 Open Access Article
Open Access ArticleFungal pretreatment methods for organic wastes: advances and challenges in biomass valorization
Pankaj Kumar
Chaurasia†
 *a,
Shashi Lata
Bharati†
*a,
Shashi Lata
Bharati†
 *b,
Sunita
Singh
c,
Azhagu Madhavan
Sivalingam
d,
Shiv
Shankar
e and
Ashutosh
Mani
*b,
Sunita
Singh
c,
Azhagu Madhavan
Sivalingam
d,
Shiv
Shankar
e and
Ashutosh
Mani
 *f
*f
aChemical, Biological and Environmental Laboratory, P.G. Department of Chemistry, L.S. College, B.R. Ambedkar Bihar University, Muzaffarpur-842001, Bihar, India. E-mail: pankaj.chaurasia31@gmail.com
bDepartment of Chemistry, North Eastern Regional Institute of Science and Technology, Nirjuli, Itanagar, Arunachal Pradesh-791109, India. E-mail: shashilatachem@gmail.com
cDepartment of Chemistry, Navyug Kanya Mahavidyalaya, University of Lucknow, Lucknow 226004, Uttar Pradesh, India
dNatural Products & Nanobiotechnology Research Lab, Department of Community Medicine, Saveetha Medical College, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Thandalam, Chennai 602105, Tamil Nadu, India
eDepartment of Environmental Science, School of Vocational Studies and Applied Sciences, Gautam Buddha University, Greater Noida, UP 201312, India
fDepartment of Biotechnology, Motilal Nehru National Institute of Technology Allahabad, Prayagraj-211004, Uttar Pradesh, India. E-mail: amani@mnnit.ac.in
First published on 13th January 2025
Abstract
Food wastes, municipal solid wastes, sewage sludge, plant materials, animal biomasses, aquatic and terrestrial wastes, agricultural and forestry wastes, industrial and domestic wastes and many other lignocellulosic biomasses are grouped under the category of organic wastes or bio-wastes. Various techniques, mainly mechanical (high-pressure homogenization and ultra-sonication), thermal (temperature-based), microwave-assisted, chemical, and biological pretreatments, have been found to be effective in organic waste valorization. Fungal pretreatment of organic wastes is a promising biological technology because of its excellent efficiency in the decomposition of various types of organic wastes, such as food wastes, ligno-cellulosic biomasses, hemicellulose, agricultural wastes, hardwoods, softwoods, switchgrass, spent coffee grounds, park wastes, cattle dung, and solid digestate, which are specifically reviewed. Fungal pretreatment of organic waste materials can generate advantageous products such as biogas, alternative energy sources, monomeric or oligomeric sugar products, and different types of acids. However, the major challenge associated with fungal pretreatment technology is the requirement of a longer time to achieve a greater degree of biomass valorization, which increases the cost and vulnerability to contamination. However, the use of fungal pretreatment with other pretreatment techniques may shorten the time and enhance the functionality of the method with a higher rate of biomass valorization. Heat generation in the fungal pretreatment process and need for feedstock sterilization before fungal pretreatment are some other challenges that need to be properly addressed for its efficient application on an industrial scale. In this review, the use of different fungal pretreatment methods for the valorization of different types of biomasses and production of valuable products is evaluated and discussed. We performed a comprehensive assessment of the fungal pre-treatment of various types of organic wastes together with a concise but effective discussion on organic solid wastes and different pretreatment techniques involved in bio-waste digestion processes. Furthermore, techno-economic analysis, challenges and future perspectives are discussed.
Sustainability spotlightSustainable treatment of biowaste or organic waste such as food waste, municipal solid waste, sewage sludge, plant materials, animal biomass, aquatic and terrestrial waste, agricultural and forestry waste, and many others is the need of the hour. Thus, different countries are working on various related projects to treat this biowaste and sustainably produce energy, biogases, methane, ethanol, carbohydrates, protein and several other useful chemicals. This type of sustainable literature review may be helpful to better understand the goals of sustainable development. This critical review is well-aligned with various sustainable development goals of the United Nations, including ‘affordable and clean energy’ (UN's SDG 7), ‘sustainable cities and communities’ (UN's SDG 11) and ‘responsible consumption and production’ (UN's SDG 12). |
1 Introduction
Solid waste, organic waste or bio-waste materials are composed of large-size organic molecules (macromolecules) such as lignocellulose, lignin, hemicellulose, proteins, fats, and vitamins. These materials are produced owing to various reasons; however, their decomposition produces harmful gases and constituents, which need to be properly treated. The appropriate management of this bio-waste may also produce several beneficial products such as biogas, sugars, electricity (via biogas or other fuel sources derived from biowaste valorization), short chain carboxylic acids, fertilizers and several other products. The generation of municipal solid wastes is expected to increase from 2.1 billion tonnes in 2023 to 3.8 billion tonnes by 2050.1 Besides, food loss and associated waste are global challenges. It is predicted that 13% of the globally generated food is wasted annually from harvest up to, but not counting retail, accounting for an economic value of about four hundred billion USD.2 Additionally, households, restaurants and many other food services are responsible for about 19% of food loss or food waste generation.2 Disposed food waste reached 38.1 million tons in 2017.3 Therefore, it is crucial to carefully manage food waste to avoid health issues and environmental contamination.4,5 The biggest waste produced in the process of sewage treatment is sewage sludge, which is rich in organic content, and considered organic municipal solid waste.6 The generation of sewage sludge has increased annually. For instance, the dry mass of sewage sludge has increased annually from ten million tons (in 2010) to 13.5 million tons (in 2020) in the twenty seven countries of the European Union.7 In this case, the process of composting and anaerobic digestion is preferred because waste can be processed by these ways into safe products such as organic fertilizers and soil improvers.8Currently, a series of management strategies is being promoted by various countries and technologies for food waste treatment are being developed. In Germany, there were greater than nine thousand pertinent food waste anaerobic digestion projects in action by 2015, accounting for greater than 80% of the biogas-based projects in Europe.9 Additionally, food waste produces around 5 million tons of fertilizer annually.10,11 By 2025, the rate of recycling of food waste is intended to be increased in the UK from the current 10% to 70%.12,13 Organic macromolecules such as proteins, sugars and fats are found in food waste materials. Food wastes also have trace elements such as Fe (iron) and Co (cobalt), which facilitate the growth of microorganisms.14
The huge generation of bio-waste is a serious problem worldwide, particularly in developing countries where its management is not as good as developed countries. However, it may also be converted into a useful form by the proper management of bio-waste via pretreatment because the bio-pretreatment of this type of waste may generate a large amount of energy and useful products. There are some important pretreatment techniques available for the pretreatment of organic bio-waste (Fig. 1), i.e. mechanical pretreatment techniques including high-pressure homogenization and ultrasonication, thermal pretreatment, microwave-assisted pretreatment, combined pretreatment technology, chemical pretreatment technology, and biological pretreatment methods including bacterial pretreatment, enzymatic pretreatment, and fungal pretreatment techniques. However, considering the complexity of the lignocellulosic structures and problems linked with pretreatment via chemical and physical methods, biological techniques are very useful because they are environmentally friendly, economical, effective and do not require or release any toxic chemicals.15 Among them, aerobic pretreatment, temperature-phased anaerobic digestion, and enzyme-mediated pretreatment processes are promising biological pretreatment processes,16 which can be used as favorable alternatives to chemical, physical, and thermal processes. The aerobic process is dependent on the inherent enzymatic activity of sludge, which requires oxygen.17 Alternatively, temperature-phased anaerobic digestion is done through a configuration composed of two digesters in a series, where thermophilic conditions are applied for the first digester, while mesophilic conditions for the second digester.16 The enzyme-mediated process of pretreatment causes am improvement in the sludge bioconversion with the assistance of exogenous enzymes, contributing to the degradation of refractory compounds.16 Regarding the production of biogas from sludge, a critical review on various pretreatment methods has been presented by Mitraka et al.18
 | ||
| Fig. 1 Different pretreatment techniques involved in the pretreatment process of organic or bio-waste. | ||
Fungi and their enzymatic systems are known for their huge biotechnological application due to their versatile capability and ability to produce several enzymes.19–22 Fungi also play great roles in environment restoration by eliminating or degrading several toxic environmental pollutants such as organic molecules and heavy metals.23,24 Together with these applications, fungi also show strong potential in the biological pretreatment of organic waste or bio-waste. Among the numerous physical, chemical and biological pretreatment techniques,18,25 fungal-based biological methods may play an emerging role in this field. The fungal pretreatment method is an important alternative to conventional pretreatments, which is mostly performed in the temperature range of 25–30 °C, with the minimum use of water, at atmospheric pressure, and without the use of any chemicals.26 Wood rot fungi such as soft, white, and brown fungi play the most important role in the fungal pretreatment technique because of their potential to change the constituents of lignocellulosic biomass.27 After fungal pretreatment, detoxification and/or washing are not generally necessary because the mild conditions of fungal treatment are unlikely to generate microbial inhibitory compounds. At higher feedstock particle sizes, the fungal pretreatment technique is more effective than most conventional pretreatment methods.26 However, fungal pretreatment also has some disadvantages together with the aforementioned advantages. These shortcomings include the requirement of a long reaction time, lower sugar yields and the need for sterilization of the feedstock in comparison with the traditional pretreatment methods.28
As a solid-state procedure with low energy and chemical consumption, the fungal pretreatment technique is considered a low-cost technique27 but its above-described shortcomings, mainly the longer time requirement, may make it more costly, which can be eliminated using combined pretreatment technology. The sterility requirement, long residence time, significant heat generation by the fungal metabolic rate and need for a high rate of aeration for efficient delignification may play a significant role in the techno-economic study of fungus-based pretreatment at the commercial scale, which still need to be studied.27 However, despite the shortcomings or challenges of fungal pretreatment methods, several studies have shown that fungal pretreatment techniques may be a noteworthy biological pretreatment method for the treatment of diverse biowaste or biomass29,30 because they provide a gentle, ecofriendly, and low cost future solution for biomass conversion. Also, they are widely utilized in the production of chemicals and biofuels due to their role in the degradation of lignin, saccharification, lipid accumulation and fermentation.29
Considering the crucial involvement of fungi in the pretreatment of lignocellulosic biomass, agricultural waste, food waste, saccharification process, biogas production, bioethanol production, and sugar production, etc., we decided to prepare a comprehensive review on the fungal pretreatment of these biowaste materials. The principle objective of this review is to analyze the significance of the fungal pretreatment in the management of the problem of huge organic waste or bio-waste materials and present a techno-economic analysis, potential future challenges and its applicability. During the fungal pretreatment process, the production of biogases, bioethanol, sugar molecules, and other biologically valuable components has a great future owing to their use in the welfare of humanity. Several recent reports are discussed herein in detail to highlight and understand the promising possibilities of fungal pretreatment methods. Furthermore, a brief but effective discussion is also presented on the topics of organic waste and various pretreatment techniques.
2 Organic waste or bio-waste or sources of biomass
A wide range of materials come under the topic of organic solid waste or bio-waste. Waste from food, sewage sludge, plants and animal biomass, fungal and algal biomass, several aquatic and terrestrial waste, agricultural waste, industrial waste, and many other waste materials that are rich in organic components such as polysaccharides, lignin, proteins, fats, and vitamins are considered organic waste or organic solid waste. Fig. 2 presents a picture of huge plant biomass from nature (dead plant materials and green plants). In a comprehensive critical review, Lizundia et al. well-described the valorization of organic waste.31 Among the great diversity of aquatic organisms, algae and crustaceans are considered a good source of organic waste. Vast probabilities to get varying polysaccharides are offered by the high quantity of marine algae reaching the coast and discarded. About 9.1 million tonnes are discarded by fisheries annually.32 whereas seafood accounts for 31% of the consumer-level food losses in the United States of America (USA).33 Waste derived from forestry (softwood, grass, sawdust, and hardwood) offers biomass with a high lignocellulosic nature at a low cost. Compared to waste from agriculture, forestry-lignocellulosic waste requires intensive physico-chemical treatments given the more complex structure of the cell wall.34 Thus, to extract important and valuable materials, hydrolysis or thermo-chemical processes are applied to this type of waste. Considering the projected global production of eggs of ninety million tons by 2030,35 eggshells are a classic example of terrestrial animal waste with portions still useable after they are discarded. Eggshells are a source of hydroxyapatite after calcination and following treatment with salts, for instance Ca3(PO4)2.36,37 Porcine or bovine bones also offer good access to hydroxyapatite. After the removal of residual protein through alkaline process and calcination at a high temperature, yields of ∼65 wt% are attained.38 Compared to synthetic hydroxyapatite, bio-derived hydroxyapatite displays traces of Na+, Mg2+, K+, Zn2+, Al3+, Sr2+, F−, Cl−, SO42−, and CO32−, which are valuable for stimulating the proliferation functions of cell.39 Bovine bones may be utilized for the extraction of collagen fibres that are mineralized.40There are numerous sources of bio-waste with promising compositions from aquatic origin such as fishes (hyaluronic acid, skin collagen, and gelatin after denaturation), red algae (carrageenans and agarose in the cell wall), brown algae (cell wall-based alginate), chitin (chitosan found upon chitin deacetylation), cephalopod endoskeleton (β-chitin, proteins, and lipids), and cuticles from marine arthropods (varying protein-enfolded alpha-chitin nanofibrils). Agricultural bio-waste include cereals (rice, corn, wheat, rye, barley, oats, etc.), fruits (grapes, oranges, apples, coffee, mangoes, bananas, apricots, pineapples, etc.), vegetables (carrots, tomatoes, olive husks, onions, potatoes, and red beet), and legumes (lentils, cow peas, lupins, beans, chickpeas, etc.). Bio-waste from forestry origin includes (grass, softwood, hardwood, sawdust, cellulose, lignin, and hemicelluloses). Fungal bio-waste is composed of glucans, chitin, glycoproteins, and melanin. Bio-waste from terrestrial animal origin include eggshells (CaCO3, organic matter, MgCO3, and Ca3(PO4)2), bones with natural hydroxyapatite and collagen fibres, which are mineralized, feathers with beta-keratin, manure (carbon, oxygen, hydrogen, nitrogen, and sulphur), wool with alpha-keratin, fat, impurities, etc., structural proteins derived from animals (silk, collagen, and gelatin), and exoskeletons of terrestrial-arthropods (protein-enfolded chitin fibres).31
Parts of plants such as fruits, tubers, and seeds, from different crops such as sunflower, rapeseed, palm, cotton, corn and soybean have been utilized in the generation of first-generation ethanol and biodiesel (Fig. 3),41 while complete biomass of above-ground plants called lignocellulosic feedstock (inedible leaves and stems) has been utilized for the creation of second-generation biofuels.41 Switchgrass, sorghum, miscanthus, and eucalyptus are some biofuel crops and a few of them have adaptability to deprived soils and marginal agronomic lands.42 Olives, coconut and endocarps/shells of eastern black walnut are feedstocks with a high density and contain the maximum content of lignin among the recognized organs of plants, where endocarp-derived energy is equivalent to coal.43 Worldwide, several million tons of biomass from drupe endocarp are available (24–31 million tons),44 which is greatly underutilized, and thus countries with energy scarcity can benefit from its proper management.43 Agricultural and industrial waste are also important sources of lignocellulosic materials and can be used for the generation of biofuels. Lignin, cellulose, and hemicellulose are the major components of the plant cell wall, while proteins, organic acids, and tannins together with secondary metabolites are its minor components. The composition of lignocellulosic materials varies with species, plant parts and ecological conditions.43,45
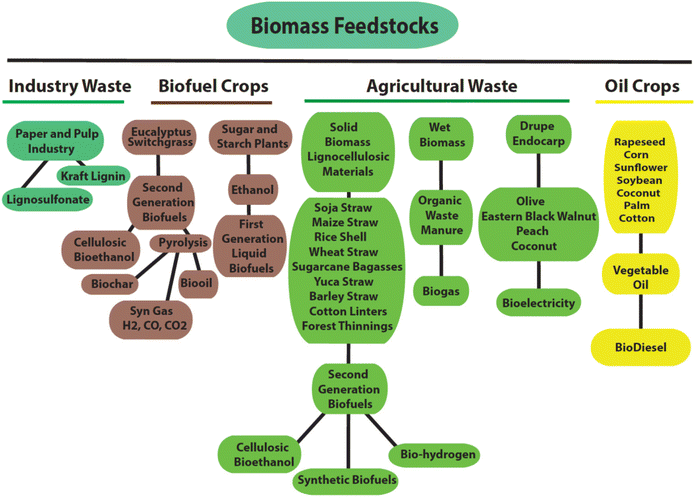 | ||
| Fig. 3 Different types of biomass and their application in biofuel, bioenergy and bio-product generation.41 | ||
3 Methods for the pretreatment of organic waste
Mainly two types of waste are generated by daily garbage, in which the first type includes non-biodegradable wastes (i.e. metals, plastics, and glass), while the second includes biodegradable wastes such as leftover foods, dried leaves, and fruits.46 Several animal-based products, plant-linked products, garden waste, food waste, and degradable carbon are examples of biodegradable organic waste. Energy may be provided as biogas by using the organic waste recycling method of anaerobic digestion.46 The efficiency of non-organic recycling is also improved by the separation of organic and non-organic wastes.47 Minimizing the pollution in the water, air, and land by recycling organic waste is its one of the promising advantages. In the case of less effective techniques (disposal and incineration), the amount of garbage left over is also minimized by the recycling of organic waste. Organic waste stabilization offers value by enhancing the content of nutrients and availability for utilization as fertilizer in the agriculture sector. Popular concepts such as zero-waste strategies, cleaner production, sustainability, and bio-based circular economy are supported by the recycling of organic waste.46,48,49 Different barriers in the management of organic waste have also been assessed by Kharola et al. (2022).46The generation of biogas from organic waste may be a promising factor for a sustainable future. Organic waste utilization for biogas generation applying the methods of mono- and co-digestion has been broadly reported.25 The reactions in the production of biogas occur in many stages, among which hydrolysis is crucial as the 1st step and helps in enhancing the complete yield. The optimization of the hydrolysis step causes the decomposition of complex organic matter into large quantities of monomeric/oligomeric components, which can simply be used under anaerobic conditions for biogas production. The aim of pretreatment approaches is making the existing nutrients available to the maximum species of microbes that accelerate the use of biomass for the duration of anaerobic digestion.50 In the review by Mitraka et al. (2022), they comprehensively discussed several pretreatment methods for the increased production of biogas from sewage sludge.18 There are many methodologies for the pretreatment of organic wastes for biogas production,18,25 which are concisely discussed herein (Table 1).
