Open Access Article
Open Access Articleβ-Cyclodextrin and reduced graphene oxide loaded Ag–TiO2 composites for enhanced photocatalytic oxidation of urea under sunlight†
Palak
Soni
a,
Bonamali
Pal
*ab and
Raj Kumar
Das
 *ab
*ab
aDepartment of Chemistry and Biochemistry, Thapar Institute of Engineering & Technology, Patiala, 147004, Punjab, India. E-mail: bpal@thapar.edu; rkdas@thapar.edu
bTIET-Virginia Tech Center of Excellence in Emerging Materials, Thapar Institute of Engineering and Technology, Patiala, 147004, India
First published on 21st March 2025
Abstract
Urea oxidation is important to increase agricultural growth, which can meet food requirements across the world. It is pivotal for converting nitrogen to nitrate that is usable by crops, thus preventing nitrogen loss to the atmosphere. This study focuses on improving the photodegradation efficiency of TiO2 by incorporating β-CD (beta-cyclodextrin), RGO (reduced graphene oxide), and Ag to enhance nitrate conversion. FT-IR, DRS, PL, EDX, XRD, XPS, HR-TEM DLS, and FESEM were conducted to characterize these materials. Among all the catalysts, the quaternary composite, β-CD/Ag–TiO2/RGO, exhibited superior performance, achieving an 86.2% degradation efficiency with a 27.8% nitrate yield under sunlight irradiation within 150 min of reaction time. Several factors contribute to the enhanced photoactivity of β-CD/Ag–TiO2/RGO, including the high surface area and absorptive power of β-CD, the large electronic mobility of RGO, and the localized surface plasmonic resonance effect of Ag, extending the catalyst's response to visible light. An intriguing aspect of this study is the encapsulation of gaseous nitrogen into the hydrophobic interior cavity of β-CD, contributing to the enhancement of urea oxidation. These findings can be very substantial for both agriculturists and chemists, providing valuable insights into designing novel photocatalysts for improved urea oxidation, thereby enhancing agricultural productivity.
1. Introduction
With the projected increase in the global population, there is a growing reliance on fertilizers to meet the escalating demand for crops.1 Nitrogen-rich fertilizers are fundamental for promoting crop growth, the use of which remains a cornerstone of agricultural practices.2 Being an easily assimilable form of nitrogen, urea is an extensively used fertilizer that accommodates 46% N content engaged in the mineralization process.3 Plants primarily uptake nitrogen as nitrate (NO3−) and ammonium (NH4+) ions. For urea to become assimilable, it undergoes hydrolysis and subsequently forms NH4+ ions through protonation, and a further nitrification process begins where nitrite and nitrate form.4 Plants absorb these ions through specialized transporters that use proton gradients for transportation.5,6 However, urea exhibits significant losses upon contact with soil compared to other fertilizers, leading to ammonia volatilization, denitrification, runoff, leaching, and other pathways, resulting in the accumulation of reactive nitrogen (organic nitrogen and inorganic nitrogen compounds excluding N2).7,8 Moreover, this unintended ammonia emission diminishes crop yields due to nitrogen loss and poses long-term negative health hazards and environmental consequences as agriculture expands.9,10 Consequently, the oxidation of urea into beneficial products like NO3− is imperative for sustainable agriculture.11 Hence, there is an urgent need to develop efficient photocatalysts with robust oxidizing capabilities to enhance overall NO3− production efficiency. However, urea oxidation to nitrate is an 8-electron transfer process that is very challenging and often affords molecular nitrogen (N2) as the major product due to partial oxidation. As to increase crop yield, fertilizers are often over-applied, thereby increasing production costs. This can also result in an enhancement of urea concentration in groundwater, resulting in various adverse effects. As a result, different inhibitors have been used to control nitrogen leaching, but their high cost, as well as lack of stability in ambient conditions, limit their practical application.In this regard, a semiconductor-based photocatalytic process using solar light could be more effective for NO3− production yield during urea oxidation to enhance crop yield in agricultural land.12 Different metal (M)–TiO2 hybrid nanocatalysts display superior photocatalytic activity, stability and reusability for potential applications in removal of environmental pollutants and production of green energy.13 Researchers are still attempting to fabricate a better M–TiO2 nanointerface to improve the photogenerated charge separation efficiency utilizing various synthetic protocols for maximum photocatalytic performance under sunlight. In addition to numerous attempts to reduce the photoexcited electron–hole pair charge recombination process, tuning the M–TiO2 size, shape, surface structural morphology and band energetics is attracting a great deal of current research interest14 for highly enhanced photocatalytic activity of urea oxidation to NO3− (ref. 15) under visible/sunlight irradiation.
