CAS PubMed.
- Y. Tang, G. Pu, Y. Tang, T. Sun, M. Wang and J. Wang, J. Phys. Chem. Lett., 2024, 15, 7036–7044 CrossRef CAS PubMed.
- T. Haposan, A. Arramel, P. Y. D. Maulida, S. Hartati, A. A. Afkauni, M. H. Mahyuddin, L. Zhang, D. Kowal, M. E. Witkowski, K. J. Drozdowski, M. Makowski, W. Drozdowski, L. J. Diguna and M. D. Birowosuto, J. Mater. Chem. C, 2024, 12, 2398–2409 RSC.
- D. Huang, Z. Cheng, Q. Ouyang, H. Lian and J. Lin, J. Mater. Chem. C, 2023, 11, 6220–6226 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2410659. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc04390a |
| This journal is © The Royal Society of Chemistry 2025 |