| S. N. | Pretreatment techniques | Potential features | Potential drawbacks | Potential types |
|---|---|---|---|---|
| 1 | Mechanical treatment | (i) Size reduction without producing any products and chemical alteration | High energy consumption | High pressure homogenization technique, ultrasonication, etc. |
| (ii) No chemical needed | ||||
| (iii) Its use before biological or other pretreatment methods is needed and enables the easy handling, transport and processing of even a big density of lignocellulosic materials | ||||
| 2 | Thermal pretreatment | (i) Helps in complex structures' hydrolysis | Mostly high temperature requirement | Low temperature-based treatment, high temperature-based pretreatment |
| (ii) Helps in enhancing the anaerobic digestion | ||||
| 3 | Microwave-assisted pretreatment | A type of heat pretreatment and helpful in the stabilization of wastes | Electromagnetic radiation required | — |
| 4 | Chemical based pretreatments | (i) Chemical constituents of bio-wastes are broken down using oxidants, alkali, and acids | Harmful/toxic chemicals or reagents required | Pretreatment using alkali, pretreatment using acids, ozonation, peroxidation, etc. |
| (ii) Helping in the solubilisation of sludge | Used chemicals may be harmful for environment and human health | |||
| 5 | Biological methods of pretreatment | (i) Microbial utilization for the degradation of organic waste | Comparatively time consuming process, contamination possibility, need of sterilization, works in optimized conditions like pH, temperature, concentrations, etc. | Bacterial method of pretreatment, fungal method of pretreatment, enzymatic ways, combined methods |
| (ii) No adverse conditions are required | ||||
| (iii) Efficient in biodegradation, valuable products formation, bioconversion and biogas production | ||||
| (iv) Bacterial, fungal and enzymatic methods are very effective techniques | ||||
| 6 | Combination of methods | (i) Utilization of two or more different methods for the pretreatment of organic waste | Depends on methods combined for pretreatment | Thermal-chemical methods, chemical–biological techniques, physical–chemical–biological methods, other possible methods |
| (ii) More effective and efficient |
3.1 Mechanical pretreatment
Mechanical pretreatment causes a reduction in the size of organic waste particles and does not generate any products.25 It is a process that consumes energy, which is its main drawback. Developments in milling approaches display that compared with the dry milling procedure, wet milling is better because of its greater pulverization properties with minimal consumption of energy.51 de Oliveira et al. (2022) investigated two wet mechanical pre-treatments on bio-waste from urban household, which were air-compressed press and worm screw press.52 In each experiment, they studied two liquid/solid ratios. An enhancement in the biodegradable organic matter proportion extracted from bio-waste was observed with an increase in the ratio of liquid to solid in the pre-treatment up to 949 g COD per kg TS from the household bio-waste. In a constantly stirred tank-based reactor, a very good COD load conversion (81%) and high methane production of up to 345 L CH4 per kg VS were achieved by anaerobic digestion.52 Cesaro et al. (2021) assessed the potential of press-extrusion pretreatment to enhance the anaerobic degradation of the organic part of solid municipal waste.53 Among the mechanical pretreatment methods, recently press-extrusion attracted great interest for its probable use to either increase the organic weight in the digester or enhance the overall stability of the process and methane yield.53 To improve the production of bio-methane, Chevalier et al. (2023) successfully evaluated the influence of the mechanical treatment method via twin-screw extrusion using different types of lignocellulosic biomass and they tested two dissimilar shear stress screw profiles.54 The specific rate was found to be enhanced by both extrusion mechanical treatments, which was proven by the kinetic assessment of the production of methane.3.2 Thermal pretreatment
The thermal pretreatment method helps in hydrolyzing the complex organic components in organic waste and has been employed to increase their anaerobic digestion.25 El Gnaoui et al. (2022)77 evaluated the effect of thermal pretreatment, including thermal pretreatment at temperatures of 60 °C and 80 °C for 60 min, and thermal pretreatment at 100 °C, 120 °C, and 140 °C for 30 min, as well as pre-hydrolysis (biological) at temperatures of 37 °C, 55 °C, 37 °C, followed by temperature of 55 °C and 55 °C followed by 37 °C for 40 h on the anaerobic breakdown of food waste in a batch test. The pre-hydrolysis (biological) and thermal pretreatment method resulted in an enhancement in the soluble COD and efficiency of hydrolysis. There was an increase in the yield of methane from 371.17 mL CH4 per g VS for untreated food waste to 471.95 mL CH4 per g VS. The greatest methane yield was observed for biological pre-hydrolysis at a temperature of 37 °C for 20 h, followed by 55 °C for 20 h. The rate of formation of biogas increased and the lag phase decreased applying the pretreatments.77 There are also many other recent reports in the literature on thermal pretreatment.78–803.3 Microwave pretreatment
Microwave pretreatment is also a type of heat pretreatment method. Besides the generally utilized thermal pretreatment method, microwave pretreatment is effective in the stabilization of organic waste and biogas formation.25 This method has a few benefits, which include quick heating and penetration, simple handling and control, pathogen elimination, and effective dewaterability of sludge and sludge reduction,81 due to which it is more efficient in comparison with the conventional thermal pretreatment methods. The effect of microwave pretreatment on the anaerobic fermentation of model food waste to organic acids with small chains and ethanol was investigated.82 Microwave pretreatment was studied at 120 °C, 150 °C, and 180 °C (three temperatures) and residence times of 2, 5 and 8 min. The highest decrease in the volatile suspended solids (VSS) was 20%, representing the solubilisation of organic matter. There was a greater (17.5% COD per COD) total product yield in the fermentation batch tests in comparison with the untreated substrate (11.1% COD per COD).82 The influence of microwave-based pretreatment on the anaerobic co-digestion of sludge and food waste was described by Liu et al. (2020).83 The results displayed that microwave pretreatment was beneficial for the dissolution of organic materials, protein conversion to NH4+–N, cumulative production of CH4, unit yield of bio-methane, and methane formation reaction rate in the sludge and food-based waste anaerobic system of co-digestion. In the co-digestion system, the maximum cumulative production of CH4 reached 3446.3 ± 172.3 mL (35 days), which was 19.93% greater than that of the control. Moreover, the microwave pretreatment method considerably enhanced the accumulation of volatile fatty acids and content of butyric acid in the anaerobic-digested effluent.83 Hydrolysis of the cassava pulp was studied by Prasertsilp et al. (2023)84 using the microwave method for the effective utilization of natural materials and four different factors such as liquid–solid ratio, acid type, watt power and time were investigated. A high glucose content was provided by this study, exhibiting 88.1% conversion. There are several other relevant studies on microwave-assisted pretreatment technology.85,863.4 Chemical pretreatment methods
In the chemical pretreatment method, oxidants, alkali, and/or acids are used to breakdown the organic components of organic bio-waste, which has been found to be very effective. Ozonation and peroxidation (oxidation) are found to be beneficial in the pretreatment, causing the solubilisation of sludge.25 Up to a certain limit, there is a dose-dependent association between the concentration of oxidant and solubilization of sludge. Therefore, the peroxidation and ozonation process tends to display a greater rate of sludge degradation, this compromising the biogas yield.87,88 In the study by Alino et al. (2022),89 they assessed the effectiveness of a low-cost and more sustainable method to enhance the biodegradability of sugarcane bagasse and enhance the generation of methane by its pre-collection with acidic types of organic bio-waste (such as cheese whey, fruit waste, and vegetable waste). They obtained the best result with sugarcane bagasse plus fruit and vegetable waste (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 ratio) of 520 ± 7 NL CH4 per kg VS (27.6% greater than the control) with a decrease in the degradation time (T90) from 13 days to 7 days. The yield of methane increased by 21.2% and 34.1% upon alkaline pretreatment with sodium hydroxide at concentrations of 5% and 10%, respectively.89 Jankovičová et al. (2022)90 performed a study on the hydrolysis of materials in NaOH (0.5% and 5%) and H2SO4 (0.5% and 5%) at 90–100 °C for 2 h. The influence of these techniques on the lignocellulosic constitution of rapeseed straw, maize based waste, and wheat straw and the biogas yield was compared. The 0.5% NaOH pretreatment enhanced the production of biogas the most (for rapeseed straw by 159%, wheat straw by 240% and maize waste by 59%); furthermore, the solubilization degrees were greater.90 The study by Sreevathsan et al., (2023)91 on the effects of ozonation on the biodegradability improvement and bio-methanation ability of wastewater pretreatment and study by Qiao et al. (2023)92 on the pretreatment of landfill leachate state the advantages of chemical pretreatment.
95 ratio) of 520 ± 7 NL CH4 per kg VS (27.6% greater than the control) with a decrease in the degradation time (T90) from 13 days to 7 days. The yield of methane increased by 21.2% and 34.1% upon alkaline pretreatment with sodium hydroxide at concentrations of 5% and 10%, respectively.89 Jankovičová et al. (2022)90 performed a study on the hydrolysis of materials in NaOH (0.5% and 5%) and H2SO4 (0.5% and 5%) at 90–100 °C for 2 h. The influence of these techniques on the lignocellulosic constitution of rapeseed straw, maize based waste, and wheat straw and the biogas yield was compared. The 0.5% NaOH pretreatment enhanced the production of biogas the most (for rapeseed straw by 159%, wheat straw by 240% and maize waste by 59%); furthermore, the solubilization degrees were greater.90 The study by Sreevathsan et al., (2023)91 on the effects of ozonation on the biodegradability improvement and bio-methanation ability of wastewater pretreatment and study by Qiao et al. (2023)92 on the pretreatment of landfill leachate state the advantages of chemical pretreatment.
3.5 Combination of chemical pretreatment with other pretreatment methods
Chemical pretreatment methods including the utilization of alkali and acid are typically used in combination with additional treatment methods.93–95 The combination of pretreatment techniques is beneficial for improving the solubilization, sanitation, dewaterability and anaerobic digestion of sludge.25 The influence of thermal and combined thermal-chemical pretreatments was investigated by Ahmed et al. (2022)96 on the solubilization of organics, yield of biogas, formation of recalcitrant, and energy efficiency at varying temperatures and alkali dosages. The organic fractions of municipal solid waste were subjected to thermal pretreatment (i.e. 100–200 °C temperature, 1.6–15.8 bar pressure, and 30–120 min reaction time) alone and in conjugation with different dosages of alkali (1–7 g per L NaOH). Compared to the control (331 mL per g VSadded), the maximum biogas production increase of 43% (474 mL per g VSadded) and 87% (618 mL per g VSadded) was observed at a temperature of 125 °C and 125 °C + 3 g per L sodium hydroxide (NaOH) dose, respectively.96 Pham et al. (2021)97 performed acid-catalyzed hydrolysis at temperatures of 120 °C, 150 °C, and 180 °C utilizing H2SO4 (sulfuric acid) with concentrations in the range of 0–0.5 M within 90–180 min reaction time to yield bio-based chemicals from sewage sludge. The maximum yield of xylose was 7.69 mol%, while the maximum yield of glucose was 5.22 mol% at a temperature of 120 °C, H2SO4 concentration of 0.5 M within 180 min reaction time. Moreover, under acid-catalyzed hydrolysis at a temperature of 180 °C and 180 min, at 0.5 M H2SO4, levulinic acid production reached the highest level of 0.48 mol%, while at 0.1 M H2SO4, the maximum production of 5-hydroxymethylfurfural was 1.66 mol%.97 Qiao et al. (2023)92 described the importance of combined physicochemical methods for the landfill leachate pretreatment process. Combined pretreatment methods are more useful techniques in pretreatment processes compared with individual pretreatment methods. In another study, the effect of combined pretreatment on plant waste was studied using physical, enzymatic and chemical techniques, which also proved that combined methods are more favorable.983.6 Biological pretreatment methods
The biological pretreatment of organic waste or bio-waste is a promising ecofriendly method, which is based on the use of mainly fungi, bacteria, and enzymes. Their use in the pretreatment of organic waste is very effective in the decomposition of large cellulosic or other organic materials into monomeric or smaller units and generating valuable biogas. Table 2 (ref. 78) shows the variation in the production of biogas from some lignocellulosic biomass after a low-temperature pretreatment process under biological conditions.99–106 In biological pretreatment, among the various microorganisms, fungi are of the utmost significance and filamentous fungi, specifically Basidiomycetes, have great potential in delignification and lignocellulosic biomass conversion owing to the effective involvement of their enzymatic machinery.15 In bio-waste processing, microbial play promising roles in the improvement of the production of biogas and the process can be enhanced by using various metagenomics approaches.107 The reports by Mitraka et al. (2022)18 and Salihu and Alam (2016)25 also presented a detailed discussion on biological pretreatment. Bacterial pretreatments are concisely discussed herein with a few recent reports because the focus is to comprehensively discuss fungal pretreatment techniques.| S. N. | Lignocellulosic sources | AD condition/mode | Temperature/time | Yield of biogas/methane | Increase | Reference |
|---|---|---|---|---|---|---|
| 1 | 67% wheat straw and 33% sunflower meal | Mesophilic/batch mode (45 days) | 120 °C/1 hour | -/370 mL per g VS | 8.8% | 99 |
| 140 °C/1 hour | -/390 mL per g VS | 14.7% | ||||
| 2 | Bean straw | Mesophilic/continuous mode (hydraulic retention time 4.5 days) | 121 °C/1 hour | 145 mL per g COD/- | — | 100 |
| 3 | Rice straw | Mesophilic/batch mode (30 days) | 80 °C/6 hours | 372.5 mL per g VS/- | 12.4% | 101 |
| 4 | Rice straw | Mesophilic/batch mode (35 days) | 100 °C/150 minutes | 128 L per kg TS/- | 22.8% | 102 |
| 130 °C/150 minutes | 125 L per kg TS/- | 19.8% | ||||
| 5 | Rice straw | Mesophilic/batch mode (50 days) | 90 °C/15 minutes | 307 mL per g TS/- | 3.0% | 103 |
| 6 | Wheat straw | Mesophilic/batch mode (45 days) | 120 °C/60 minutes | 496 mL per g VS/- | 22.8% | 104 |
| 7 | Wheat straw | Mesophilic/batch mode (30 days) | 121 °C/60 minutes | — | 29% | 105 |
| 8 | Sugarcane bagasse | Mesophilic/batch mode (30 days) | 121 °C/60 minutes | — | 11% | 105 |
| 9 | Switchgrass | Mesophilic/batch mode (1100 hours) | 100 °C/6 hours | — | 25.9% | 106 |
4 Fungal pretreatment of organic waste
Due to their minimum energy condition and negligible toxicity, fungal pretreatment processes have attracted significant attention for their role in biomass conversion. They avoid the use of chemicals, hinder the production of compounds and show good selectivity towards lignin degradation.111,112 Several choices are provided by the useful and copious species of fungi for biomass conversion. Rice straw, wheat straw, corn straw, peanut shells, coconut shells, bagasse, spent coffee grounds, food digestate, food waste, hardwoods, softwoods, switchgrass, etc. are the recognized biowaste successfully subjected to fungal treatment techniques. Table 3 shows the major chemical compositions of various types of biowaste/organic waste.113–121 After the degradation of the lignocellulose structure, the cellulose and hemicellulose degradation efficiency in the biomass increases.29Fig. 4 shows the complex structure of lignin,122 while Fig. 5 shows a schematic presentation of the degradation of cellulose. The competence of subsequent fermentation and accumulation of lipid is promoted by saccharification, and thus various downstream products can be formed from the conversion of biomass.123,124 However, its popularization has been hindered due to the low efficacy of the fungal pretreatment process, but applying useful strategies may result in an improvement in the efficiency of fungal technology.29 This section presents a comprehensive approach for fungal pretreatment technology in biomass conversion based on recent literature studies. Different methods based on fungi have been adopted and the process of biomass/bio-waste valorization successfully performed in several studies, which are reviewed and their results presented in this section. Fungal pretreatment of organic waste or biomass is discussed here as the main heading because the principle target of this review is to comprehensively evaluate the various studies reported on fungal pretreatment technology in solving the problem of different organic waste or bio-waste and production of many useful products such as biogas, bioethanol, sugars, acids, and others.| S. N. | Common bio-waste | Composition | Reference | |
|---|---|---|---|---|
| 1 | Wheat straw | Cellulose: 44.2%, hemicellulose: 26.5%, lignin: 22.4%, ash: 2.8% | 113 | |
| 2 | Rice straw | Cellulose: 36.1%, hemicellulose: 27.2%, lignin: 19.7%, ash: 12.1% | 113 | |
| 3 | Rice husk | Cellulose: 50%, lignin: 25–30%, moisture: 10–15% and silica: 15–20% | 114 | |
| 4 | Poplar | Cellulose: 39.2%, hemicellulose: 18.8%, lignin: 29.6%, ash: 1.5% | 113 | |
| 5 | Corn straw | Cellulose: 33%, lignin: 19.47%, cellulose sugar: 23.58%, ash: 5.12% | 115 | |
| 6 | Peanut shell | Cellulose: 44.8%, lignin: 36.1% and hemicellulose: 5.6% | 116 | |
| 7 | Bagasse | Cellulose: 35.2%, hemicellulose: 24.5%, lignin: 22.2%, ash: 20.9% | 117 | |
| 8 | Coconut shell | Cellulose: 36.13%, lignin: 32.33%, hemicellulose: 20.36%, content soluble in water: 11.17% | 118 | |
| 9 | Walnut shells | Polysaccharides: 49.7%, lignin: 30.1%, extractives: 10.6% | 119 | |
| 10 | Almond shells | Polysaccharides: 56.1%, lignin: 28.9%, extractives: 5.7% | 119 | |
| 11 | Pine nut shells | Polysaccharides: 48.7%, lignin: 40.5%, extractives: 4.5% | 119 | |
| 12 | Corn stover fractions | Cobs | Cellulose: 37.8%, lignin: 13.5% | 120 |
| Husk | Cellulose: 38.1%, lignin: 12.6% | |||
| Leaves | Cellulose: 39.3%, lignin: 17.6% | |||
| Stalks | Cellulose: 44.9%, lignin: 19.9% | |||
| 13 | Food wastes | Fruits and vegetables wastes | Protein: 5.20%, fat: 1.36%, carbohydrates: 39.01% | 121 |
| Waste of mixed vegetables | Protein: 15.3%, fat: 0.87%, carbohydrates: 83.83% | |||
| Dairy related products | Protein: 14.05%, fat: 28.43%, carbohydrates: 57.51% | |||
| Waste of cereal products | Protein: 11.71%, fat: 3.83%, carbohydrates: 84.98% | |||
| Bakery wares related wastes | Protein: 12.92%, fat: 6.03%, carbohydrates: 81.05% | |||
| Wastes of meat related products | Protein: 25.17%, fat: 57.74%, carbohydrates: 17.10% | |||
| Wastes of fish related products | Protein: 27.48%, fat: 65.53%, carbohydrates: 6.98% | |||
| Wastes from egg related products | Protein: 19%, fat: 73.06%, carbohydrates: 7.94% | |||
| Wastes from restaurants | Protein: 15.59%, fat: 19.05%, carbohydrates: 65.36% | |||
| 14 | Rice wastes | Carbohydrates: 91%, protein: 8% | 121 | |
| 15 | Spent coffee grounds | Protein: 39.88%, fat: 60.12% | 121 | |
| 16 | Tea wastes | Carbohydrates: 76.59%, protein: 23.04% | 121 | |
 | ||
| Fig. 4 Model structure of lignin.122 | ||
4.1 Fungal pretreatment of lignocellulosic biomass of agricultural wastes