The loading of carbon materials like reduced graphene oxide (RGO) over TiO2 could potentially solve these problems.16 RGO is recognized for its excellent properties, including specific surface area, physicochemical stability, surface flexibility, and low production cost. Moreover, it has high electron mobility and electric capacitance facilitating efficient multi-electron transfer reactions.17 As a result, it could promote photocatalytic urea oxidation which is an 8-electron transfer process. However, its effectiveness is hindered by agglomeration and restacking due to π–π stacking and van der Waals forces, that reduce its surface area,18,19 limiting its practical application.
β-Cyclodextrin (β-CD), a well-known supramolecule, is a cyclic oligosaccharide composed of seven glucose units. It is highly adsorbent, non-toxic, and acts as a capping agent to stabilize metals. Urea oxidation frequently results in the formation of N2 as a primary product due to incomplete oxidation. To further improve the efficiency of photocatalysts, it is necessary to design a hybrid system with a strong affinity for N2 and significant oxidizing power, which helps to prevent leaching and improves nitrate production efficiency.20 β-CD has a toroidal shape with a hydrophilic exterior, exposing –OH groups for hydrogen bonding, and a hydrophobic interior that can encapsulate non-polar guests.21,22 This distinctive structure of β-CD provides numerous adsorption sites and synergistic interactions, extending its absorption spectrum towards the visible region and reducing the recombination rate.23 β-CD binds with urea through covalent forces and the hydrophobic interior encapsulates molecular nitrogen very effectively.24 This mechanistic approach offers stronger interactions, less leaching, and high nitrate yield.
For better charge separation, a photocatalyst should strongly absorb visible light. Adding plasmonic metals such as Ag, Au, or Cu can enhance this ability through localized surface plasmonic resonance (LSPR), which broadens the absorption into the visible spectrum.25 These transitions enable nanoparticles to absorb sunlight, generating strong electric fields and photogenerated e−/h+ pairs. These pairs can disseminate through phonons, causing a rise in lattice temperatures.26 High energy charge carriers, electric fields, and elevated temperatures can significantly boost photoactivity.27 Therefore, the deposition of coinage metals is an effective way to elevate the potential of a prepared material. Interestingly, plasmonic metal-loaded semiconductors are also emerging as new-generation catalysts for nitrogen fixation to yield ammonia.28–31
It has been found that literature reports on photocatalytic urea oxidation to yield nitrate are quite limited. Among the reported materials, β-CD/TiO2@Ag NC achieves the highest nitrate yield at 17.8(3)%, while TiO2/RGO yields 9.8(1)% with a NaF additive under neutral pH, which can disrupt ecosystems.32 This reaction necessitates high electron mobility to maximize the transfer of electrons for enhancing nitrate yield. Consequently, it is vital to develop a new photocatalyst with high capacitance as well as optimum nitrogen binding ability to facilitate urea oxidation. Inspired by these observations, highly proficient ternary (β-CD/Ag–TiO2, Ag–TiO2@RGO, β-CD/TiO2/RGO) and quaternary (β-CD/Ag–TiO2/RGO) heterojunction systems were constructed by using photodeposition and hydrothermal methods. These innovative nanocomposites, with their promising attributes, offer high electron mobility, increased surface area, enhanced optical response, and improved charge separation, thus facilitating a multi-electron transfer pathway of β-CD/Ag–TiO2/RGO for the photooxidation of urea to afford nitrate under visible/sunlight irradiation.
