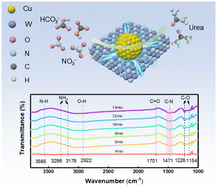
The electrocatalytic reduction of carbon dioxide (CO2) and different nitrogenous substances has shown a broad prospect in replacing the traditional urea synthesis process, but there are still serious challenges in mass transfer at the gas–liquid–solid interface. The conversion of bicarbonate (HCO3−) and nitrate (NO3−) into urea via the C–N coupling process under environmental conditions is a promising alternative to traditional industrial urea synthesis, which uses CO2 as the carbon source. However, initiating the C–N coupling reaction through the adsorption and activation of HCO3− and NO3− is considerably challenging. Designing and engineering highly selective and active electrocatalysts are necessary to accelerate electrochemical urea synthesis. Herein, we constructed a Mott–Schottky heterogeneous catalyst by loading Cu nanoparticles onto WN nanosheets (Cu-WN), achieving an excellent faradaic efficiency (FE) of 15.9% and urea yield rate of 421 μg h−1 mgcat.−1 at −0.3 V vs. RHE, outperforming the majority of reported electrocatalysts. Results show that the spatial charge region induced by the Mott–Schottky heterostructure facilitates the simultaneous adsorption and activation of HCO3− and NO3−, accelerating the multiple-electron transfer process. This work furnishes a promising impetus for the advancement of urea electrosynthesis via electrochemical C–N coupling under ambient conditions.