Fungi play a significant role in the decomposition of the various cellulosic materials (such as wheat straw, willow chips, rice straw, sugar bagasse, corn stover, and plant materials) and agricultural waste. Lignocellulosic biomass contains a large amount of complex carbohydrates, i.e. 55–75% in total solids (TS), and is renewable as well as widely available, and thus it can be excellent feedstock for the production of energy.125 It is well established that WRF may be involved in the improvement of enzyme-based hydrolysis and its subsequent sugar yield.126 Therefore, its involvement in pretreating substrates, mainly for the generation of bioethanol,125,127 has been studied but hardly for anaerobic digestion.128 In the case of submerged fermentation for pretreatment, the solid-state fermentation (SSF) process has been found to be better, which permits greater loads of feedstock, prefers fungal enzyme attachment to the substrate, and the diffusion of oxygen. SSF needs less aeration, mixing, water, and heating, and consequently less expensive than liquid culture.129Pretreatment of lignocellulosic materials such as wheat straw, woody willow chips, and corn stover was studied by Kovács et al. (2022)130 with the help of four filamentous fungi, namely, Penicillium aurantiogriseum, Gilbertella persicaria (SZMC11086), Trichoderma reesei (DSM768), and Rhizomucor miehei (SZMC11005). The excellent production of a hydrolytic enzyme and maximum yield of biogas from the partly decomposed substrates were shown by P. aurantiogriseum. Corn stover was the best material for the breakdown of biomass and generation of the biogas. The most effective strain for the pretreatment and biogas production was P. aurantiogriseum. All the tested fungi preferred the corn stover substrate for the productivity of methane.130 In 60 mL batch fermentation, within the first 20 days, a noteworthy portion of the generated methane (95%) was found to be evolved. The maximum yield of methane was observed in the corn stover-fed reactors. The maximum average production of methane (281 mL N per g oTS) was observed in the reactors pretreated with P. aurantiogriseum. During 300 mL batch fermentation, the influence of a 5-fold enhancement in volumetric scaling was also studied in the succeeding step. However, it was notable that only a 13.3% maximum difference (for the case of P. aurantiogriseum reactors) was observed between the outcomes of the two reactor sizes, showing the insensitivity of the overall process to scaling up. G. persicaria, T. reesei, and R. miehei-based pretreatment of all the substrates also showed the same behavior.130 Biogas production from corn silage was found to be enhanced by Pleurotus ostreatus and Dichomitus squalens, while a negative impact was shown by Trametes versicolor and Irpex lacteus.131 The cumulative production of CH4 increased by 1.55-fold with P. ostreatus after 10 days at 28 °C, while a longer pretreatment duration (30 and 60 days) showed a lower effect. With distinctive corn silage, the CH4 production increased from 0.301 to 0.465 m3 per kg VS due to the depolymerization of lignin.131
Pallín et al. (2024)132 utilized Irpex lacteus for the pretreatment of lignocellulosic biomass (wheat straw) and assessed its feasibility at a demonstration scale. Similar to submerged cultures, scaling up SSFs is not straightforward. Before choosing the best bioreactor design, several issues such as the microbe growth kinetics, agitation requirement, physical properties of the solid substrate, sterilization conditions, and agitation-generated mechanical stress should be addressed. During the process, the agitation-based design of the SSF reactor was ruled out because of the agglomeration of Irpex lacteus into wheat straw. After considering the several factors associated with the SSF reactor, an autoclavable vertical bioreactor of 22 L capacity was designed.132 The digestibility of the wheat straw after 21 days of pretreatment in a solid-state fermentations bioreactor (60.6%) was similar to that found on a small scale, i.e. 57.9%. In the three bioreactor experiments (B1, B2 and PB), the sugar evolution was completely different after 21 days of treatment.132 A 26.5% reduction in lignin was observed. There was greater reduction in lignin in experiment B2, i.e. 34.90% ± 0.87%, in comparison with that in experiment B1, i.e. 26.7% ± 3.08%. In the case of the PB fermentation, an increase was noticed for all the compounds in except lignin, which decreased by 52.3% ± 0.69%. This was the largest reduction in all the biomass constituents obtained in the three solid-state fermentation reactor experiments. There was similar reduction in lignin, i.e. 53.2% ± 0.60%, in the flask-scale pretreatment.132
Two isolated species of fungi, namely, Trichoderma harzianum and Aspergillus terreus, were used to investigate the degradation of cellulose, followed by bioethanol generation from acid-thermal pretreated rice straw, and the experiment was conducted in two phases.133 In the first phase, Aspergillus terreus and Trichoderma harzianum, isolated cellulose degrading fungi, were used for the enzymatic hydrolysis of pretreated rice straw, which was divided into HL (hydrolysate liquid) and RP (residual pulp), while in the second phase of the experiments, the substrate (enzymatically hydrolyzed) was subjected to yeast fermentation for the generation of bioethanol. The results showed the degradation of 80% cellulose by these fungi. The performance of Aspergillus terreus in the degradation of cellulose with hydrolysate liquid and residual pulp was 92% and 80%, while that for Trichoderma harzianum was 93% and 82%, respectively. With the use of A. terreus, the glucose formation from cellulose during enzyme hydrolysis was 12.15 g L−1 and 16 g L−1, while it was 10.8 g L−1 and 21.6 g L−1 using T. harzianum, respectively. The underlying mechanism of this process involves the action on the β-1,4-glycosidic bonds by the cellulose-degrading fungi through enzymatic activities only after the pretreatment process. The treatment of rice straw RP with T. harzianum led to a greater bioethanol yield of 5.4 g L−1, while that for Aspergillus terreus was 4.7 g L−1.133 The pH of the enzymatically pretreated reactors using A. terreus was 3.8 for the control, 4.8 for the hydrolysate liquid, and 4.5 for the residual pulp, while in the case of the T. harzianum-based reactors, the pH values were 5.0, 4.9, and 4.8, respectively. The substrate and inoculum types are the major factors in the production of bioethanol in the pH range of 3.5–6. In the reactors based on hydrolysate liquid as the substrate, the performance of A. terreus and T. harzianum in the production of bioethanol was the same at 4.5 g L−1.133
A genuine challenge in the production of bioethanol from rice straw is to remove lignin properly from biomass through a pretreatment process because lignin removal is necessary to generate bioethanol from rice straw effectively by the saccharification and fermentation process.134 This challenge can be addressed through the use of alkali-assisted pretreatment and acid thermal pretreatment of rice straw. Devi and Munjam133 successfully used A. terreus and T. harzianum in the production of bioethanol from rice straw, which was pretreated using an acid-thermal method. Alternatively, the study by Takano and Hoshino134 showed the use of an enzyme cocktail (optimized) and Mucor circinelloides (xylose fermenting fungus) in the production of ethanol from alkali-pretreated rice straw by concurrent saccharification and solid state fermentation. Abo-State et al.135 subjected rice straw to steam treatment (autoclaving) and various gamma irradiation doses of 50 and 70 mrad. Subsequently, different fungal isolates were used to enzymatically treat the steam-treated rice straw throughout the SSF process.135 Therefore, any pretreatment processes that are effective in the removal of lignin from rice straw can be used and combined with the fungal treatment process for the production of bioethanol by saccharification and SSF process.
Furthermore, the effect of alkali sodium hydroxide (NaOH)/hydrogen peroxide (H2O2)-based various pretreatment approaches on willow sawdust biomass was described by Atitallah et al. (2022)136 utilizing the conventional yeast, i.e. Saccharomyces cerevisiae, and three non-conventional strains of yeasts including Pachysolen tannophilus, Wickerhamomyces anomalus X19, and Pichia stipitis. The results showed that greater delignification, i.e. 38.3% ± 0.1%, saccharification efficiency, i.e. 31.7% ± 0.3%, and ethanol yield were achieved by the 2-stage pretreatment method, i.e. 0.5% w/v NaOH for 24 h and 0.5% v/v H2O2 for 24 h. Ethanol yields in the range of 11.67 ± 0.21 to 13.81 ± 0.20 g/100 g TS were observed by the Saccharomyces cerevisiae or Wickerhamomyces anomalus X19 monocultures and co-cultures with Pichia stipitis. W. anomalus was selected as the non-conventional strain due to its high efficiency in bioethanol production, whereas S. cerevisiae was utilized as the highest exploited strain of yeast for the production of bioethanol from sugar fermentation. There was reduction in hemicellulose to 1.3%, 18.9%, 25.1%, and 21.4% when willow sawdust was subjected to different pretreatment approaches, i.e. A (sodium hydroxide), C (sodium hydroxide and hydrogen peroxide mixture), D (initially sodium hydroxide, followed by hydrogen peroxide) and E (initially hydrogen peroxide, followed by sodium hydroxide), respectively (approach B, i.e. H2O2, is omitted here). Among them, the maximum removal of lignin (38.3%) was observed for approach D.136 The use of co-cultures for bioethanol fermentation is generally considered beneficial over monocultures because of the synergistic action of the metabolic pathways of the involved microorganisms.137 In another study, filamentous fungi such as Rhizomucor miehei, Aspergillus nidulans, Gilbertella persicaria, and Trichoderma reesei were tested for the pretreatment of dry CS (corn stover), WS (wheat straw) and WWC (willow wood chip).138A. nidulans-based pretreatment doubled the yield of methane compared with the untreated corn stover. Pretreatment with G. persicaria and T. reesei also showed noteworthy differences in the production of bio-methane in comparison with the samples having only untreated plant substrates, respectively. Outstanding activity by endo-(1,4)-β-D-glucanase on willow wood chip and corn stover, and great activity by β-glucosidase on willow wood chip were shown in the case of A. nidulans. Consequently, the A. nidulans-based pretreatment of the samples generated the highest biogas yield for all the involved raw substances. This study recommended the use of a short pretreatment time for cellulose-abundant substances, which in definite cases may double the yield of biogas.138
Phanerochaete chrysosporium, among the WRF, is recognized for its selective lignin breakdown and numerous applications in biotechnology.139 Pretreatment of the abundant wheat straw (WS) can be done by applying WRF, which transforms cellulose (complex plant biomass) into glucose.140 This sugar can be used by Pichia fermentans and IAA (indole-3-acetic acid) may be produced in the presence of tryptophan. Besides effective WS pretreatment in the course of primary fermentation, Phanerochaete chrysosporium may also generate IAA in the presence of tryptophan,141 which may further participate in enhancing the production of IAA in the course of secondary fermentation.142 In one study, the P. chrysosporium (150 μg mL−1)-based pretreatment of WS resulted in a 9-fold enhancement in IAA in comparison with untreated WS (16.44 μg mL−1).142 IAA was produced in the range of 1.99–129.33 μg mL−1.142 The WS was pretreated with Phanerochaete chrysosporium for releasing the sugar in medium, which could be used by Pichia fermentans for the production of IAA. A considerable amount of sugar was released by P. chrysosporium from the 2nd day onwards and was the highest on the 9th day (0.89 mg mL−1).142 The production of IAA using yeasts was also described earlier.143 Less than 25 μg mL−1 was produced by Pichia guilliermondii and Hanseniaspora uvarum when they were inoculated in the medium based on yeast extract-dextrose after incubation for seven days.144 Furthermore, an endophytic yeast found in roots of maize, Williopsis saturnus, was observed to generate indole-3-acetic acid (22.51 μg mL−1) in vitro in GPB medium (glucose-peptone broth).145
In the degradation of natural substrates, a consortium of effective microbes has been found to be more effective than a single organism.146,147 Ramarajan and Manohar (2017)148 observed good lignocellulolytic by the fungal isolates, namely, GK1 (Chaetomium globosum), GK2 (Chaetomium brasiliense), G4 (Engyodontium album), G10 (Metarhizium anisopliae), G13 (Engyodontium album), M155 (Acremonium persicinum), M158 (Acremonium minutisporum), and M2E (Inonotus tropicalis). They evaluated the activity of these isolates and isolate 2a, Cerrena unicolor,149 in the liquid culture media, where good growth and ligninolytic activity were shown by M2E and 2a, while exceptional cellulolytic activity was shown by the isolates GK1 and GK2 on the lignocellulosic substrates, i.e. RS (rice straw) and SCB (sugarcane bagasse). Upon treatment with individual isolates, the highest sugar yield observed from SCB with GK2 was 1.35 g L−1, while the sugar yield was less than 1 g L−1 from RS and SCB after treatment with individual isolates except GK2. Amongst the different consortia, the highest yield of sugar (4.39 g L−1) was produced by M2E + GK2 on sugarcane bagasse, followed by the yield of 2.64 g L−1 on rice straw by 2a + GK2. This enhanced yield of sugar in the case of the M2E + GK2 and 2a + GK2 consortia could be due to the high manganese peroxidase activity on SCB by the M2E + GK2 consortium and enhanced activity of laccase by the 2a + GK2 consortium on RS, followed by noteworthy cellulolytic nature. Thus, the developed lignolytic and cellulolytic marine-derived fungal consortium shows potential for application in agricultural waste.148 A comparative study between biological and physical pretreatment was also performed by researchers. The yield of sugar moderately increased from the substrates via physical pretreatment and a combination of physical and biological pretreatment but it was less than the developed consortium-based biological pretreatment, which demonstrates the potential of fungal isolates in biological pretreatment.148 Rouches, Zhou et al. (2016)126 performed a study on the pretreatment of wheat straw using different strains of fungi to determine the probability of increasing the production of methane. Anaerobic digestion was found to be improved up to 20% by Polyporus brumalis BRFM 985 even after the mass loss. Using this strain, they obtained up to 43% extra methane (CH4)/g of pretreated VS in comparison with the control straw. Considering the dry weight loss studied in the pretreatment course under non-optimized conditions, there was up to 21% extra methane per g of initial TS (total solids). In the case of the fixed culture condition, there was a decrease in delignification with an increase in glucose between 50 and 400 mg per g straw in a strain-dependent way.126
4.2 Pretreatment of hardwoods, softwoods and switchgrass
The lignin degradation ability was found to be increased by co-culturing of Paracremonium sp. LCB1 and Clonostachys compactiuscula LCN1 and pronounced drop of 76.37% in the weight of lignin was observed for the pretreatment of bamboo culms using this co-culture at a temperature of 30 °C, 40 days of culture time and pH 5. There was a high loss ratio of lignin/cellulose (>10).150 It was also observed that co-culturing of two or three fungi gave higher degree of weight loss of lignin in comparison with a single fungal strain culture. During the process of pretreatment, the co-cultivation of interacting fungi results in the over-expression of lignolytic enzymes, which may generate a synergistic and combinatorial influence for effective delignification.151 Consequently, the combination of LCB1 + LCN1 gave the maximum loss of lignin weight.150Trametes versicolor (a white rot fungus), and Gloeophyllum trabeum and Rhodonia placenta (two brown rot funguses) were used for the pre-treatment of two softwoods, namely, Pinus yunnanensis and Cunninghamia lanceolata, and two hardwoods, namely, Populus yunnanensis and Hevea brasiliensis, with different conversion periods.152 Selective degradation in softwood was shown by T. versicolor, where lignin and hemicellulose were converted preferentially, while cellulose was selectively retained. Alternatively, in hardwood, simultaneous conversion was achieved for cellulose, hemicellulose and lignin by T. versicolor. Carbohydrates were converted preferentially by the brown rot fungal species but cellulose conversion was selectively shown by R. placenta. The accessibility to wood cells was improved and the porosity was enhanced by the fungal pre-treatment. It was concluded that the cellulose content may be maximized by the use of T. versicolor pretreatment, while pretreatment using brown rot fungi (especially R. placenta) may be beneficial for the production of biofuels, chemicals based on gasoline and other bio-chemicals.152 Both brown rot fungi caused higher mass loss of softwoods compared to T. versicolor of 28.59%, 36.19%, and 13.09%, decaying by G. trabeum, R. placenta, and T. versicolor in P. yunnanensis, while in the wood of Cunninghamia lanceolata, 66.52%, 45.87%, and 35.57% decay was observed by G. trabeum, R. placenta, and T. versicolor, respectively. However, the case was reverse for hardwoods, where the hardwood mass conversion by white rot fungi was higher than brown rot fungi. The nature of lignin and different pathways of bio-degradation between hard woods and softwoods may be the reasons for this discrepancy in the degradation percentage by the different groups of fungi.152
Alternatively, a technoeconomic analysis was done by Olughu et al. (2023)153 for the fungal pretreatment-dependent production of cellulosic ethanol, where the processing capacity of the plant was 2000 tonnes switchgrass per day. The ethanol yield of the plant was projected to be 211.9 L per t of switchgrass and fungal pretreatment was the main contributor to the total capital investment. The profitability of switchgrass-based ethanol production was observed to be sensitive to the changes in the cost of the feedstock, yield of glucose and yield of xylose. An increase in the yield of glucose from 60% to 80% resulted in a 5-fold enhancement in the net present value. Additionally, a study on the optimization of the fermentation time in the course of fungi-based pretreatment and subsequent glucose yield optimization upon enzyme-catalysed hydrolysis will be essential to improve the economic feasibility of this type of ethanol plant.153
4.3 Fungal pretreatment of spent coffee grounds (SCGs)