2. Experimental section
2.1 Materials
Graphite powder (98% extra pure); conc. sulfuric acid (H2SO4, 98%); potassium permanganate (KMnO4, 99% extra pure); sodium nitrate (NaNO3, 99%); hydrochloride acid (HCl, 35.4%); L-ascorbic acid (99.5%); hydrogen peroxide (H2O2, 30% (w/v) extra pure); ammonia (NH3, 28%); ethanol (C2H5OH, 99.9%); isopropanol (C2H5O, 99.5%); urea (CO(NH2)2, 99% extra pure) were received from Loba Chemie, India. TiO2 was supplied from Degussa Corporation, Germany. Silver(I) nitrate (AgNO3, ≥99%) was bought from Sigma-Aldrich. β-CD (98.00%) was purchased from GLR innovations. Deionized water (DI) was received from Organo Biotech Laboratories and utilized during all the experimental studies.2.2 Synthesis
Preparation of RGO was done by using a reported procedure.202.3 Material characterization techniques
The optical absorption of the prepared hybrids was assessed using a Jasco V-750 spectrophotometer with barium sulfate as the reference. Emission properties were analyzed via photoluminescence (PL) spectra upon excitation at 340 nm, utilizing a spectrofluorimeter (Shimadzu RF-6000). Structural defects were investigated through Raman spectroscopy using a Horiba Labram HR confocal micro-Raman spectrophotometer. X-ray diffraction (XRD) patterns were recorded with a Smart Lab SE diffractometer. Field-emission scanning electron microscopy (FESEM) images were recorded with a JEOL JSM-7600F electron microscope. Elemental composition and distribution were analyzed using energy dispersive spectroscopy (EDS, Bruker). High-resolution transmission electron microscopy (HR-TEM) studies were performed with a JEOL JSM-F200. X-ray photoelectron spectroscopy (XPS, PHI 5000 VERSA Probe III) was used to study the chemical environment and oxidation states of the samples. Total organic carbon (TOC) measurements were conducted with a Thermo Scientific HiPer TOC-TN-CLD module to evaluate the demineralization efficiency of CTA, ATR, CTR, and CTAR. Sample aliquots were mixed with H3PO4 (5% w/v) and Na2S2O8 (10% w/v). Then, the reaction mixture was heated in a glass vessel to oxidize all the organic carbon to CO2, which was detected using a non-dispersive infrared (NDIR) detector. The nitrate yield was further estimated using the ultraviolet spectrophotometer screening method (Varian UV0910M156) by monitoring the absorbance at 220 nm.2.4 Photodegradation analysis
The photocatalytic performance of the ternary and quaternary nanocomposites (NCs) was investigated using urea as the model molecule at an initial concentration of 1.8 mM. Each test was conducted in a series of test tubes, holding 10 mg of catalyst suspended in 10 mL of urea solution. To attain adsorption–desorption equilibrium, the suspensions were stirred vigorously in the dark for 30 min. For urea degradation, the test tubes containing catalysts were kept under sunlight for 150 min in Patiala, India (1–30 May 2023; 12:00 pm–2:30 pm), with average solar radiation of approximately 785 W m−2 and temperatures around 35 °C. After 30 min fixed interval, 2 mL of supernatant was collected, and the catalyst was removed by centrifugation at 8000 rpm from the degraded solution. Additionally, urea concentration was estimated using the p-dimethylaminobenzaldehyde (DMAB) method.35Urea photodegradation efficiency using the various photocatalysts was calculated using the following equation:
 | (1) |
TOC measurements were conducted with a Thermo Scientific HiPer TOC-TN-CLD module to evaluate the demineralization efficiency of CTA, ATR, CTR, and CTAR. The demineralization efficiency (%) was calculated according to the following equation:36
 | (2) |
3. Results and discussion
3.1 Characterizations
The band gap energy of the synthesized ternary and quaternary NCs was determined using Tauc plot (eqn (3)):
| αhv = A(hv − Eg)n | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 The quaternary NC shows a significantly reduced band gap of 1.85 eV. The reduction in Eg is apparent with the successive addition of RGO, Ag, and β-CD to the TiO2 surface, causing a shift in the optical response towards the visible spectrum. Consequently, this enhancement could improve the photocatalytic performance of the fabricated quaternary NC.
41 The quaternary NC shows a significantly reduced band gap of 1.85 eV. The reduction in Eg is apparent with the successive addition of RGO, Ag, and β-CD to the TiO2 surface, causing a shift in the optical response towards the visible spectrum. Consequently, this enhancement could improve the photocatalytic performance of the fabricated quaternary NC.