SCGs are biowaste materials produced after coffee brewing, which are generated in a noteworthy volume each year globally. The exact volume of SCGs generated each year is not well known but it is estimated that 6 million tons are produced globally each year on a wet basis.154,155 Approximately, 50% production comes from small-scale coffee shops, cafeterias, individuals, and restaurants.156 However, discarded SCGs pose a considerable threat to the environment. Presently, new technologies and policies are devoted to developing SCGs as worthwhile feedstock for the synthesis of bioproducts, platform for the production of chemicals, and generation of value-added energy materials.157,158 Furthermore, given that they are a rich source of polysaccharides, proteins, and lipids, SCGs are promising feedstock for bio-based and chemical processes to get great-value products for the cosmetics, pharmaceutical, and food industries.159 The contents of hemicellulose and lignin in SCGs were found to be 39.75%, and 23.1%, while protein and caffeine were found to be 10.82% and 1.83%, respectively.160 Actually, the valorization of SCGs can be done in many ways, including via the production of SCOAs (short-chain organic acids).161 These organic acids with small chains are monocarboxylic acids (aliphatic) with 2 to 6 C-atoms (i.e. acetic, propionic, butyric, isobutyric, valeric, caproic, and lactic acids), having industrial application either by direct involvement or use as building blocks for further transformations.162 Usually, petrochemical processes are used for the production of these molecules but their production via biological processes is being promoted due to the growing cost and environmental impact of crude oil, specially utilizing organic waste as the substrate.163A promising work demonstrated the biological pretreatment of coffee waste by acidogenic fermentation161 using two fungi, i.e. Paecilomyces variotii NRRL-115 and Trametes versicolor CBS 109428. The production of SCOA (short chain organic acid) was positively influenced by the utilization of SCG_TvSmF (spent coffee ground_submerged fermentation by T. versicolor) as the pretreatment, getting the maximum of 2.44 g COD per L, which was a significant enhancement (87%) compared to the control. Also, the generation of acetic acids, propionic acids, and butyric acids in an average proportion of 59.9%/33.8%/6.3% was observed. The production of acetic acid happened throughout the assay, while the appearance of butyric acids and propionic acids occurred after the 9th day and 18th day, respectively.161 As observed before in other studies,125 celluloses and hemicelluloses of spent coffee grounds were possibly broken down and consumed. A study showed that the inclusion of a pretreatment step may assist to make spent coffee grounds an appropriate material for valorization and this work is a nice contribution towards lessening the cost of enzymatic hydrolysis as a complex feedstock pretreatment.161 Afriliana et al. (2021)160 studied composting spent coffee grounds using aerobic static batch composting with temperature control using Aspergillus sp. and Penicillium sp. The basis for selecting these activator fungi in compositing was the high contents of hemicellulose and lignin in the substrate. The study was performed via the analysis of three samples (control, C1, and C2), where the greatest degradation was observed for lignin in C2. In comparison with the similar rates of 35.56% in C1 and 31.1% in the control, this led to the improved global breakdown of lignin of 40.28% in C2. The percentage protein decomposition, i.e. 85.44% (sample C2) and 83.02% (sample C1), was greater than that of the control (81.82%). The macromolecule decomposition rate was more than 40% in the case of lignin, while 70% in the case of cellulose. With the help of this method, the composting time can be sped up and the results of the produced compost can be optimized.160
The capacity of Pleurotus ostreatus in the degradation of the lignocellulosic nature of combined spent coffee grounds (SCG) and olive pruning residues (OLPR) was assessed by Fayssal et al. (2021).164 They adopted the complete randomized design with 5 treatments, i.e. S1: 100% wheat straw (control), S2: 33% wheat straw + 33% spent coffee grounds + 33% olive pruning residues, S3: 66% wheat straw + 17% spent coffee grounds + 17% olive pruning residues, S4: 17% wheat straw + 66% spent coffee grounds + 17% olive pruning residues, and S5: 17% wheat straw + 17% spent coffee grounds + 66% olive pruning residues, with 10 replicates per treatment. Among them, only S1, S2, and S3 were observed to be productive. With an increase in the OLPR and SCG proportions, the loss of organic matter decreased. The lignin loss percentage was greater in S1 compared with S2 and S3, i.e. 53.51%, 26.25%, and 46.15%, respectively. The combined production yield of mushrooms harvested from 2 flushes of Pleurotus ostreatus cultured in grass and coffee pulp created a biological efficiency change of 59.9% and 93%, respectively.165 To access holocellulose, the fungus needs to firstly break lignin,125 where a greater loss of lignin means greater mycelial activity.166 In all the studied substrates, the degradation of hemicellulose favorably occurred with respect to cellulose, which was consistent with the early results reported by Thompson et al. (2003)167 for WS.164
The previous discussion demonstrated the efficiency of fungal systems in the pretreatment of SCGs as a biological method. In recent years, the utilization of SCGs as a bio-resource for many value-added bio-products has attracted considerable attention but there are certain noteworthy challenges, which need to be solved for its effective industrial application. The heterogeneity of SCGs from different sources and collection from coffee shops, consumers and other small-scale sources are the primary challenges. Inconsistency in the SCG composition, factors such as type of coffee, the method used for brewing and conditions required for processing are some of the factors generating difficulties to standardize the process of extraction and optimize the creation of value-added bio-products. Furthermore, the development of effective techniques is necessary to sort and preprocess SCGs to ensure their reliable excellence and composition.158 A large quantity of SCGs is produced by coffee shops and domestic consumers, and thus logistical challenges are encountered by bioprocessing plants in concentrating the huge volumes of SCGs to the level of processing. Thus, innovative approaches and more research are required to overcome these challenges.158
4.4 Fungal digestion of food wastes
Various sources are responsible for the generation of food waste such as canteens, households, hotels, function halls, gated communities, different food processing industries, and many others.168 Thus, its decentralized treatment at the source utilizing the best probable anaerobic digestion (AD) technique makes it remunerative,169 and consequently the diversion of food waste to landfills may be avoided to a high extent.170 There are three configurations of the AD process based on the TS concentration in organic waste, i.e. wet anaerobic digestion (total solids ≤10%), semi dry anaerobic digestion (total solids in the range of 10–15%), and dry anaerobic digestion (total solids >15%).171–173 Dry anaerobic digestion (solid-state digestion) is a positive technique, owing to its various benefits compared with wet anaerobic digestion (total solids <10%), making it especially striking for food waste treatment, municipal solid waste organic fraction treatment, and treatment of agricultural waste.174Different pretreatment methods (namely, autoclaving, acid based, alkali-based, aeration, and fungi-based methods of pretreatment) were applied by Bhurat et al. (2023)175 for the pretreatment of food waste. In comparison with the control, a 3.8-fold improvement in the yield of hydrogen and 1.7-fold enhancement in the yield of methane were shown by fungal treatment. To study the fungal succession and their ecological and engineering value, food waste AD bioreactors were investigated by Yang et al. (2022).176 It was observed that deterministic procedures slowly dominated the fungal assembly succession (i.e. at the final stage up to 84.85%), signifying the varying environmental status accountable for the dynamics of the fungal community, and specially, the structure, diversity and biomass of the fungal community were controlled by the various environmental variables or the same variables with opposite influences.176 A work on fungal mash enzymatic pretreatment combined with pH adjustment was performed by Zhang et al. (2022)177 using Aspergillus awamori (CICC 41363) to generate fungal mash enzymes via SSF. Complex amylase (CA) was the crude enzyme produced from this fungus, which was added to the food waste fermentation short-term anaerobic system. There was 116.9% enhancement in the concentration of SCOD with the addition of CA relative to the control. After 24 h, the TOC and SCOD concentrations considerably increased with the addition of complex amylase (CA) under an extensive range of pH conditions. Here, the total organic carbon and SCOD mean concentration were 12.5 g L−1 and 34.5 g L−1, which were 1.65 and 1.81-times greater than the control (7.6 g L−1 and 19.1 g L−1), respectively. pH 8 was the optimal pH condition for the yield of VFAs, which was consistent with the finding by Chen and co-workers.178 This study may be an economical way to increase the yield of VFAs for the valorization of FW in the course of anaerobic fermentation.177
Furthermore, fungal mash (in situ produced) was also utilized by Yin et al. (2016),179 showing the nice presence of hydrolytic enzymes to pretreat activated sludge, FW, and their combination before AD. The enzyme-catalyzed pretreatment of activated sludge combined with FW resulted in the generation of 3.72 g per L glucose and 51 mg per L free amino nitrogen, equivalent to SCOD (7.65 g L−1) within 24 h, accompanied with 19.9% reduction in VS (volatile solids). The decrease in VS was found to be 19.1% and 21.4% after the activated sludge and FW pretreatment, respectively, through fungal mash. Moreover, the yield of bio-methane from the fungal mash pretreated mixed waste was 2.5-times greater than the activated sludge receiving no pretreatment, with a further decrease in the volatile solids of 34.5%. This resulted in a total reduction in volatile solids of 54.3% in the suggested anaerobic system with fungal mash pretreatment. This study demonstrates that in the enhancement of the production of bio-methane and the maximization of the mixed waste volume reduction via anaerobic co-digestion, in situ-produced fungal mash-based combined activated sludge and FW pretreatment is a promising option.179
The effective role of fungi in the pretreatment of food biowaste and its conversion into several useful bio-products has been well demonstrated. However, to reduce the harmful impacts of food waste on the environment and human health and conversion of food waste into value-added bio-products, certain challenges need to be resolved such as the bulk collection of food waste from various sources, their proper separation from other types of inessential materials, their bulk storage and processing at biorefinery plants and further research on an industrial scale from the laboratory-scale experiments.
4.5 Saccharification of grain stillage
In terms of the composition of fiber, grain stillage is mainly made of hemicellulose (15–25%) and cellulose (35–45%), depending on its source such as rice, corn, sorghum, and wheat.180 It is also considered a feedstock for bio-refineries because of its large content of carbohydrates.181 Pretreatment of grain stillage using the microwave-assisted hydrothermal (MH) pretreatment, fungus-based pretreatment, and their combination was done by Ren et al. (2020).181 A superior reducing sugar yield (25.51 g/100 g) and saccharification efficiency (66.28%) were achieved by the microwave-assisted hydrothermal + Phanerochaete chrysosporium (microwave-assisted hydrothermal prior to Phanerochaete chrysosporium) pretreatment. A considerable loss of mass, i.e. 23.54% and 39.43%, was caused by the joint pretreatment of Phanerochaete chrysosporium + microwave-assisted hydrothermal and microwave-assisted hydrothermal + Phanerochaete chrysosporium, respectively. The degrees of delignification were considerably enhanced to 32.80% (for Phanerochaete chrysosporium + microwave-assisted hydrothermal) and 43.34% (for microwave-assisted hydrothermal + Phanerochaete chrysosporium) after the combined pretreatment. Furthermore, the degree of delignification by microwave-assisted hydrothermal + Phanerochaete chrysosporium was considerably greater than by Phanerochaete chrysosporium + microwave-assisted hydrothermal pretreatment.181 This may be because microwave-assisted hydrothermal pretreatment results in hydrogen bond breakage and lignocellulose structure destruction through explosion and disruption, which stimulates the subsequent attack of P. chrysosporium for delignification.182,183 To enable the utilization of the cost-effective grain stillage, the use of joint microwave-assisted hydrothermal and Phanerochaete chrysosporium pretreatment may be an excellent method.1814.6 Symbiotic digestion of lignocellulose
In tropical and subtropical areas of the world, fungus-growing termites have the ability to consume 20–90% of dead plant materials.184–186 Lignocellulosic materials can be completely degraded and digested by Termitomyces fungi with a resulting ecological influence on the processes of the ecosystem, chiefly the carbon cycle.187 To investigate the digestion of lignocellulose in a fungus-growing termite O. formosanus (Shiraki) symbiotic system and to equate the bacterial communities across various phases during the degradation process, Ahmad et al. (2022)188 performed many analytical works on the fate of the components of plant biomass and performed 16S rRNA gene amplicon sequencing. The digestive tract of the young workers initiates the degradation of lignocellulose but leaves the maximum cellulose, lignin, and hemicellulose, which are principally decomposed in the fresh fungus comb. The consumed samples of lignocellulose (fresh, mature, and old comb) from three colonies were compared188 with the original wood of mulberry through compositional analysis of lignocellulose using the fiber detergent technique.189 It was shown by the examination of the comb material that in all three colonies, the considerable degradation of the lignocellulosic constituents occurred.188 There was on average a reduction in lignin, cellulose, and hemicellulose in the fresh comb by 18.9%, 11.1%, and 15.0%, in the mature comb by 56.9%, 41.0%, and 32.5%, and in the old comb by 63.0%, 65.5%, and 53.4%, respectively.1884.7 Fungal pretreatment of solid digestate
The fate of the digestate fractions generally involves agricultural aims such as soil amendment and organic fertilizer.190 Zanellati et al. (2020)191 studied fungal pretreatment on non-sterile solid digestate and inoculated the fungi Coprinopsis cinerea MUT 6385, Cephalotrichum stemonitis MUT 6326, and Cyclocybe aegerita MUT 5639 in the digestate non-sterile solid fraction with aim to reuse it as feedstock for anaerobic digestion. In the Cyclocybe aegerita, Cephalotrichum stemonitis, and Coprinopsis cinerea pretreated samples, there were noteworthy reductions in the concentration of total solids (TS), i.e. 23.8%, 25.4%, and 28.5%, respectively. In the C. cinerea samples pretreated for 10 days and Cephalotrichum stemonitis samples pretreated for 20 days, the NDF (neutral detergent fiber) loss percentage ranged from 1.6% to 10.4%, respectively. Similar behavior was shown by the different strains towards the PCWP (plant cell wall polymer), causing a greater reduction in hemicellulose (18.5–59.3%) compared with cellulose (0.2–8.2%) and lignin (1.0–9.6%). C. stemonitis-based pretreatment for 20 days gave the maximum reduction in all the PCWP components and resulted in the reduction of hemicellulose, lignin, and cellulose of 59.3%, 9.6%, and 8.2%, respectively. The anaerobic digestion showed a superior function with solid fraction of digestate treated by the fungal strain Cephalotrichum stemonitis for 20 days, which led to about 3-fold greater yield of biogas and CH4, i.e. +182% and +214%, respectively, compared with the untreated solid fraction of digestate. The cumulative methane formed with the fungal strain C. stemonitis was considerably greater than that attained with the fungal strains Cyclocybe aegerita and Coprinopsis cinerea for both 10 and 20 days.191M. isabellina ATCC 42613 was applied by Zhong et al. (2016)192 for accumulating lipids on detoxified hydrolysate medium. The characteristics of the digestates (solid and liquid) and AD effluent showed that the solid digestate had a 30.60% TS content and carbohydrate content, i.e. cellulose (26%), xylan (13%), and lignin (30%), to be utilized for fungal lipid accumulation as the lignocellulosic feedstock. After the pretreatment and hydrolysis processes, the mixture feed with the total solids of 10% produced a hydrolysate having glucose (13.85 g L−1), xylose (8.95 g L−1), and acetate (2.67 g L−1). This study showed the substrate consumption by Mortierella isabellina on hydrolysates.192 Without detoxification, there was no consumption of sugars and acetate in the hydrolysate during the culture period of 89 hours. In comparison with the culture of synthetic medium (consumption of all sugars and acetate in 66 h), a delay (23 h) of the consumption of the substrate was noticed from the cultures on detoxified hydrolysates. There was complete consumption of glucose and acetate in 49–54 h, respectively. At the end of the batch culture (77 h), 1.79 g per L xylose remained in the broth, and 8.98 g per L biomass and 1.50 g per L lipid were accumulated. The corresponding yields of lipid and biomass were 0.07 g g−1 and 0.42 g g−1, respectively.192 Conclusively, fungi show promise in the pretreatment of solid digestate with anaerobic digestion process.4.8 Fungal pretreatment of park waste and cattle dung
To treat cellulosic biomass and enhance its digestibility, Ali and Sun (2015)193 studied the influence of physico-chemical pretreatment on the degradation of cellulose, followed by treatment utilizing fungi Trichoderma viride and Aspergillus terreus. In their experimental set up, a mixture of new leaves (125 g), dry leaves (125 g), and cattle dung (250 g) was present in each of the two digesters. Park waste fungal treatment was applied for 7 days at 25 °C, followed by the incubation of both digesters for 70 days on an incubator shaker (temperature of 35 °C and 120 rpm) to help the mixing. The pre-treated and untreated substrate biogas and CH4 yields were measured. The three pre-treatment stages improved the production yields of daily biogas and CH4 from the substrate. In comparison with the untreated substrate, the pretreated substrate gave maximum yields of biogas and CH4 of 2.6 and 1.9 L per kg VS, respectively, on the 28th day. There was 102.6 L per kg VS biogas cumulative production for the untreated substrate, which was found to be improved to 125.9 L per kg VS for the pretreated substrate. Consequently, there was 22.7% enhancement in comparison with the yield of biogas from the untreated substrate. The pretreated and untreated substrate cumulative production of CH4 was 79.8 and 61.4 L per kg VS, respectively, and in comparison with the yield of methane from the untreated substrate, there was a 30% enhancement.193 This study may be useful for the treatment of cattle dung and park waste and in the production of biogas with further improvement, optimization and/or with combined technology.4.9 Cardboard waste fungal pretreatment
Cardboard waste is also a good source of hemicellulose, lignin, and cellulose. Suthar and Singh (2022) studied the fungal pretreatment of waste cardboard in a monoculture and mixed culture, and then composted for 35 days after mingling with cow dung in various ratios.194 They utilized fungi Oligoporus placenta and Trametes hirsuta for fungal pretreatment. There was considerable decrease in the contents of cellulose (28.3–35.8%), hemicellulose (61.4–68.4%), and lignin (67.5–69.3%) in the waste cardboard. The pretreated waste cardboard displayed better reduction rates in TOC (26.02–47.92%), C–N ratio (19.4–23.5), and contents of lignocellulose, in addition to an increase in total N (40.48–63.31%), total K (51.92–73.91%), germination index (88.5–102.0%), and levels of elements i.e. copper, iron, zinc, chromium, and manganese. Thus, after the pretreatment with a white rot fungi consortium, waste cardboard could be utilized as an important substrate for the preparation of value-added compost.194Therefore, now, it is very clear that fungi have a great future in biological pretreatment technology for the management of the aforementioned organic waste and the production of valuable energy, biogases and compounds. However, there are also several associated challenges before these pretreatment technologies. There are some other reports that may be significant for readers in the field of bio-waste degradation as well as opportunities and challenges.195–208Table 4 summarizes the effective brief descriptions of the myco-pretreatment of different types of organic waste. Together with being an advantageous biological solution to the problems of bio-waste management, fungal-based bio-pretreatment processes may also generate various types of valuable products after the pretreatment processes from macromolecules lignins, cellulose, hemicellulose, starch, pectins, etc.
| S. N. | Fungi | Enzyme activity | Substrates | Some outputs | References |
|---|---|---|---|---|---|
| 1 | Trametes versicolor (WRF), Gloeophyllum trabeum and Rhodonia placenta (brown rot fungi) | — | Softwoods and hardwoods | • Softwoods mass conversion by two brown fungal species was greater than white rot fungi | 152 |
| • Hardwoods mass conversion by WRF exceeded that by BRF | |||||
| 2 | Fungal source | — | Switchgrass | • Yield of ethanol was projected to be 211.9 L per t of switchgrass and fungal pretreatment was the main contributor to the total capital investment | 153 |
| • Growing yield of glucose from 60 to 80% resulted in a five-fold enhancement in the net present value | |||||
| 3 | Paracremonium sp. LCB1 and Clonostachys compactiuscula LCN1 | Hemicellulase and ligninolytic enzyme | Bamboo culms | • Significant lowering in the lignin weight (76.37%) | 150 |
| • Lignin/cellulose ratio showed high loss (>10) | |||||
| 4 | Penicillium aurantiogriseum, Gilbertella persicaria (SZMC11086), Rhizomucor miehei (SZMC11005), and Trichoderma reesei (DSM768) | Endoglucanase, β-glucosidase, and cellobiohydrolase | Wheat straw, woody willow chips, and corn stover | • Excellent production of hydrolytic enzyme and maximum yield of biogas from the partly decomposed substrates were shown by P. aurantiogriseum | 130 |
| • Maximum yield of methane was for corn stover fed reactors | |||||
| • All the tested fungi preferred the corn stover substrate for the productivity of methane | |||||
| 5 | Pleurotus ostreatus and Dichomitus squalens | — | Corn silage | • Biogas production was enhanced | 131 |
| • Methane gas was found to increase by 1.55 fold | |||||
| • Lignin de-polymerization increased the production of methane to 0.301 to 0.465 m3 per kg VS | |||||
| 6 | Irpex lacteus | — | Wheat straw | • Wheat straw digestibility found after 21 days of the pretreatment in the solid state fermentations bioreactor (60.6%) was alike to that found on a small scale i.e. 57.9% | 132 |
| • In the 03 bioreactor experiments, sugars evolution was completely different after 21 days of treatment | |||||
| • Greater lowering of lignin in experiment B2 i.e. 34.90 ± 0.87% in comparison with that of experiment B1 i.e. 26.7 ± 3.08% | |||||
| • Alike reduction of lignin i.e. 53.2 ± 0.60% in the flask-scale pretreatment | |||||
| 7 | Aspergillus terreus and Trichoderma harzianum | — | Rice straw | • Degradation of cellulose | 133 |
| • Production of bioethanol | |||||
| • The 80% degradation of cellulose by the isolated fungi A. terreus and T. harzianum | |||||
| • A. terreus performance on the decrease of cellulose with HL and RP as substrate was 92% and 80%, respectively | |||||
| • T. harzianum performance on the decrease of cellulose with HL and RP as substrate was 93% and 82%, respectively | |||||
| • Rice straw's RP treated with T. harzianum has resulted in greater bioethanol of 5.4 g L−1 | |||||
| 8 | Saccharomyces cerevisiae, Pichia stipitis, Pachysolen tannophilus, and Wickerhamomyces anomalus X19 | — | Willow sawdust | • Bioethanol production | 136 |
| • Yields of ethanol ranging from 11.67 ± 0.21 to 13.81 ± 0.20 g/100 g TS was shown by the S. cerevisiae or W. anomalus X19 monocultures and co-cultures with P. stipitis | |||||
| • For the approach D, there was maximum removal of lignin (38.3%) | |||||
| • Co-cultures was useful | |||||
| 9 | Rhizomucor miehei, Aspergillus nidulans, Gilbertella persicaria, and Trichoderma reesei | β-Glucosidase and endoglucanase | Dry CS (corn stover), WS and WWC | • β-Glucosidase and endo-(1,4)-β-D-glucanase activity | 138 |
| • Yield of methane | |||||
| 10 | Pichia fermentans, Phanerochaete chrysosporium | — | Wheat straw | • Auxin production by P. fermentans | 142 |
| • Indole-3-acetic acid (IAA) production | |||||
| • The wheat straw was pretreated by P. chrysosporium for releasing the sugar in the medium which may be used by P. fermentans for the production of IAA | |||||
| • Sugar amount was released by P. chrysosporium from 2nd day onwards and was highest on 9th day | |||||
| 11 | GK1 (Chaetomium globosum), GK2 (Chaetomium brasiliense), G4 (Engyodontium album), G10 (Metarhizium anisopliae), G13 (Engyodontium album), M155 (Acremonium persicinum), M158 (Acremonium minutisporum), and M2E (Inonotus tropicalis) | LiP (lignin peroxidase), laccase, CMCase, MnP (manganese peroxidase), and xylanase | Rice straw and sugarcane bagasse | • LiP (lignin peroxidase), laccase, CMCase, MnP (manganese peroxidase), and xylanase activity | 148 |
| • Amongst the different consortia, the highest yield of sugar (4.39 g L−1) was given by M2E + GK2 | |||||
| • Ligninolytic and cellulolytic marine-derived fungal consortium were effective for agricultural wastes | |||||
| 12 | Polyporus brumalis BRFM 985 | — | Wheat straw | • Enhancement in the production of methane | 126 |
| • They obtained up to 43% extra methane (CH4) per gram of pretreated VS in comparison with control straw | |||||
| 13 | Paecilomyces variotii NRRL-115 and Trametes versicolor CBS 109428 | Enzymatic extracts | Spent coffee grounds | • Biological pretreatment for coffee waste's acidogenic fermentation | 161 |
| • Generation of acetic acids, propionic acids, and butyric acids in an average proportion (59.9/33.8/6.3%) | |||||
| • Production of SCOA in the course of acidogenic fermentation | |||||
| 14 | Aspergillus sp., and Penicillium sp. | — | Spent coffee grounds | • Chemical composition of SCG (%) as hemicellulose (39.75% ± 0.007%), lignin (23.1% ± 0.007%), caffeine (1.83% ± 0.007%), and protein (10.82% ± 0.007%) | 160 |
| • Study via three samples analysis (control, C1, and C2) | |||||
| • Greater degradation of lignin in C2 | |||||
| • Composting time can be speed up and results of the produced compost can be optimized by this composting method | |||||
| 15 | Pleurotus ostreatus | — | Spent coffee grounds and olive pruning residues | • Adopted the complete randomized design with 5 treatments | 164 |
| • They observed only S1, S2, and S3 as productive | |||||
| • Lignin loss percentage was greater in S1 in compare with S2 and S3 i.e. 53.51%, 26.25%, and 46.15%, respectively | |||||
| • Degradation of hemicellulose preferentially occurred with respect to cellulose | |||||
| 16 | Aspergillus awamori (CICC 41363) | Complex amylase (CA) | Food wastes | • Solubility and degradability of the organics in food waste were considerably enhanced by CA addition | 177 |
| • The 116.9% increase in concentration of SCOD with the CA addition relative to the control | |||||
| • TOC and SCOD mean concentration were 12.5 g L−1 and 34.5 g L−1, respectively | |||||
| • Under weakly basic and neutral conditions, a greater VFAs concentration was found | |||||
| • In enhancing FW hydrolysis, the pretreatment method of adding CA could be an effective method | |||||
| 17 | Fungal mash | Hydrolytic enzymes | Food wastes | • Enzymatic pretreatment of activated sludge mixed with FW caused in the production of glucose (3.72 g L−1) | 179 |
| • The reduction of VS was found as 19.1% and 21.4% after the activated sludge and FW pretreatment, respectively by the fungal mash | |||||
| • Yield of bio-methane of fungal mash pretreated mixed waste was 2.5 times greater than the activated sludge receiving no pretreatment, with as further reduction of VS of 34.5% | |||||
| 18 | Phanerochaete chrysosporium | Ligninolytic enzyme activity | Grain stillage | • Pretreatment of grain stillage | 181 |
| • The maximum activities of ligninolytic enzyme was achieved by the fungal pretreatment with Phanerochaete chrysosporium digestion (PC) in six days with 10% inoculum size at which yield of reducing sugar and efficiency of saccharification reached 19.74 g/100 g and 36.29%, respectively | |||||
| • The degrees of delignification were considerably enhanced to 32.80% (for PC + MH) and 43.34% (for MH + PC) after the combined pretreatment | |||||
| • Use of combined MH and PC pretreatment could be an excellent method | |||||
| 19 | Coprinopsis cinerea MUT 6385, Cephalotrichum stemonitis MUT 6326, and Cyclocybe aegerita MUT 5639 | — | Solid digestate | • In the C. aegerita, C. stemonitis, and C. cinerea pretreated samples, there was significant decrease in the concentration of total solids (TS) i.e. 23.8%, 25.4%, and 28.5%, respectively | 191 |
| • The anaerobic digestion functioned superior with SFD treated by the fungal strain C. stemonitis for twenty days | |||||
| • Cumulative yields of biogas and methane also studied | |||||
| 20 | Mortierella isabellina | — | Anaerobic digestate | • Production of lignocellulosic biodiesel | 192 |
| • Accumulation of lipid | |||||
| • After the process of pretreatment and hydrolysis, the mixture feed at the total solids of 10% produced a hydrolysate having glucose (13.85 g L−1), xylose (8.95 g L−1), and acetate (2.67 g L−1) | |||||
| • Complete consumption of glucose and acetate in 49–54 hours, respectively | |||||
| 21 | Aspergillus terreus and Trichoderma viride | — | Park wastes | • Physico-chemical pretreatment's influence on the degradation of cellulose followed by fungal treatment | 193 |
| • TS, VS, TOC, etc. were studied | |||||
| • Study on biogas and CH4 yields | |||||
| 22 | Oligoporus placenta and trametes hirsuta | — | Card wastes | • Fungal pretreatment of waste cardboard | 194 |
| • Considerable lowering in cellulose (28.3–35.8%), hemicellulose (61.4–68.4%), and lignin (67.5–69.3%) content in waste card board |
5 Techno-economic analysis and scale-up issues for industrial implementation of fungal pretreatment methods
Fungal pretreatment processes at the industrial level require large capital investments. From grasses to hardwoods, the total capital investment ranges from 700 million (dollars) to 1.2 billion (dollars), respectively, which is approximately 5- to 10-times greater than the previously estimated cost for conventional treatment on a parallel scale.209 This high cost is due to the expenses related to purchasing large equipment, installation, construction, engineering, requirement of many units for each of the main processes such as autoclaving, fungal pretreatment and enzymatic hydrolysis and other requirements. Packed-bed bioreactors utilized in the fungal pretreatment process are responsible for the majority of the cost mainly because of their longer residence time. In the fungal pretreatment process, the estimated price of fermentable sugar was 1.6–2.8 dollars per kg, which is 4–5 times greater than the previously stated production cost of sugar utilizing the conventional pretreatment methods.209,210 Due to its minimum requirement of energy and chemicals, the fungal pretreatment process is believed to be an inexpensive pretreatment method but the analysis showed that it has a noticeably higher cost than conventional pretreatments and about one order of magnitude over that anticipated for a pretreatment to be feasible at the commercial level.210The high cost of facility arrangement for fungal pretreatment is primarily responsible for the need of high capital investment, while the feedstock cost is the second highest contributor to the cost of sugar production, which contributed 18–22% of the total cost of sugar production.27 However, continuous advancements in technologies may reduce the total cost of sugar production using fungal pretreatment techniques, making them a commercially profitable environmentally safe and green technique. The longer time required for fungal pretreatment, enhancement of the yield of sugar, and sterilization requirement of the feedstock before fungal pretreatment are several bottlenecks that need to be overcome to expand and improve the fungal-based pretreatment technique. Studies on its combination with other pretreatments with low severity achieved a greater yield of sugar with a significantly reduced need for energy and chemicals.211 Optimization of these processes is not only required on the laboratory scale from a technical perspective but also at the commercial scale utilization from a techno-economic perspective.27 High temperature may affect fungal-based pretreatment processes because fungal enzymes are strongly involved in the enzymatic degradation process of lignocellulosic materials or other biowaste, which are inactivated at higher temperatures, and thus during the fungal-based treatment plant set up, special attention should also be given to the control of temperature increase during the process.
6 Conclusions, challenges and future perspectives
Fungi are effective in the decomposition of lignocellulosic biomass, food waste, sewage sludge, polysaccharides, lignin, hemicelluloses, etc., and found to be efficiently involved in saccharification, biogas productions, glucose production, ethanol production, and bio-fertilizer development utilizing organic waste. They have a significant contribution to the valorization of biomass29via the generation of alcohol,202 biodiesel,203 organic acids,204 and gas fuels.205 However, there are several challenges in the efficient use of pretreatment technologies for the digestion of organic waste or bio-waste and solid waste management. The anaerobic decomposition of organic waste occurs when dumped in landfills.46 Harmful greenhouse gases such as methane are produced by the decomposition of green waste under anaerobic condition, which are the main contributors to global warming.206 The rapidly depleting landfill space is another problem with direct landfill disposal. In the proper management of organic waste, there are various considerable barriers. Poor infrastructure, strategy planning, staff capacity, registration, programme engagement, information system, and unsystematic management of waste make it a difficult job.46 Also, there is a lack of participation in the initiatives of the separation of garbage and inadequate communication between homeowners and the municipality,207 which make the management of organic waste very difficult. To allow a more effective value extraction and recycling process, the separation of waste should occur at the source.207 The separation of different types of wastes such as dry wastes and wet wastes (biodegradable) makes the pretreatment process more effective and significant. Thus, this is the main responsibility of producers of wastes together with effective government involvement. There is a need for combined efforts from urban local bodies, governments, private sectors, and non-governmental bodies for long-term waste management, and visionary project developments are strongly needed in this regard. There is also a strong need for well-defined roles and responsibility to work on waste management with continuous monitoring and assessment.207 Kumar et al. (2017)207 nicely reviewed the challenges and opportunities related to the management of waste materials in India. Thus, to perfectly implement fungal pretreatment technology either individually or in combination with other technologies for a green and sustainable environment, the above-mentioned barriers need to be tackled because in poor countries organic waste disposal is a very difficult task. The consistent and properly managed involvement of the system (government, industry and public) in organic waste management with appropriate methodologies is essential to pretreat organic or bio-waste biologically. A longer pretreatment time is generally required by fungal technology to achieve high removal rates of lignin and saccharification of cellulose, which generates problems regarding cost increase and contamination by bacteria.125 Besides a longer pretreatment time, the requirement of feedstock sterilization before the pretreatment process, heat generation during the fungal pretreatment process, and lower yield of sugar are some other challenges and shortcomings of fungal pretreatment, which needed to be properly tackled in the future to make fungal pretreatment technology more efficient and cost-effective. The large solid content in biomass is the principal reason restraining its solid fermentation, which generates ethanol. Similar to glucose fermentation, the yield of ethanol and titer can be achieved through the amalgamation of cosolvent-improved lignocellulose fractionation, and subsequently saccharification and fermentation.208 Fungal pretreatment in combination with other pretreatment methods may increase the enzymatic digestibility and shorten the time for fungal pretreatment.29 Fungal technology has great advantages in the field of biomass conversion because several derived products from biomass hydrolyzed sugar are important from the point of energy generation. For example, 5-hydroxymethylfurfural, levulinic acid, and furfural are considered value-added chemicals produced via fungal technology.29 Future development will require further upgraded fungal technology with a shorter pretreatment time, little or no sterilization requirements, enhanced enzymatic digestibility, reduced chance of contamination, and greater product yields. These upgraded fungal pretreatment technologies should more efficiently break the complex structures of biomass, enhancing the production of various valuable products.29 Biogases (methane) and other useful organic components (bioethanol, monomeric sugars, etc.) produced during the process of fungal pretreatment technology may be utilized in human welfare using consistent management techniques.Data availability
This is a review article and during the preparation of this article, no new data were generated or analysed as part of this review.Author contributions
Dr Pankaj Kumar Chaurasia and Dr Shashi Lata Bharati prepared the original manuscript's draft, wrote major sections, supervised, and edited the manuscript; Dr Sunita Singh wrote specific sections and performed formal analysis and editing; Dr Azhagu Madhavan Sivalingam, and Dr Shiv Shankar performed formal analysis and editing. Dr Ashutosh Mani was involved in supervision, editing and formal analysis. All authors also reviewed the manuscript.Conflicts of interest
No competing interests.Acknowledgements
Dr Pankaj Kumar Chaurasia is grateful to the P.G. Department of Chemistry, L.S. College, Muzaffarpur and Dr Shashi Lata Bharati is grateful to the Department of Chemistry, NERIST, Nirjuli (Arunachal Pradesh) for support during the work. The other authors are also thankful to their respective departments for providing facilities during the work.References
- United Nations Environment Programme (UNEP), Global Waste Management Outlook 2024–Beyond an Age of Waste, Turning Rubbish into a Resource- International solid waste association ISWA, 2024, https://www.unep.org/resources/global-waste-management-outlook-2024.
- Food and Agricultural Organization of the United Nations, (FAO 2024), 2024 Food loss and waste masterclass: the basics. https://www.fao.org/platform-food-loss-waste/flw-events/events/events-detail/2024-food-loss-and-waste-masterclass--the-basics/en.
- Environmental Protection Agency (EPA), “Composting at home”, 2020, https://www.epa.gov/recycle/composting-home/.
- H. C. Moon and I. S. Song, Enzymatic hydrolysis of Food Waste and methane production using UASB bioreactor, Int. J. Green Energy, 2011, 8(3), 361–371, DOI:10.1080/15435075.2011.557845.
- M. Rajin, A. Z. Yaser, S. Saalah, Y. Jagadeson and M. Ag Duraim, The effect of enzyme addition on the anaerobic digestion of food waste, Green Engineering for Campus Sustainability, Springer, Berlin, Germany, 2020 Search PubMed.
- A. Z. Yaser, J. Lamaming, E. Suali, M. Rajin, S. Saalah, Z. Kamin, N. N. Safie, N. A. S. Aji and N. Wid, Composting and Anaerobic Digestion of Food Waste and Sewage Sludge for Campus Sustainability: A Review, Int. J. Chem. Chem. Eng., 2022, 2022, 14, DOI:10.1155/2022/6455889.
- J. Havukainen, A. Saud, T. F. Astrup, P. Peltola and M. Horttanainen, Environmental performance of dewatered sewage sludge digestate utilization based on life cycle assessment, Waste Manag., 2022, 137, 210–221, DOI:10.1016/j.wasman.2021.11.005.
- A. Kwarciak-Kozłowska, Industrial and Municipal Sludge, Butterworth-Heinemann, Oxford, UK, 2019, pp. 337–360, DOI:10.1016/B978-0-12-815907-1.00015-5.
- S. Wang, C. Xu, L. Song and J. Zhang, Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives, Int. J. Environ. Res. Publ. Health, 2022, 19(15), 9519, DOI:10.3390/ijerph19159519.
- F. Appel, A. Ostermeyer-Wiethaup and A. Balmann, Effects of the German Renewable Energy Act on structural change in agriculture–The case of biogas, Util. Pol., 2016, 41, 172–182, DOI:10.1016/j.jup.2016.02.013.
- A. Pazera, R. Slezak, L. Krzystek, S. Ledakowicz, G. Bochmann, W. Gabauer, S. Helm, S. Reitmeier, L. Marley and F. Gorga, Biogas in Europe: Food and beverage (FAB) waste potential for biogas production, Energy Fuels, 2015, 29, 4011–4021, DOI:10.1021/ef502812s.