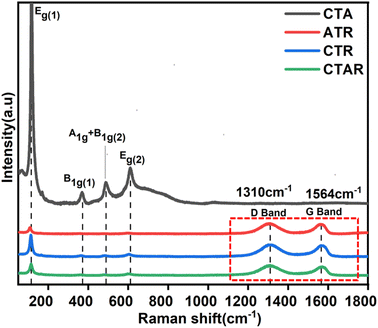
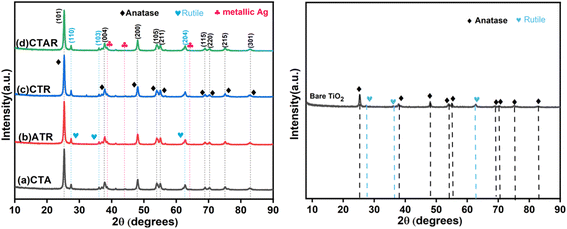
HRTEM analysis was conducted to investigate the shape, size, and interactions between the components of the quaternary NC. Fig. 6a provides evidence of the layered morphology of RGO sheets. Fig. 6b shows the accumulation of clustered, spherical, and agglomerated metallic Ag deposited on TiO2 over the RGO layers. The dark spots of metallic Ag, which appear black, range in size from 7 to 21 nm. Fig. 6c and d clearly depicts the white lining of β-CD around the TiO2 particles and the black spots of metallic Ag firmly attached to the TiO2 surface.38 The RGO sheets serve as a substrate for the integrated photodeposited Ag on the TiO2 nanoparticles.49Fig. 6e shows two sets of lattice fringes: 0.36 nm for the (101) plane of TiO2 and 0.23 nm for the (111) plane of Ag(0). Fig. 6f presents the SAED pattern, displaying concentric rings with bright spots analogous with the TiO2 (110), (101), and metallic Ag (111), (200) diffraction planes of the CTAR nanocomposite.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1582 cm−1 (C
O) and 1582 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), confirming the presence of RGO, along with Ti–O–C bonds suggesting TiO2-RGO interactions. β-CD/TiO2@RGO exhibits –OH stretching band at 3343 cm−1 and C
C), confirming the presence of RGO, along with Ti–O–C bonds suggesting TiO2-RGO interactions. β-CD/TiO2@RGO exhibits –OH stretching band at 3343 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks, reflecting contributions from β-CD and RGO, coupled with strong Ti–O–C signals. In the spectrum of β-CD/TiO2/Ag/RGO, the presence of –OH, C–H, and Ti–O–C peaks highlights enhanced interactions between β-CD, TiO2, RGO, and Ag nanoparticles, demonstrating the composite's robust structural integration.
C peaks, reflecting contributions from β-CD and RGO, coupled with strong Ti–O–C signals. In the spectrum of β-CD/TiO2/Ag/RGO, the presence of –OH, C–H, and Ti–O–C peaks highlights enhanced interactions between β-CD, TiO2, RGO, and Ag nanoparticles, demonstrating the composite's robust structural integration.
3.2 Photooxidation of urea
The efficiencies of the CTA, ATR, CTR, and CTAR NCs were assessed in photocatalytic urea oxidation. Fig. 8 shows the adsorption and photodegradation curves for the various nanocomposites after 150 min of reaction time. The adsorption capacity of the catalysts showed a progressive increase in the following sequence: CTA < ATR < CTR < CTAR. This improvement is likely due to the incorporation of new guest binding sites arising from the deposition of β-CD, RGO, and Ag nanoparticles (Fig. S4† and 8a). Once equilibrium was achieved, the catalytic activity was examined under sunlight (Fig. S5† and 8b). Sunlight exposure enhanced the photoactivity of all the catalysts, where the quaternary composite (CTAR) exhibited the highest degradation after 150 min of sunlight exposure.The oxidation of urea involves the transfer of 8 electrons to form nitrate.20 The composites incorporate RGO, which has high electron mobility, facilitating electron transfer and enhancing photoinduced charge separation. β-CD provides a substantial number of sites for urea binding, which results in reduced nitrogen leaching. Moreover, the deposition of Ag nanoparticles enhances light absorption due to the LSPR effect.32,50
The modification with different components in all the catalysts altered the oxidation trend, as shown in Fig. 8c, following the order: CTAR (86.2%) > CTR (75.8%) > ATR (70.5%) > CTA (55.7%). The observed data were analyzed according to a pseudo-first-order kinetic equation:51
 | (4) |
The rate constant (min−1) for urea oxidation followed the order: CTA (0.0069) < ATR (0.0092) < CTR (0.0133) < CTAR (0.017). Among all the nanocomposites, CTAR exhibits the highest rate of photocatalytic reaction. The combined components in the CTAR nanocomposite contributed to the improved photocatalytic activity.
3.3 Demineralization efficiency and nitrate yields
It is clear that the oxidation of urea does not necessarily lead to complete mineralization into CO2 and H2O. Therefore, it is crucial to assess the demineralization efficiency52,53 (eqn (2)). Fig. 9a presents the results of urea oxidation using bare CTA (54%), ATR (69.7%), CTR (75%), and CTAR (85%) over 150 min under sunlight irradiation. The CTAR nanocomposite demonstrates the highest demineralization efficiency (85%) of urea which aligns closely with the degradation efficiency suggesting its complete photodegradation. | ||
| Fig. 9 Comparison of (a) demineralization efficiency and (b) nitrate yield for the different hybrid composites. | ||
The principal aim of this study is to improve the overall yield of NO3− using potential photocatalysts. The oxidation of urea was conducted using CTA, ATR, CTR, and CTAR under sunlight for 150 min to determine the NO3− yield. The results depicted in Fig. 9b illustrate the NO3− yield achieved by these photocatalysts. Remarkably, the CTAR nanocomposite exhibits the highest NO3− yield at 27.8%. The synergistic effect of silver, RGO, and β-CD loading demonstrates greater solar light absorption, increases electron–hole pair lifetime, and minimizes nitrogen leaching.54
It found that the CTAR quaternary hybrid exhibits the highest urea degradation efficiency and nitrate yield (%) compared to previously reported photocatalysts (Table 1).