- A. Whiting and A. Azapagic, Life cycle environmental impacts of generating electricity and heat from biogas produced by anaerobic digestion, Energy, 2014, 70, 181–193, DOI:10.1016/j.energy.2014.03.103.
- A. D. Bees and I. D. Williams, Explaining the differences in household food waste collection and treatment provisions between local authorities in England and Wales, Waste Manag., 2017, 70, 222–235, DOI:10.1016/j.wasman.2017.09.004.
- L. Zhang and D. Jahng, Long-term anaerobic digestion of food waste stabilized by trace elements, Waste Manag., 2012, 32, 1509–1515, DOI:10.1016/j.wasman.2012.03.015.
- A. Anu, R. C. Kuhad, A. Rapoport, V. Kumar, D. Singh, V. Kumar, S. K. Tiwari, S. Ahlawat and B. Singh, Biological pretreatment of lignocellulosic biomass: An environment-benign and sustainable approach for conversion of solid waste into value-added products, Crit. Rev. Environ. Sci. Technol., 2024, 54(10), 771–796, DOI:10.1080/10643389.2023.2277670.
- U. Brémond, R. de Buyer, J.-P. Steyer, N. Bernet and H. Carrere, Biological pretreatments of biomass for improving biogas production: An overview from lab scale to full-scale, Energy Rev., 2018, 90, 583–604, DOI:10.1016/j.rser.2018.03.103.
- F. Girotto, W. Peng, R. Rafieenia and R. Cossu, Effect of Aeration Applied During Different Phases of Anaerobic Digestion, Waste Biomass Valorization, 2018, 9, 161–174, DOI:10.1007/s12649-016-9785-9.
- G.-C. Mitraka, K. N. Kontogiannopoulos, M. Batsioula, G. F. Banias, A. I. Zouboulis and P. G. Kougias, A Comprehensive Review on Pretreatment Methods for Enhanced Biogas Production from Sewage Sludge, Energies, 2022, 15, 6536, DOI:10.3390/en15186536.
- P. K. Chaurasia, S. L. Bharati and S. Yadava, Nano-reduction of gold and silver ions: A perspective on the fate of microbial laccases as potential biocatalysts in the synthesis of metals (gold and silver) nano-particles, Curr. Res. Microb. Sci., 2022, 3, 100098, DOI:10.1016/j.crmicr.2021.100098.
- P. K. Chaurasia and S. L. Bharati, Research Advances in the Fungal World: Culture, Isolation, Identification, Classification, Characterization, Properties and Kinetics, Publisher: Nova Science Publishers, Inc., USA, 2020, pp. 1–419, ISBN: 978-1-53617-197-6 Search PubMed.
- P. K. Chaurasia, S. Yadava, S. L. Bharati and S. K. Singh, Selective oxidation and N-coupling by purified laccase of Xylaria polymorpha MTCC-1100, Russ. J. Bioorg. Chem., 2014, 40(4), 455–460, DOI:10.1134/S1068162014040025.
- P. K. Chaurasia, S. L. Bharati and S. K. Singh, Role of laccase from Coriolus versicolor MTCC-138 in selective oxidation of aromatic methyl group, Russ. J. Bioorg. Chem., 2014, 40(3), 288–292, DOI:10.1134/S1068162014020034.
- P. K. Chaurasia, Nagraj, N. Sharma, S. Kumari, M. Yadav, S. Singh, A. Mani, S. Yadava and S. L. Bharati, Fungal assisted bio-treatment of environmental pollutants with comprehensive emphasis on noxious heavy metals: Recent updates, Biotechnol. Bioeng., 2023, 120(1), 57–81, DOI:10.1002/bit.28268.
- P. K. Chaurasia, N. Sharma, Nagraj, D. M. Rudakiya, S. Singh and S. L. Bharati, Fungal-Assisted Bioremediation of Agricultural Organic Pollutants (Pesticides and Herbicides), Curr. Green Chem., 2022, 9(1), 14–25, DOI:10.2174/2213346109666220927121948.
- A. Salihu and M. Z. Alam, Pretreatment methods of organic wastes for biogas production, J. Appl. Sci., 2016, 16, 124–137, DOI:10.3923/jas.2016.124.137.
- C. Wan and Y. Li, Fungal pretreatment of lignocellulosic biomass, Biotechnol. Adv., 2012, 30, 1447–1457, DOI:10.1016/j.biotechadv.2012.03.003.
- J. Vasco-Correa and A. Shah, Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass, Fermentation, 2019, 5, 30, DOI:10.3390/fermentation5020030.
- J. Vasco-Correa, X. Ge and Y. Li, Biological pretreatment of lignocellulosic biomass, in Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, ed. S. I. Mussatto, Elsevier, Amsterdam, The Netherlands, 2016, pp. 561–586, ISBN 9780128023235 Search PubMed.
- Y. Chai, M. Bai, A. Chen, L. Peng, J. Shao, S. Luo, Y. Deng, B. Yan and C. Peng, Valorization of waste biomass through fungal technology: Advances, challenges, and prospects, Ind. Crops Prod., 2022, 188, 115608, DOI:10.1016/j.indcrop.2022.115608.
- N. Nadir, N. L. Ismail and A. S. Hussain, Fungal Pretreatment of Lignocellulosic Materials, in Biomass for Bioenergy - Recent Trends and Future Challenges, IntechOpen, 2019, DOI:10.5772/intechopen.84239.
- E. Lizundia, F. Luzi and D. Puglia, Organic waste valorisation towards circular and sustainable biocomposites, Green Chem., 2022, 24, 5429–5459, 10.1039/D2GC01668K.
- E. Gilman, A. Perez Roda, T. Huntington, S. J. Kennelly, P. Suuronen, M. Chaloupka and P. A. H. Medley, Benchmarking global fisheries discards, Sci. Rep., 2020, 10, 14017, DOI:10.1038/s41598-020-71021-x.
- D. Gunders and J. Bloom, Wasted: How America Is Losing up to 40 Percent of its Food from Farm to Fork to Landfill, Natural Resources Defense Council, 2017 Search PubMed.
- K. C. Badgujar and B. M. Bhanage, Dedicated and waste feedstocks for biorefinery: an approach to develop a sustainable society, in Waste Biorefinery: Potential and Perspectives, ed. T. Bhaskar, A. Pandey, S. V. Mohan, D.-J. Lee and S. K. Khanal, Elsevier, 2018, pp. 3–38, DOI:10.1016/B978-0-444-63992-9.00001-X.
- Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT) Production: Livestock Primary: Eggs Primary, 2019 Search PubMed.
- S.-C. Wu, H.-C. Hsu, S.-K. Hsu, Y.-C. Chang and W.-F. Ho, Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment, J. Asian Ceram. Soc., 2016, 4, 85–90, DOI:10.1016/j.jascer.2015.12.002.
- S. Mignardi, L. Archilletti, L. Medeghini and C. De Vito, Valorization of Eggshell Biowaste for Sustainable Environmental Remediation, Sci. Rep., 2020, 10, 2436, DOI:10.1038/s41598-020-59324-5.
- N. A. M. Barakat, M. S. Khil, A. M. Omran, F. A. Sheikh and H. Y. Kim, Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods, J. Mater. Process. Technol., 2009, 209, 3408–3415, DOI:10.1016/j.jmatprotec.2008.07.040.
- P. Shi, M. Liu, F. Fan, C. Yu, W. Lu and M. Du, Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts, Mater. Sci. Eng., C, 2018, 90, 706–712, DOI:10.1016/j.msec.2018.04.026.
- F. A. Sheikh, M. A. Kanjwal, J. Macossay, M. A. Muhammad, T. Cantu, I. S. Chronakis, N. A. M. Barakat and H. Y. Kim, Fabrication of Mineralized Collagen from Bovine Waste Materials by Hydrothermal Method as Promised Biomaterials, J. Biomim. Biomater. Tissue Eng., 2011, 1, 194–197, DOI:10.1166/jbt.2011.1017.
- C. M. Welker, V. K. Balasubramanian, C. Petti, K. M. Rai, S. DeBolt and V. Mendu, Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts, Energies, 2015, 8(8), 7654–7676, DOI:10.3390/en8087654.
- Z. Qin, Q. Zhuang, X. Zhu, X. Cai and X. Zhang, Carbon Consequences and Agricultural Implications of Growing Biofuel Crops on Marginal Agricultural Lands in China, Environ. Sci. Technol., 2011, 45, 10765–10772, DOI:10.1021/es2024934.
- V. Mendu, A. E. Harman-Ware and M. Crocker, et al., Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production, Biotechnol. Biofuels, 2011, 4, 43, DOI:10.1186/1754-6834-4-43.
- V. Mendu, T. Shearin, J. E. Campbell, J. Stork, J. Jae, M. Crocker, G. Huber and S. DeBolt, Global bioenergy potential from high-lignin agricultural residue, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4014–4019, DOI:10.1073/pnas.1112757109.
- V. K. Thakur and M. K. Thakur, Recent advances in green hydrogels from lignin: a review, Int. J. Biol. Macromol., 2015, 72, 834–847, DOI:10.1016/j.ijbiomac.2014.09.044.
- S. Kharola, M. Ram, N. Goyal, S. K. Mangla, O. P. Nautiyal, A. Rawat, Y. Kazancoglu and D. Pant, Barriers to organic waste management in a circular economy, J. Clean. Prod., 2022, 362, 132282, DOI:10.1016/j.jclepro.2022.132282.
- M. S. Aini, A. Fakhru’l-Razi, S. M. Lad and A. H. Hashim, Practices, attitudes and motives for domestic waste recycling, Int. J. Sustain. Dev. World Ecol., 2002, 9(3), 232–238, DOI:10.1080/13504500209470119.
- B. Sharma, B. Vaish, U. K. Singh, P. Singh and R. P. Singh, Recycling of organic wastes in agriculture: an environmental perspective, Int. J. Environ. Res., 2019, 13(2), 409–429, DOI:10.1007/s41742-019-00175-y.
- A. Kumar, S. K. Mangla and P. Kumar, An Integrated Literature Review on Sustainable Food Supply Chains: Exploring Research Themes and Future Directions, Sci. Total Environ., 2022, 821, 153411, DOI:10.1016/j.scitotenv.2022.153411.
- P. N. Patil, P. R. Gogate, L. Csoka, A. Dregelyi-Kiss and M. Horvath, Intensification of biogas production using pretreatment based on hydrodynamic cavitation, Ultrason. Sonochem., 2016, 30, 79–86, DOI:10.1016/j.ultsonch.2015.11.009.
- D. W. Fuerstenau and A. Z. M. Abouzeid, The energy efficiency of ball milling in comminution, Int. J. Miner. Process., 2002, 67, 161–185, DOI:10.1016/S0301-7516(02)00039-X.
- M. M. de Oliveira, P. Moretti, C. Malinowsky, R. Bayard, P. Buffière, A. B. de Castilhos Júnior, J. de Araujo Morais Júnior, G. B. A. Júnior and R. Gourdon, Mechanical pre-treatment of source-collected municipal biowaste prior to energy recovery by anaerobic digestion, Chemosphere, 2022, 292, 133376, DOI:10.1016/j.chemosphere.2021.133376.
- A. Cesaro, V. Cieri and V. Belgiorno, Press-extrusion pretreatment of the organic fraction of municipal solid waste for enhanced methane production, J. Mater. Cycles Waste Manag., 2021, 23, 130–138, DOI:10.1007/s10163-020-01105-3.
- A. Chevalier, P. Evon, F. Monlau, V. Vandenbossche and C. Sambusiti, Twin-Screw Extrusion Mechanical Pretreatment for Enhancing Biomethane Production from Agro-Industrial, Agricultural and Catch Crop Biomasses, Waste, 2023, 1, 497–514, DOI:10.3390/waste1020030.
- C. L. Rai and P. G. Rao, Influence of sludge disintegration by high pressure homogenizer on microbial growth in sewage sludge: An approach for excess sludge reduction, Clean Technol. Environ. Policy, 2009, 11, 437–446, DOI:10.1007/s10098-009-0202-y.
- D. Sun, M. Wu, C. Bi, F. Gao, W. Wei and Y. Wang, Using high-pressure homogenization as a potential method to pretreat soybean protein isolate: Effect on conformation changes and rheological properties of its acid-induced gel, Innov. Food Sci. Emerg. Technol., 2022, 82, 103195, DOI:10.1016/j.ifset.2022.103195.
- M. Nabi, D. Gao, J. Liang, Y. Cai and P. Zhang, Combining high pressure homogenization with free nitrous acid pretreatment to improve anaerobic digestion of sewage sludge, J. Environ. Manag., 2022, 318, 115635, DOI:10.1016/j.jenvman.2022.115635.
- A. Pirozzi and F. Donsì, Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues, Molecules, 2023, 28, 5657, DOI:10.3390/molecules28155657.
- T. Malik, R. Sharma, K. Ameer, O. Bashir, T. Amin, S. Manzoor and I. A. M. Ahmed, Potential of high-pressure homogenization (HPH) in the development of functional foods, Int. J. Food Prop., 2023, 26(1), 2509–2531, DOI:10.1080/10942912.2023.2249262.
- S. Carpentieri, G. Ferrari and F. Donsì, High-Pressure Homogenization for Enhanced Bioactive Recovery from Tomato Processing By-Products and Improved Lycopene Bioaccessibility during In Vitro Digestion, Antioxidants, 2023, 12(10), 1855, DOI:10.3390/antiox12101855.
- H. Xiao, J. Liang, Y. Zhang, J. Chang, R. Zhang and P. Zhang, Conversion of Materials and Energy in Anaerobic Digestion of Sewage Sludge with High-Pressure Homogenization Pretreatment, Processes, 2023, 11, 2467, DOI:10.3390/pr11082467.
- S. Pilli, P. Bhunia, S. Yan, R. J. LeBlanc, R. D. Tyagi and R. Y. Surampalli, Ultrasonic pretreatment of sludge: A review, Ultrason. Sonochem., 2011, 18, 1–18, DOI:10.1016/j.ultsonch.2010.02.014.
- E. Elbeshbishy, S. Aldin, H. Hafez, G. Nakhla and M. Ray, Impact of ultrasonication of hog manure on anaerobic digestability, Ultrason. Sonochem., 2011, 18, 164–171, DOI:10.1016/j.ultsonch.2010.04.011.
- O. G. Apul and F. D. Sanin, Ultrasonic pretreatment and subsequent anaerobic digestion under different operational conditions, Bioresour. Technol., 2010, 101, 8984–8992, DOI:10.1016/j.biortech.2010.06.128.
- N. A. Oz and C. C. Yarimtepe, Ultrasound assisted biogas production from landfill leachate, Waste Manag., 2014, 34, 1165–1170, DOI:10.1016/j.wasman.2014.03.003.
- Y. Wu, S. Yao, B. A. Narale, A. Shanmugam, S. Mettu and M. Ashokkumar, Ultrasonic Processing of Food Waste to Generate Value-Added Products, Foods, 2022, 11, 2035, DOI:10.3390/foods11142035.
- F. Karouach, M. Bakraoui, Y. El Gnaoui, N. Lahboubi and H. El Bari, Effect of combined mechanical–ultrasonic pretreatment on mesophilic anaerobic digestion of household organic waste fraction in Morocco, Energy Rep., 2020, 6, 310–314, DOI:10.1016/j.egyr.2019.11.081.
- K. Zhao, G. Song, C. Lu, J. Wang, R. Liu and C. Hu, Ultrasonication as anaerobic digestion pretreatment to improve sewage sludge methane production: Performance and microbial characterization, J. Environ. Sci., 2024, 146, 15–27, DOI:10.1016/j.jes.2023.04.022.
- M. Buvaneshwaran, M. Radhakrishnan and V. Natarajan, Influence of ultrasound-assisted extraction techniques on the valorization of agro-based industrial organic waste – A review, J. Food Process. Eng., 2023, 46(6), e14012, DOI:10.1111/jfpe.14012.
- N. Wang, L. Li, K. Wang, X. Huang, Y. Han, X. Ma, M. Wang, X. Lv and X. Bai, Study and Application Status of Ultrasound in Organic Wastewater Treatment, Sustainability, 2023, 15, 15524, DOI:10.3390/su152115524.
- O. Sarkar, L. Matsakas, U. Rova and P. Christakopoulos, Ultrasound-controlled acidogenic valorization of wastewater for biohydrogen and volatile fatty acids production: Microbial community profiling, iScience, 2023, 26(4), 106519, DOI:10.1016/j.isci.2023.106519.
- C. Arce and L. Kratky, Mechanical pretreatment of lignocellulosic biomass toward enzymatic/fermentative valorization, iScience, 2022, 25(7), 104610, DOI:10.1016/j.isci.2022.104610.
- J. Chen, K. Adjallé, T. T. Lai, S. Barnabé, M. Perrier and J. Paris, Effect of mechanical pretreatment for enzymatic hydrolysis of woody residues, corn stover and alfalfa, Waste Biomass Valorization, 2020, 11, 5847–5856, DOI:10.1007/s12649-019-00856-x.
- Q. Liu, H. Fan, H. Mou, J. Liu, J. Huang, X. Dong and H. Song, Preparation and characterization of xylan by an efficient approach with mechanical pretreatments, Ind. Crop. Prod., 2021, 165, 113420, DOI:10.1016/j.indcrop.2021.113420.
- M. Garuti, E. Sinisgalli, M. Soldano, F. G. Fermoso, A. J. Rodriguez, M. Carnevale and F. Gallucci, Mechanical pretreatments of different agri-based feedstock in full-scale biogas plants under real operational conditions, Biomass Bioenergy, 2022, 158, 106352, DOI:10.1016/j.biombioe.2022.106352.
- L. Luo, Y. Qu, W. Gong, L. Qin, W. Li and Y. Sun, Effect of particle size on the aerobic and anaerobic digestion characteristics of whole rice straw, Energies, 2021, 14, 3960, DOI:10.3390/en14133960.
- Y. El Gnaoui, A. Frimane, N. Lahboubi, C. Herrmann, M. Barz and H. EL Bari, Biological pre-hydrolysis and thermal pretreatment applied for anaerobic digestion improvement: Kinetic study and statistical variable selection, Cleaner Waste Systems, 2022, 2, 100005, DOI:10.1016/j.clwas.2022.100005.
- M. Wang, J. Wang, Y. Li, Q. Li, P. Li, L. Luo, F. Zhen, G. Zheng and Y. Sun, Low-Temperature Pretreatment of Biomass for Enhancing Biogas Production: A Review, Fermentation, 2022, 8, 562, DOI:10.3390/fermentation8100562.
- J. Rühl, S. Agrawal and M. Engelhart, Efficacy of thermal hydrolysis for boosting specific methane yield depending on temperature-normalized solids retention time in an activated sludge process, Environ. Sci.:Water Res. Technol., 2022, 8, 2971–2980, 10.1039/D2EW00206J.