3.4 Scavenger studies
Various scavengers, such as EDTA, IPA, and argon gas, were selected to reveal the roles of various reactive species.56 Scavenging experiments were conducted during the urea oxidation over the CTAR photocatalyst under similar conditions (Fig. 10). As depicted in Fig. 10, the degradation rate significantly decreased in the presence of IPA and argon gas, indicating that ˙OH and O2− act as the essential active species in the oxidation of urea. Conversely, the rate of degradation also decreased with the EDTA scavenger, although the reduction was lesser, suggesting that the holes (h+) have minor participation in the oxidation process. | ||
| Fig. 10 Effect of scavenger addition in the photocatalytic urea degradation efficiencies of the CTAR quaternary heterostructure. | ||
3.5 Mechanistic details
The band gap energy (Eg) values obtained from the DRS analysis were utilized to calculate the valence band (VB) and conduction band (CB) positions at the point of zero charge using the following equations (Scheme 2):| EVB = χ − Ee + 0.5 Eg | (5) |
| ECB = EVB − Eg | (6) |
 | ||
| Scheme 2 Schematic representation showing the relative band positions and the photocatalytic activity of the CTAR composite. | ||
The photodegradation mechanism of the β-CD-containing CTAR composite for urea degradation can be explained based on the band positions of TiO2. The CB is positioned at −0.385 eV and the VB is at +2.235 eV, relative to the NHE. Upon sunlight irradiation, TiO2 does not absorb significantly due to a large band gap. However, the incorporation of Ag substantially enhanced the visible light sensitization of TiO2 due to the SPR phenomenon. The interaction of sunlight with Ag induced a collective oscillation of its conduction electrons, generating a localized electric field. This effect facilitated the efficient separation of photogenerated electron–hole (e−/h+) pairs on the TiO2 surface, thereby transforming it into a visible-light-active photocatalyst. In the presence of Ag nanoparticles and RGO, these electrons are efficiently separated and transported, reducing recombination rates. Ag acts as an electron sink, while RGO facilitates charge transfer due to its excellent conductivity. As the CB edge potential is much more negative than that of O2/O2˙− (−0.046 eV), there is a facile reduction of dissolved oxygen to form superoxide anions. Similarly, the VB edge potential is more positive than that of ˙OH/H2O (+2.68 eV), allowing the oxidation water to produce hydroxyl (˙OH) radicals. These reactive oxygen species (ROS) effectively break down urea into NO3− and less harmful byproducts. Additionally, β-CD enhances the photocatalytic efficiency by forming inclusion complexes with urea molecules, increasing their local concentration near the photocatalyst's active sites and improving the degradation efficiency. This synergistic effect makes the composite highly effective for the photodegradation of urea.
The mechanism involves the synergistic interaction between Ag, Ti, and RGO as shown in Scheme 3. Photoexcited electrons from the TiO2 CB swiftly migrate to Ag and RGO, preventing photoelectron pair recombination and increasing charge carriers to form reactive species (O2−, ˙OH), thus boosting photocatalytic performance. The two-dimensional RGO structure provides excellent conductivity, facilitating rapid charge transport and separation. Ag nanoparticles generate high-energy electrons with the LSPR effect, which undergo fast transfer to the RGO surface. These electrons react with adsorbed oxygen to form superoxide ions (O2−), while excited holes oxidize with H2O to produce hydroxyl radicals (˙OH). Both species are important for urea degradation.48
 | ||
| Scheme 3 Detailed mechanism for urea oxidation catalyzed by the β-CD/TiO2/Ag/RGO (CTAR) quaternary composite. | ||
Urea molecules first interact noncovalently with the hydroxyl groups of β-CD (step 1) and are oxidized to produce molecular nitrogen (N2) (step 2). This nitrogen binds to the hydrophobic region of β-CD (step 3), which helps minimize nitrogen leaching. Following this, the nitrogen bound to β-CD undergoes further oxidation, resulting in the formation of nitrate (step 4). The final step (step 5) is associated with the release of nitrate to regenerate the original photocatalyst.
3.6 Reusability and stability studies
The practical applicability of a catalyst is often assessed based on its recyclability and stability. Herein, the recyclability of the CTAR catalyst was evaluated over four cycles under sunlight. As shown in Fig. 11a, the CTAR NC's degradation efficiency dropped by only 9% after four cycles, highlighting its potential as a reusable catalyst. The slight decline in photocatalytic performance could be attributed to the loss of photocatalyst mass during successive experiments. | ||
| Fig. 11 (a) Recyclability of the CTAR NC for urea degradation under sunlight over four consecutive cycles. (b) XRD patterns showing the stability of the CTAR NC before and after degradation. | ||
Furthermore, the structural stability of the catalyst was also evaluated to support this assertion. Fig. 11b displays the XRD patterns of the CTAR nanocomposite before and after photocatalytic degradation reactions. No shifts in peak positions or changes in peak intensities were observed, indicative of structural integrity. Therefore, the CTAR NC is considered an excellent material for the photocatalytic oxidation of urea.
4. Conclusion
Novel ternary and quaternary nanocomposites incorporating Ag, RGO, and β-CD were synthesized for the first time and extensively studied with the aim of enhancing the photocatalytic oxidation of urea. Among these, the CTAR quaternary nanocomposite demonstrated exceptional photocatalytic efficiency, achieving 86.2% degradation within 150 min under solar light, surpassing that of the other ternary nanocomposites. The incorporation of Ag extended the visible light sensitivity of TiO2, β-CD facilitated nitrogen binding through hydrophobic interactions to minimize nitrogen leaching, and RGO provided high capacitance and electron mobility while preventing rapid recombination of photogenerated charge carriers. The synergistic interactions among TiO2, Ag, β-CD, and RGO contributed to the superior photocatalytic performance of the quaternary nanocomposite. Furthermore, the catalyst exhibited remarkable stability and recyclability. This study underscores the potential of the CTAR composite as a green, solar light-activated catalyst for enhancing the photocatalytic oxidation of urea.Data availability
The supporting data have been uploaded as a part of the ESI.†Author contributions
Palak soni: conceptualization, performance, methodology, experiments, execution, data collection, data correction, writing the original draft. Bonamali Pal: supervision, data investigation, data validation, reviewing, and editing. Raj Kumar Das: supervision, data investigation, data validation, reviewing, and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge Physics Department (TIET) for XRD and SEM analysis, IISER Mohali for HR-TEM facility, IIT Kanpur for XPS analysis, and ACIRD Lab for TOC and nitrate analysis. We are also grateful to TIET-VT CEEMS, CSIR India (grant number: 01(3082)/21/EMR-II), and DBT India (grant number: BT/PR36172/NNT/28/1811/2021) for financial support.References
- J. Penuelas, F. Coello and J. Sardans, A better use of fertilizers is needed for global food security and environmental sustainability, Agric. Food Secur., 2023, 12, 1–9 Search PubMed.
- S. J. Leghari, N. A. Wahocho, G. M. Laghari, A. H. Laghari, G. M. Bhabhan, K. H. Talpur and A. A. Lashari, Role of nitrogen for plant growth and development: a review, Adv. Environ. Biol., 2016, 10, 209–218 Search PubMed.
- P. Shetty, C. Acharya and N. Veeresh, Effect of urea fertilizer on the biochemical characteristics of soil, Int. J. Appl. Sci. Biotechnol., 2019, 7, 414–420 CAS.
- C. Masclaux-Daubresse, F. Daniel-Vedele, J. Dechorgnat, F. Chardon, L. Gaufichon and A. Suzuki, Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture, Ann. Bot., 2010, 105, 1141–1157 Search PubMed.
- Y. S. Ku, S. S. Cheng, M. S. Ng, G. Chung and H. M. Lam, The tiny companion matters: the important role of protons in active transports in plants, Int. J. Mol. Sci., 2022, 23, 2824 CAS.
- T. Ketehouli, K. F. I. Carther, M. Noman, F. W. Wang, X. W. Li and H. Y. Li, Adaptation of plants to salt stress: characterization of Na+ and K+ transporters and role of Cbl gene family in regulating salt stress response, Agronomy, 2019, 9, 687 CrossRef CAS.
- M. Skorupka and A. Nosalewicz, Ammonia volatilization from fertilizer urea—A new challenge for agriculture and industry in view of growing global demand for food and energy crops, Agriculture, 2021, 11, 822 CrossRef CAS.
- Z. Ma, Y. Yue, M. Feng, Y. Li, X. Ma, X. Zhao and S. Wang, Mitigation of ammonia volatilization and nitrate leaching via loss control urea triggered H-bond forces, Sci. Rep., 2019, 9, 1–9 CrossRef PubMed.