- X. Liu, Q. Wang, Y. Tang and S. G. Pavlostathis, A comparative study on biogas production, energy balance, and nutrients conversion with inter-stage hydrothermal treatment of sewage sludge, Appl. Energy, 2021, 288, 116669, DOI:10.1016/j.apenergy.2021.116669.
- D. A. Jones, T. P. Lelyveld, S. D. Mavrofidis, S. W. Kingman and N. J. Miles, Microwave heating applications in environmental engineering-a review, Resour. Conserv. Recycl., 2002, 34, 75–90, DOI:10.1016/S0921-3449(01)00088-X.
- S. Simonetti, C. F. Martín and D. Dionisi, Microwave Pre-Treatment of Model Food Waste to Produce Short Chain Organic Acids and Ethanol via Anaerobic Fermentation, Processes, 2022, 10(6), 1176, DOI:10.3390/pr10061176.
- J. Liu, M. Zhao, C. Lv and P. Yue, The effect of microwave pretreatment on anaerobic co-digestion of sludge and food waste: Performance, kinetics and energy recovery, Environ. Res., 2020, 189, 109856, DOI:10.1016/j.envres.2020.109856.
- P. Prasertsilp, K. Pattaragulwanit, B. S. Kim and S. C. Napathorn, Microwave-assisted cassava pulp hydrolysis as food waste biorefinery for biodegradable polyhydroxybutyrate production, Front. Bioeng. Biotechnol., 2023, 11, 1131053, DOI:10.3389/fbioe.2023.1131053.
- Z. Jie, C. Liu and D. Xia, et al., An atmospheric microwave plasma-based distributed system for medical waste treatment, Environ. Sci. Pollut. Res., 2023, 30, 51314–51326, DOI:10.1007/s11356-023-25793-0.
- Z. Jie, C. Liu and D. Xia, et al., Microwave plasma torches for solid waste treatment and vitrification, Environ. Sci. Pollut. Res., 2023, 30, 32827–32838, DOI:10.1007/s11356-022-24523-2.
- H. Carrère, C. Dumas, A. Battimelli, D. J. Batstone, J. P. Delgenès, J. P. Steyer and I. Ferrer, Pretreatment methods to improve sludge anaerobic degradability: A review, J. Hazard. Mater., 2010, 183, 1–15, DOI:10.1016/j.jhazmat.2010.06.129.
- I. T. Yeom, K. R. Lee, K. H. Ahn and S. H. Lee, Effects of ozone treatment on the biodegradability of sludge from municipal wastewater treatment plants, Water Sci. Technol., 2002, 46, 421–425, DOI:10.2166/wst.2002.0641.
- J. H. L. Alino, J. A. Bastos, P. V. Remor, L. M. Frare, F. Orssatto, F. M. Damaceno and T. Edwiges, Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse, Methane, 2022, 1, 189–200, DOI:10.3390/methane1030015.
- B. Jankovičová, M. Hutňan, M. N. Czölderová, K. Hencelová and Z. Imreová, Comparison of acid and alkaline pre-treatment of lignocellulosic materials for biogas production, Plant Soil Environ., 2022, 68(4), 195–204, DOI:10.17221/421/2021-PSE.
- S. Sreevathsan, I. Crasta, B. K. Bhavana and S. N. Mudliar, Effect of ozonation on biodegradability enhancement and biomethanation potential of pretreatment process wastewaters from 2G ethanol production, J. Chem. Technol. Biotechnol., 2023, 98(6), 1425–1434, DOI:10.1002/jctb.7361.
- Y. Qiao, R. Jin, J. Gao, K. Wang, Y. Jiang, J. Xiong, M. Jia, Z. He and J. Liu, Process of landfill leachate pretreatment using coagulation and hydrodynamic cavitation oxidation, RSC Adv., 2023, 13, 32175–32184, 10.1039/D3RA04259F.
- M. S. Shehu, Z. A. Manan and S. R. W. Alwi, Optimization of thermo-alkaline disintegration of sewage sludge for enhanced biogas yield, Bioresour. Technol., 2012, 114, 69–74, DOI:10.1016/j.biortech.2012.02.135.
- J. Xu, H. Yuan, J. Lin and W. Yuan, Evaluation of thermal, thermal-alkaline, alkaline and electrochemical pretreatments on sludge to enhance anaerobic biogas production, J. Taiwan Inst. Chem. Eng., 2014, 45, 2531–2536, DOI:10.1016/j.jtice.2014.05.029.
- G. Zhen, X. Lu, Y. Y. Li and Y. Zhao, Combined electrical-alkali pretreatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion, Appl. Energy, 2014, 128, 93–102, DOI:10.1016/j.apenergy.2014.04.062.
- B. Ahmed, V. K. Tyagi, A. A. Kazmi and A. Khursheed, New insights into thermal-chemical pretreatment of organic fraction of municipal solid waste: Solubilization effects, recalcitrant formation, biogas yield and energy efficiency, Fuel, 2022, 319, 123725, DOI:10.1016/j.fuel.2022.123725.
- V. T. Pham, C. Y. Guan, P. C. Han, B. M. Matsagar, K. C. W. Wu, T. Ahamad, C. Y. Chang and C. P. Yu, Acid-catalyzed hydrothermal treatment of sewage sludge: effects of reaction temperature and acid concentration on the production of hydrolysis by-products, Biomass Convers. Biorefin., 2023, 13, 7533–7546, DOI:10.1007/s13399-021-01495-w.
- A. Bendaoud, A. Belkhiri, M. Maai, T. Moubchir, A. Hmamou, S. Tlemcani, N. Eloutassi and A. Lahkimi, Simple and Combined Pretreatment of a Mixture of Forestry and Aromatic-Medicinal Plant Waste by Chemical, Physical and Enzymatic Methods, J. Econ. Eng., 2023, 24(4), 376–383, DOI:10.12911/22998993/160094.
- A. A. Rajput, Zeshan and M. Hassan, Enhancing Biogas Production through Co-Digestion and Thermal Pretreatment of Wheat Straw and Sunflower Meal, Renewable Energy, 2021, 168, 1–10, DOI:10.1016/j.renene.2020.11.149.
- J. J. Montoya-Rosales, M. Peces, L. M. González-Rodríguez, F. Alatriste-Mondragón and D. K. Villa-Gómez, A Broad Overview Comparing a Fungal, Thermal and Acid Pre-Treatment of Bean Straw in Terms of Substrate and Anaerobic Digestion Effect, Biomass Bioenergy, 2020, 142, 105775, DOI:10.1016/j.biombioe.2020.105775.
- J. Du, Y. Qian, Y. Xi and X. Lü, Hydrothermal and Alkaline Thermal Pretreatment at Mild Temperature in Solid State for Physicochemical Properties and Biogas Production from Anaerobic Digestion of Rice Straw, Renewable Energy, 2019, 139, 261–267, DOI:10.1016/j.renene.2019.01.097.
- T. Luo, H. Huang, Z. Mei, F. Shen, Y. Ge, G. Hu and X. Meng, Hydrothermal Pretreatment of Rice Straw at Relatively Lower Temperature to Improve Biogas Production via Anaerobic Digestion, Chin. Chem. Lett., 2019, 30, 1219–1223, DOI:10.1016/j.cclet.2019.03.018.
- D. Wang, F. Shen, G. Yang, Y. Zhang, S. Deng, J. Zhang, Y. Zeng, T. Luo and Z. Mei, Can Hydrothermal Pretreatment Improve Anaerobic Digestion for Biogas from Lignocellulosic Biomass?, Bioresour. Technol., 2018, 249, 117–124, DOI:10.1016/j.biortech.2017.09.197.
- A. A. Rajput, Zeshan and C. Visvanathan, Effect of Thermal Pretreatment on Chemical Composition, Physical Structure and Biogas Production Kinetics of Wheat Straw, J. Environ. Manag., 2018, 221, 45–52, DOI:10.1016/j.jenvman.2018.05.011.
- S. Bolado-Rodríguez, C. Toquero, J. Martín-Juárez, R. Travaini and P. A. García-Encina, Effect of Thermal, Acid, Alkaline and Alkaline-Peroxide Pretreatments on the Biochemical Methane Potential and Kinetics of the Anaerobic Digestion of Wheat Straw and Sugarcane Bagasse, Bioresour. Technol., 2016, 201, 182–190, DOI:10.1016/j.biortech.2015.11.047.
- G. Jin, T. Bierma and P. M. Walker, Low-Heat, Mild Alkaline Pretreatment of Switchgrass for Anaerobic Digestion, J. Environ. Sci. Health, Part A, 2014, 49, 565–574, DOI:10.1080/10934529.2014.859453.
- A. Das, S. Das, N. Das, P. Pandey, B. Ingti, V. Panchenko, V. Bolshev, A. Kovalev and P. Pandey, Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials, Agriculture, 2023, 13(9), 1689, DOI:10.3390/agriculture13091689.
- D. Mu, K. Ma, L. He and Z. Wei, Effect of microbial pretreatment on degradation of food waste and humus structure, Bioresour. Technol., 2023, 385, 129442, DOI:10.1016/j.biortech.2023.129442.
- G. Liu, D. Han and S. Yang, Combinations of mild chemical and bacterial pretreatment for improving enzymatic saccharification of corn stover, Biotechnol. Biotechnol. Equip., 2022, 36(1), 598–608, DOI:10.1080/13102818.2022.2112910.
- C. Song, W. Li, F. Cai, G. Liu and C. Chen, Anaerobic and Microaerobic Pretreatment for Improving Methane Production From Paper Waste in Anaerobic Digestion, Front. Microbiol., 2021, 12, 688290, DOI:10.3389/fmicb.2021.688290.
- A. Chen, W. Li, X. Zhang, C. Shang, S. Luo, R. Cao and D. Jin, Biodegradation and detoxification of neonicotinoid insecticide thiamethoxam by white-rot fungus Phanerochaete chrysosporium, J. Hazard. Mater., 2021, 417, 126017, DOI:10.1016/j.jhazmat.2021.126017.
- X. Zhang, J. Shao, A. Chen, C. Shang, X. Hu, S. Luo, M. Lei, L. Peng and Q. Zeng, Effects of cadmium on calcium homeostasis in the white-rot fungus Phanerochaete chrysosporium, Ecotoxicol. Environ. Saf., 2018, 157, 95–101, DOI:10.1016/j.ecoenv.2018.03.071.
- H. Z. Chen and Z. H. Liu, Multilevel composition fractionation process for high-value utilization of wheat straw cellulose, Biotechnol. Biofuels, 2014, 7, 137, DOI:10.1186/s13068-014-0137-3.
- B. Singh, Rice husk ash, in Waste and Supplementary Cementitious Materials in Concrete, 2018, pp. 417–460, DOI:10.1016/B978-0-08-102156-9.00013-4.
- B. Niu and B. H. Kim, Method for Manufacturing Corn Straw Cement-Based Composite and Its Physical Properties, Materials, 2022, 15, 3199, DOI:10.3390/ma15093199.
- P. Pączkowski, A. Puszka and B. Gawdzik, Effect of Eco-Friendly Peanut Shell Powder on the Chemical Resistance, Physical, Thermal, and Thermomechanical Properties of Unsaturated Polyester Resin Composites, Polymers, 2021, 13, 3690, DOI:10.3390/polym13213690.
- C. A. Rezende, M. A. de Lima and P. Maziero, et al., Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility, Biotechnol. Biofuels, 2011, 4, 54, DOI:10.1186/1754-6834-4-54.
- W. A. Rizal, K. Nisa, R. Maryana, D. J. Prasetyo, D. Pratiwi, T. H. Jatmiko, D. Ariani and A. Suwanto, Chemical composition of liquid smoke from coconut shell waste produced by SME in Rongkop Gunungkidul, IOP Conf. Ser.: Earth Environ. Sci., 2020, 462, 012057, DOI:10.1088/1755-1315/462/1/012057.
- C. S. G. P. Queirós, S. Cardoso and A. Lourenço, et al., Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition, Biomass Convers. Biorefin., 2020, 10, 175–188, DOI:10.1007/s13399-019-00424-2.
- D. Wojcieszak, J. Przybył, I. Ratajczak, P. Goliński, D. Janczak, A. Waśkiewicz, K. Szentner and M. Woźniak, Chemical composition of maize stover fraction versus methane yield and energy value in fermentation process, Energy, 2020, 198, 117258, DOI:10.1016/j.energy.2020.117258.
- K. Slopiecka, F. Liberti, S. Massoli, P. Bartocci and F. Fantozzi, Chemical and physical characterization of food waste to improve its use in anaerobic digestion plants, Energy Nexus, 2022, 5, 100049, DOI:10.1016/j.nexus.2022.100049.
- Y. Lu, Y.-C. Lu, H.-Q. Hu, F.-J. Xie, X.-Y. Wei and X. Fan, Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods, J. Spectrosc., 2017, 2017, 15, DOI:10.1155/2017/8951658.
- D. Deswal, R. Gupta, P. Nandal and R. C. Kuhad, Fungal pretreatment improves amenability of lignocellulosic material for its saccharification to sugars, Carbohydr. Polym., 2014, 99, 264–269, DOI:10.1016/j.carbpol.2013.08.045.
- G. Valdés, R. T. Mendonça and G. Aggelis, lignocellulosic biomass as a substrate for oleaginous microorganisms: a review, Appl. Sci., 2020, 10(21), 7698, DOI:10.3390/app10217698.
- C. Wan and Y. Li, Fungal pretreatment of lignocellulosic biomass, Biotechnol. Adv., 2012, 30, 1447–1457, DOI:10.1016/j.biotechadv.2012.03.003.
- E. Rouches, S. Zhou, J. P. Steyer and H. Carrere, White-Rot Fungi pretreatment of lignocellulosic biomass for anaerobic digestion: Impact of glucose supplementation, Process Biochem., 2016, 51(11), 1784–1792, DOI:10.1016/j.procbio.2016.02.003.
- A. Vaidya and T. Singh, Pre-treatment of Pinus radiata substrates by basidiomycetes fungi to enhance enzymatic hydrolysis, Biotechnol. Lett., 2012, 34, 1263–1267, DOI:10.1007/s10529-012-0894-7.
- E. Rouches, I. Herpoël-Gimbert, J. P. Steyer and H. Carrere, Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review, Renew. Sustain. Energy Rev., 2016, 59, 179–198, DOI:10.1016/j.rser.2015.12.317.
- X.-F. Tian, Z. Fang and F. Guo, Impact and prospective of fungal pre-treatment of lignocellulosic biomass for enzymatic hydrolysis, Biofuels Bioprod. Biorefining, 2012, 6, 335–350, DOI:10.1002/bbb.346.
- E. Kovács, C. Szűcs, A. Farkas, M. Szuhaj, G. Maróti, Z. Bagi, G. Rákhely and K. L. Kovács, Pretreatment of lignocellulosic biogas substrates by filamentous fungi, J. Biotechnol., 2022, 360, 160–170, DOI:10.1016/j.jbiotec.2022.10.013.
- P. Basinas, J. Rusín, K. Chamrádová, K. Malachová, Z. Rybková and C. Novotný, Fungal pretreatment parameters for improving methane generation from anaerobic digestion of corn silage, Bioresour. Technol., 2022, 345, 126526, DOI:10.1016/j.biortech.2021.126526.
- M. Á. Pallín, S. González-Rodríguez, G. Eibes, M. López-Abelairas, M. T. Moreira, J. M. Lema and T. A. Lú-Chau, Towards industrial application of fungal pretreatment in 2G biorefinery: scale-up of solid-state fermentation of wheat straw, Biomass Convers. Biorefin., 2024, 14, 593–605, DOI:10.1007/s13399-022-02319-1.
- M. H. Devi and S. Munjam, Bioethanol Production from Rice Straw and Cellulose Degradation using Aspergillus terreus and Trichoderma harzanium, Biosci., Biotechnol. Res. Asia, 2022, 19(3), 699–711, DOI:10.13005/bbra/3022.
- M. Takano and K. Hoshino, Bioethanol production from rice straw by simultaneous saccharification and fermentation with statistical optimized cellulase cocktail and fermenting fungus, Bioresour. Bioprocess., 2018, 5, 16, DOI:10.1186/s40643-018-0203-y.
- M. Abo-State, A. Ragab, N. EL-Gendy, L. Farahat and H. Madian, Bioethanol Production from Rice Straw Enzymatically Saccharified by Fungal Isolates, Trichoderma viride F94 and Aspergillus terreus F98, Soft, 2014, 3, 19–29, DOI:10.4236/soft.2014.32003.
- I. B. Atitallah, G. Antonopoulou, I. Ntaikou, A. S. Beobide, V. Dracopoulos, T. Mechichi and G. Lyberatos, A Comparative Study of Various Pretreatment Approaches for Bio-Ethanol Production from Willow Sawdust, Using Co-Cultures and Mono-Cultures of Different Yeast Strains, Molecules, 2022, 27, 1344, DOI:10.3390/molecules27041344.
- I. Ntaikou, N. Menis, M. Alexandropoulou, G. Antonopoulou and G. Lyberatos, Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipites, Bioresour. Technol., 2018, 263, 75–83, DOI:10.1016/j.biortech.2018.04.109.
- C. Szűcs, E. Kovács, Z. Bagi, G. Rákhely and K. L. Kovács, Enhancing biogas production from agroindustrial waste pre-treated with filamentous fungi, Biol. Futura., 2021, 72(3), 341–346, DOI:10.1007/s42977-021-00083-3.
- R. K. Sharma, P. Chandra and D. S. Arora, Antioxidant properties and nutritional value of wheat straw bioprocessed by Phanerochaete chrysosporium and Daedalea flavida, J. Gen. Appl. Microbiol., 2011, 56, 519–523, DOI:10.2323/jgam.56.519.
- P. K. Sadh, S. Duhan and J. S. Duhan, Agro-industrial wastes and their utilization using solid state fermentation: a review, Bioresour. Bioprocess., 2018, 5, 1, DOI:10.1186/s40643-017-0187-z.
- P. Chandra, D. S. Arora, M. Pal and R. K. Sharma, Antioxidant potential and extracellular auxin production by white rot fungi, Appl. Biochem. Biotechnol., 2019, 187, 531–539, DOI:10.1007/s12010-018-2842-z.
- R. Giri and R. K. Sharma, Fungal pretreatment of lignocellulosic biomass for the production of plant hormone by Pichia fermentans under submerged conditions, Bioresour. Bioprocess., 2020, 7, 30, DOI:10.1186/s40643-020-00319-5.
- R. Pandi, G. Velu, P. Devi and B. Dananjeyan, Isolation and screening of soil yeasts for plant growth promoting traits, Madras Agric. J., 2019, 106, 439–443, DOI:10.29321/maj.2019.000289.