- Y. Liu, Y. Ge, J. Tan, H. Wang and Y. Ding, Research on ammonia emissions characteristics from light-duty gasoline vehicles, J. Environ. Sci., 2021, 106, 182–193 CrossRef CAS.
- M. Anas, F. Liao, K. K. Verma, M. A. Sarwar, A. Mahmood, Z. L. Chen, Q. Li, X. P. Zeng, Y. Liu and Y. R. Li, Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency, Biol. Res., 2020, 53, 1–20 Search PubMed.
- Y. Jiang, Y. Zhu, W. Lin and J. Luo, Urea fertilization significantly promotes nitrous oxide emissions from agricultural soils and is attributed to the short-term suppression of nitrite-oxidizing bacteria during urea hydrolysis, Microorganisms, 2024, 12, 685 Search PubMed.
- F. Zhang, X. Wang, H. Liu, C. Liu, Y. Wan, Y. Long and Z. Cai, Recent advances and applications of semiconductor photocatalytic technology, Appl. Sci., 2019, 9, 2489 Search PubMed.
- S. A. Mousa, H. Abdallah and S. A. Khairy, Low-cost photocatalytic membrane modified with green heterojunction TiO2/ZnO nanoparticles prepared from waste, Sci. Rep., 2023, 13, 1–19 Search PubMed.
- L. Lu, G. Wang, Z. Xiong, Z. Hu, Y. Liao, J. Wang and J. Li, Enhanced photocatalytic activity under visible light by the synergistic effects of plasmonics and Ti3+-doping at the Ag/TiO2−x heterojunction, Ceram. Int., 2020, 46, 10667–10677 Search PubMed.
- G. Shao, Y. Zang and B. J. Hinds, TiO2 nanowires based system for urea photodecomposition and dialysate regeneration, ACS Appl. Nano Mater., 2019, 2, 6116–6123 Search PubMed.
- H. Liu, P. Li, H. Bai, C. Du, D. Wei, Y. Su, Y. Wang and L. Yang, Incorporation of reduced graphene oxide into faceted flower-like (001) TiO2 for enhanced photocatalytic activity, R. Soc. Open Sci., 2015, 5, 180613 CrossRef PubMed.
- W. Liu and G. Speranza, Tuning the oxygen content of reduced graphene oxide and effects on its properties, ACS Omega, 2021, 6, 6195–6205 CrossRef CAS PubMed.
- R. Geetha Bai, K. Muthoosamy, F. N. Shipton and S. Manickam, Acoustic cavitation induced generation of stabilizer-free, extremely stable reduced graphene oxide nanodispersion for efficient delivery of paclitaxel in cancer cells, Ultrason. Sonochem., 2017, 36, 129–138 CrossRef CAS PubMed.
- K. I. Nargatti, S. S. Ahankari, J. R. C. Dizon and R. T. Subramaniam, Environmentally friendly water-based reduced graphene oxide/cellulose nanofiber ink for supercapacitor electrode applications, ACS Omega, 2024, 9, 11730–11737 CrossRef CAS.
- P. Soni, B. Pal and R. K. Das, Enhanced photocatalytic urea oxidation under neutral medium by reduced graphene oxide coated TiO2 nanoparticles, Catal. Commun., 2023, 179, 106690 CrossRef CAS.
- M. Chen, Y. Meng, W. Zhang, J. Zhou, J. Xie and G. Diao, β-Cyclodextrin polymer functionalized reduced-graphene oxide: application for electrochemical determination imidacloprid, Electrochim. Acta, 2013, 108, 1–9 CrossRef CAS.
- X. Tu, F. Gao, X. Ma, J. Zou, Y. Yu, M. Li, F. Qu, X. Huang and L. Lu, Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide, J. Hazard. Mater., 2020, 396, 122776 CrossRef CAS PubMed.
- P. Sakthivel and P. Velusamy, Modification of the photocatalytic performance of various metal oxides by the addition of β-cyclodextrin under visible light irradiation, J. Water Process Eng., 2017, 16, 329–337 CrossRef.
- A. Szwajca and H. Koroniak, Encapsulation of fluoroaromatics by β-cyclodextrin and their derivatives theoretical studies, J. Fluorine Chem., 2014, 167, 122–127 Search PubMed.
- I. Ibrahim Zamkoye, B. Lucas and S. Vedraine, Synergistic effects of localized surface plasmon resonance, surface plasmon polariton, and waveguide plasmonic resonance on the same material: a promising hypothesis to enhance organic solar cell efficiency, Nanomaterials, 2023, 13, 2209 CrossRef CAS PubMed.