- H. Basha and B. Ramanujam, Growth promotion effect of Pichia guilliermondii in chilli and biocontrol potential of Hanseniaspora uvarum against Colletotrichum capsici causing fruit rot, Biocontrol Sci. Technol., 2015, 25, 185–206, DOI:10.1080/09583157.2014.968092.
- A. H. Nassar, K. A. El-Tarabily and K. Sivasithamparam, Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots, Biol. Fertil. Soils, 2005, 42, 97–108, DOI:10.1007/s00374-005-0008-y.
- L. Cortes-Tolalpa, D. J. Jiménez, M. J. L. Brossi, J. Falcao-Salles and J. D. van Elsas, Different inocula produce distinctive microbial consortia with similar lignocellulose degradation capacity, Appl. Microbiol. Biotechnol., 2016, 100(17), 7713–7725, DOI:10.1007/s00253-016-7516-6.
- H. Kausar, M. R. Ismail, H. M. Saud, R. Othman and S. Habib, Use of lignocellulolytic microbial consortium and pH amendment on composting efficacy of rice straw, Compost Sci. Util., 2013, 21, 121–133, DOI:10.1080/1065657X.2013.842131.
- R. Ramarajan and C. S. Manohar, Biological pretreatment and bioconversion of agricultural wastes, using ligninolytic and cellulolytic fungal consortia, Biomed. J., 2017, 21(2), 89–99, DOI:10.1080/10889868.2017.1282937.
- D. D'Souza-Ticlo, D. Sharma and C. Raghukumar, A thermostable metal-tolerant laccase with bioremediation potential from a marine-derived fungus, Mar. Biotechnol., 2009, 11(6), 725–737, DOI:10.1007/s10126-009-9187-0.
- B. Zhao, R. Liu, Q. Guo, G. Xu, L. Zhang, P. Sun, Y. Cao and S. Hu, The use of newly isolated fungal cultures for the selective delignification of bamboo culms, Front. Bioeng. Biotechnol., 2023, 11, 1265420, DOI:10.3389/fbioe.2023.1265420.
- H. Meehnian, A. K. Jana and M. M. Jana, Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification, Int. Biodeterior. Biodegrad., 2017, 117, 68–77, DOI:10.1016/j.ibiod.2016.11.022.
- J. Qi, F. Li, L. Jia, X. Zhang, S. Deng, B. Luo, Y. Zhou, M. Fan and Y. Xia, Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment, Polymers, 2023, 15, 1957, DOI:10.3390/polym15081957.
- O. O. Olughu, L. G. Tabil, T. Dumonceaux, E. Mupondwa, D. Cree and X. Li, Technoeconomic analysis of a fungal pretreatment-based cellulosic ethanol production, Results Eng., 2023, 19, 101259, DOI:10.1016/j.rineng.2023.101259.
- K. Johnson, Y. Liu and M. Lu, A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices, Front. Chem. Eng., 2022, 4, 2, DOI:10.3389/fceng.2022.838605.
- S. I. Mussatto, E. M. S. Machado, S. Martins and J. A. Teixeira, Production, Composition, and Application of Coffee and Its Industrial Residues, Food Bioprocess Technol., 2011, 4, 661–672, DOI:10.1007/s11947-011-0565-z.
- M. Taifouris, M. L. Corazza and M. Martín, Integrated Design of Biorefineries Based on Spent Coffee Grounds, Ind. Eng. Chem. Res., 2021, 60, 494–506, DOI:10.1021/acs.iecr.0c05246.
- S. K. Karmee, A Spent Coffee Grounds Based Biorefinery for the Production of Biofuels, Biopolymers, Antioxidants and Biocomposites, Waste Manag., 2018, 72, 240–254, DOI:10.1016/j.wasman.2017.10.042.
- H. Ahmed, R. S. Abolore, S. Jaiswal and A. K. Jaiswal, Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production, Biomass, 2024, 4, 286–312, DOI:10.3390/biomass4020014.
- J. McNutt and Q. He, Spent coffee grounds: A review on current utilization, J. Ind. Eng. Chem., 2019, 71, 78–88, DOI:10.1016/j.jiec.2018.11.054.
- A. Afriliana, E. Hidayat, Y. Mitoma, T. Masuda and H. Harada, Studies on Composting Spent Coffee Grounds by Aspergillus sp and Penicillium sp in Aerobic Static Batch Temperature Control, J. Agric. Chem. Environ., 2021, 10, 91–112, DOI:10.4236/jacen.2021.101007.
- J. Pereira, A. Cachinho, M. M. R. de Melo, C. M. Silva, P. C. Lemos, A. M. R. B. Xavier and L. S. Serafim, Enzymatic Potential of Filamentous Fungi as a Biological Pretreatment for Acidogenic Fermentation of Coffee Waste, Biomolecules, 2022, 12(9), 1284, DOI:10.3390/biom12091284.
- W. S. Lee, A. S. M. Chua, H. K. Yeoh and G. C. Ngoh, A review of the production and applications of waste-derived volatile fatty acids, Chem. Eng. J., 2014, 235, 83–99, DOI:10.1016/j.cej.2013.09.002.
- L. Sun, M. Gong, X. Lv, Z. Huang, Y. Gu, J. Li, G. Du and L. Liu, Current advance in biological production of short-chain organic acid, Appl. Microbiol. Biotechnol., 2020, 104, 9109–9124, DOI:10.1007/s00253-020-10917-0.
- S. A. Fayssal, Z. El Sebaaly, M. A. Alsanad, R. Najjar, M. Böhme, M. H. Yordanova and Y. N. Sassine, Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value, PLoS One, 2021, 16(9), e0255794, DOI:10.1371/journal.pone.0255794.
- D. Hernández, J. Sánchez and K. Yamasaki, A simple procedure for preparing substrate for Pleurotus ostreatus cultivation, Bioresour. Technol., 2003, 90, 145–150, DOI:10.1016/S0960-8524(03)00118-4.
- F. Zadrazil, D. N. Kamarad, O. S. Isikhuemhen, F. Shucharfdt and G. Flachwosky, Bioconversion of lignocellulose into ruminant feed with white rot fungi—review of work done at FAL, Braunschweig, J. Appl. Anim. Res., 1996, 10, 105–124, DOI:10.1080/09712119.1996.9706139.
- D. N. Thompson, T. N. Houghton, J. A. Lacey, G. Peter, P. G. Shaw and R. Hess, Preliminary investigation of fungal bioprocessing of wheat straw for production of straw-thermoplastic composites, Appl. Biochem. Biotechnol., 2003, 106, 423–436, DOI:10.1385/ABAB:106:1-3:423.
- K. Paritosh, S. K. Kushwaha, M. Yadav, N. Pareek, A. Chawade and V. Vivekanand, Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling, BioMed Res. Int., 2017, 2017, 19, DOI:10.1155/2017/2370927.
- K. Kuruti, S. Begum, S. Ahuja, G. Anupoju, S. Juntupally, B. Gandu and D. Ahuja, Exploitation of rapid acidification phenomena of food waste in reducing the hydraulic retention time (HRT) of high rate anaerobic digester without conceding on biogas yield, Bioresour. Technol., 2017, 226, 65–72, DOI:10.1016/j.biortech.2016.12.005.
- S. Jain, Global Food Waste Management: An Implementation Guide for Cities|Urban Food Actions Platform|Food and Agriculture Organization of the United Nations, 2018. Available online: http://www.fao.org/urban-food-actions/knowledge-products/resources-detail/en/c/1136010/.
- V. Arelli, S. Begum, G. Anupoju, K. Kuruti and S. Shailaja, Dry anaerobic co-Digestion of food waste and cattle manure: Impact of total solids, substrate ratio and thermal pre treatment on methane yield and quality of biomanure, Bioresour. Technol., 2018, 253, 273–280, DOI:10.1016/j.biortech.2018.01.050.
- A. Abbassi-Guendouz, D. Brockmann, E. Trably, C. Dumas, J. Delgenes, J. Steyer and R. Escudie, Total solids content drives high solid anaerobic digestion via mass transfer limitation, Bioresour. Technol., 2012, 111, 55–61, DOI:10.1016/j.biortech.2012.01.174.
- M. Madsen, J. B. Holm-Nielsen and K. M. Esbensen, Monitoring of anaerobic digestion processes: A review perspective, Renew. Sustain. Energy Rev., 2011, 15, 3141–3155, DOI:10.1016/j.rser.2011.04.026.
- V. Arelli, S. Juntupally, S. Begum and G. R. Anupoju, Significance of Pretreatment in Enhancing the Performance of Dry Anaerobic Digestion of Food Waste: An Insight on Full Scale Implementation Strategy with Theoretical Analogy, Processes, 2020, 8, 1018, DOI:10.3390/pr8091018.
- K. S. Bhurat, T. Banerjee, P. V. Bobde and S. S. Bhurat, Effect of chemical, physical, and biological pre-treatment of food wastes on bio-hydrogen production by dark anaerobic fermentation under mesophilic conditions, Energy Sources, Part A, 2023, 45(1), 1017–1029, DOI:10.1080/15567036.2023.2174615.
- X. Yang, Z. Zhang, S. Li, Q. He, X. Peng, X. Du, K. Feng, S. Wang and Y. Deng, Fungal dynamics and potential functions during anaerobic digestion of food waste, Environ. Res., 2022, 212, 113298, DOI:10.1016/j.envres.2022.113298.
- M. Zhang, D. Zhang, Y. Wei, B. Zhou, C. Yan, D. Wang, J. Liang and L. Zhou, Fungal mash enzymatic pretreatment combined with pH adjusting approach facilitates volatile fatty acids yield via a short-term anaerobic fermentation of food waste, Waste Manag., 2022, 151, 1–9, DOI:10.1016/j.wasman.2022.07.028.
- Y. Chen, J. Luo, Y. Yan and L. Feng, Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells, Appl. Energy, 2013, 102, 1197–1204, DOI:10.1016/j.apenergy.2012.06.056.
- Y. Yin, Y.-J. Liu, S.-J. Meng, E. U. Kiran and Y. Liu, Enzymatic pretreatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion, Appl. Energy, 2016, 179, 1131–1137, DOI:10.1016/j.apenergy.2016.07.083.
- H. Ren, T. Zhao, J. Xing, Y. Zhang, Z. Li, Y. Wang and W. Ma, Isolation and Characterization of Microcrystalline Cellulose from Distillers Grains, J. Biobased Mater. Bioenergy, 2015, 9, 515–522, DOI:10.1166/jbmb.2015.1550.
- H. Ren, W. Sun, Z. Wang, S. Fu, Y. Zheng, B. Song, Z. Li and Z. Peng, Enhancing the Enzymatic Saccharification of Grain Stillage by Combining Microwave-Assisted Hydrothermal Irradiation and Fungal Pretreatment, ACS Omega, 2020, 5, 12603–12614, DOI:10.1021/acsomega.9b03681.
- M. Selig, N. Weiss and Y. Ji, Enzymatic Saccharification of Lignocellulosic Biomass, National Renewable Energy Laboratory NREL/TP-510-42629, Golden: Colo, USA, 2008 Search PubMed.
- X. Kan, J. Zhang, Y. W. Tong and C. H. Wang, Overall evaluation of microwave-assisted alkali pretreatment for enhancement of bio-methane production from brewers' spent grain, Energy Convers. Manage., 2018, 158, 315–326, DOI:10.1016/j.enconman.2017.12.088.
- G. Bastien, G. Arnal, S. Bozonnet, S. Laguerre, F. Ferreira, R. Fauré, B. Henrissat, F. Lefèvre, P. Robe, O. Bouchez, C. Noirot, C. Dumon and M. O'Donohue, Mining for hemicellulases in the fungus-growing termite Pseudacanthotermes militaris using functional metagenomics, Biotechnol. Biofuels, 2013, 6, 78, DOI:10.1186/1754-6834-6-78.
- R. D. Buxton, Termites and the turnover of dead wood in an arid tropical environment, Oecologia, 1981, 51, 379–384, DOI:10.1007/bf00540909.
- M. Ohkuma, Termite symbiotic systems: efficient bio-recycling of lignocellulose, Appl. Microbiol. Biotechnol., 2003, 61, 1–9, DOI:10.1007/s00253-002-1189-z.
- J. A. Bonachela, R. M. Pringle, E. Sheffer, T. C. Coverdale, J. A. Guyton, K. K. Caylor, S. A. Levin and C. E. Tarnita, Termite mounds can increase the robustness of dryland ecosystems to climatic change, Science, 2015, 347, 651–655, DOI:10.1126/science.1261487.
- F. Ahmad, G. Yang, Y. Zhu, M. Poulsen, W. Li, T. Yu and J. Mo, Tripartite Symbiotic Digestion of Lignocellulose in the Digestive System of a Fungus-Growing Termite, Microbiol. Spectr., 2022, 10(6), e0123422, DOI:10.1128/spectrum.01234-22.
- P. J. V. Soest, Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin, J. Assoc. Off. Agric. Chem., 1963, 46, 829–835, DOI:10.1093/jaoac/46.5.829.
- R. Nkoa, Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review, Agron. Sustain. Dev., 2014, 34, 473–492, DOI:10.1007/s13593-013-0196-z.
- A. Zanellati, F. Spina, L. Rollé, G. C. Varese and E. Dinuccio, Fungal Pretreatments on Non-Sterile Solid Digestate to Enhance Methane Yield and the Sustainability of Anaerobic Digestion, Sustainability, 2020, 12(20), 8549, DOI:10.3390/su12208549.
- Y. Zhong, Z. Liu, C. Isaguirre, Y. Liu and W. Liao, Fungal fermentation on anaerobic digestate for lipid-based biofuel production, Biotechnol. Biofuels, 2016, 9, 253, DOI:10.1186/s13068-016-0654-3.
- S. S. Ali and J. Sun, Physico-chemical pretreatment and fungal biotreatment for park wastes and cattle dung for biogas production, SpringerPlus, 2015, 4, 712, DOI:10.1186/s40064-015-1466-9.
- S. Suthar and N. K. Singh, Fungal pretreatment facilitates the rapid and valuable composting of waste cardboard, Bioresour. Technol., 2022, 344, 126178, DOI:10.1016/j.biortech.2021.126178.
- M. Andlar, T. Rezić, N. Marđetko, D. Kracher, R. Ludwig and B. Šantek, Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation, Eng. Life Sci., 2018, 18(11), 768–778, DOI:10.1002/elsc.201800039.
- E. A. Moreira, G. F. Persinoti, L. R. Menezes, D. A. A. Paixão, T. M. Alvarez, J. P. L. F. Cairo, F. M. Squina, A. M. Costa-Leonardo, A. Rodrigues, D. Sillam-Dussès and A. Arab, Complementary Contribution of Fungi and Bacteria to Lignocellulose Digestion in the Food Stored by a Neotropical Higher Termite, Front. Ecol. Evol., 2021, 9, 632590, DOI:10.3389/fevo.2021.632590.
- A. Khalid, M. Arshad, M. Anjum, T. Mahmood and L. Dawson, The anaerobic digestion of solid organic waste, Waste Manag., 2011, 31(8), 1737–1744, DOI:10.1016/j.wasman.2011.03.021.
- L. M. Rodríguez-Jiménez, A. Pérez-Vidal and P. Torres-Lozada, Research trends and strategies for the improvement of anaerobic digestion of food waste in psychrophilic temperatures conditions, Heliyon, 2022, 8(10), e11174, DOI:10.1016/j.heliyon.2022.e11174.
- M. Kilucha, S. Cheng, S. Minza, S. M. Nasiruddin, K. Velempini, X. Li, X. Wang, K. M. Doroth and Z. Li, Insights into the anaerobic digestion of fecal sludge and food waste in Tanzania, Front. Environ. Sci., 2022, 10, 911348, DOI:10.3389/fenvs.2022.911348.
- M. Dashtban, H. Schraft and W. Qin, Fungal Bioconversion of Lignocellulosic Residues; Opportunities & Perspectives, Int. J. Biol. Sci., 2009, 5(6), 578–595, DOI:10.7150/ijbs.5.578.
- P. K. Singh, P. Mohanty, S. Mishra and T. K. Adhya, Food Waste Valorisation for Biogas-Based Bioenergy Production in Circular Bioeconomy: Opportunities, Challenges, and Future Developments, Front. Energy Res., 2022, 10, 903775, DOI:10.3389/fenrg.2022.903775.
- S. M. Sulfahri, D. R. Husain, A. Langford and A. C. M. A. R. Tassakka, Fungal pretreatment as a sustainable and low cost option for bioethanol production from marine algae, J. Clean. Prod., 2020, 265, 121763, DOI:10.1016/j.jclepro.2020.121763.
- X. Yu, Y. Zheng, K. M. Dorgan and S. Chen, Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid, Bioresour. Technol., 2011, 102(10), 6134–6140, DOI:10.1016/j.biortech.2011.02.081.
- A. Jimenez-Quero, E. Pollet, M. Zhao, E. Marchioni, L. Averous and V. Phalip, Fungal fermentation of lignocellulosic biomass for itaconic and fumaric acid production, J. Microbiol. Biotechnol., 2017, 27, 1–8, DOI:10.4014/jmb.1607.07057.
- W. Suksong, N. Wongfaed, B. Sangsri, P. Kongjan, P. Prasertsan, S. M. Podmirseg, H. Insam and S. O-Thong, Enhanced solid-state biomethanisation of oil palm empty fruit bunches following fungal pretreatment, Ind. Crops Prod., 2020, 145, 112099, DOI:10.1016/j.indcrop.2020.112099.
- M. El-Fadel and M. Massoud, Emissions from landfills: a methodology comparative assessment, Environ. Technol., 2000, 21(9), 965–978, DOI:10.1080/09593332108618041.
- S. Kumar, S. R. Smith, G. Fowler, C. Velis, S. J. Kumar, S. Arya, Rena, R. Kumar and C. Cheeseman, Challenges and opportunities associated with waste management in India, R. Soc. Open Sci., 2017, 4(3), 160764, DOI:10.1098/rsos.160764.
- T. Y. Nguyen, C. M. Cai, R. Kumar and C. E. Wyman, Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(44), 11673–11678, DOI:10.1073/pnas.1704652114.
- N. R. Baral and A. Shah, Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover, Bioresour. Technol., 2017, 232, 331–343, DOI:10.1016/j.biortech.2017.02.068.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, et al., Process. Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol, National Renewable Energy Laboratory, Golden, CO, USA, 2011 Search PubMed.
- E. Shirkavand, S. Baroutian, D. J. Gapes and B. R. Young, Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—a review, Renew. Sustain. Energy Rev., 2016, 54, 217–234, DOI:10.1016/j.rser.2015.10.003.
Footnote |
| † First authors. |
| This journal is © The Royal Society of Chemistry 2025 |