- I. Ahmed, L. Shi, H. Pasanen, P. Vivo, P. Maity, M. Hatamvand and Y. Zhan, There is plenty of room at the top: generation of hot charge carriers and their applications in perovskite and other semiconductor-based optoelectronic devices, Light Sci. Appl., 2021, 10, 1–28 Search PubMed.
- Y. Hattori, J. Meng, K. Zheng, A. Meier De Andrade, J. Kullgren, P. Broqvist, P. Nordlander and J. Sá, Phonon-assisted hot carrier generation in plasmonic semiconductor systems, Nano Lett., 2021, 21, 1083–1089 CrossRef CAS PubMed.
- P. Xing, S. Wu, Y. Chen, P. Chen, X. Hu, H. Lin, L. Zhao and Y. He, New application and excellent performance of Ag/KNbO3 nanocomposite in photocatalytic NH3 synthesis, ACS Sustainable Chem. Eng., 2019, 7, 12408–12418 Search PubMed.
- X. Li, L. Chen, J. Wang, J. Zhang, C. Zhao, H. Lin, Y. Wu and Y. He, Novel platinum-bismuth alloy loaded KTa0.5Nb0.5O3 composite photocatalyst for effective nitrogen-to-ammonium conversion, J. Colloid Interface Sci., 2022, 618, 362–374 Search PubMed.
- J. Zhang, L. Yue, Z. Zeng, C. Zhao, L. Fang, X. Hu, H. Lin, L. Zhao and Y. He, Preparation of NaNbO3 microcube with abundant oxygen vacancies and its high photocatalytic N2 fixation activity in the help of Pt nanoparticles, J. Colloid Interface Sci., 2023, 636, 480–491 Search PubMed.
- L. Chen, W. Zhang, J. Wang, X. Li, Y. Li, X. Hu, L. Zhao, Y. Wu and Y. He, High piezo/photocatalytic efficiency of Ag/Bi5O7I nanocomposite using mechanical and solar energy for N2 fixation and methyl orange degradation, Green Energy Environ., 2023, 8, 283–295 CrossRef CAS.
- P. Soni, B. Pal and R. K. Das, Influence of β-CD and Ag deposition over TiO2 towards photocatalytic oxidation of urea under solar irradiation, J. Environ. Chem. Eng., 2024, 12, 112150 CrossRef CAS.
- K. H. Leong, L. C. Sim, D. Bahnemann, M. Jang, S. Ibrahim and P. Saravanan, Reduced graphene oxide and Ag wrapped TiO2 photocatalyst for enhanced visible light photocatalysis, APL Mater., 2015, 3, 104503 Search PubMed.
- S. Bhardwaj, D. Sharma, P. Kumari and B. Pal, Influence of photodeposition time and loading amount of Ag co-catalyst on growth, distribution and photocatalytic properties of Ag@TiO2 nanocatalysts, Opt. Mater., 2020, 106, 109975 CAS.
- J. D. Giraldo and B. L. Rivas, Determination of urea using p-N,N-dimethylaminobenzaldehyde: solvent effect and interference of chitosan, J. Chil. Chem. Soc., 2017, 62, 3538–3542 Search PubMed.
- L. Rimoldi, D. Meroni, E. Falletta, V. Pifferi, L. Falciola, G. Cappelletti and S. Ardizzone, Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium, Photochem. Photobiol. Sci., 2017, 16, 60–66 CAS.
- W. Cao, T. Huang, X. H. N. Xu and H. E. Elsayed-Ali, Localized surface plasmon resonance of single silver nanoparticles studied by dark-field optical microscopy and spectroscopy, J. Appl. Phys., 2011, 109, 034310 Search PubMed.
- N. Attarchi, M. Montazer and T. Toliyat, Ag/TiO2/β-CD nano composite: preparation and photo catalytic properties for methylene blue degradation, Appl. Catal., A, 2013, 467, 107–116 Search PubMed.
- H. N. Tien, V. H. Luan, L. T. Hoa, N. T. Khoa, S. H. Hahn, J. S. Chung, E. W. Shin and S. H. Hur, One-pot synthesis of a reduced graphene oxide-zinc oxide sphere composite and its use as a visible light photocatalyst, Chem. Eng. J., 2013, 229, 126–133 CAS.
- J. T. Abdalla, J. Wang and D. Wang, Effect of Ag/rGO on the Optical Properties of Plasmon-Modified SnO2 Composite and Its Application in Self-Powered UV Photodetector, Crystals, 2019, 9, 648 CAS.
- S. Nayak, L. Mohapatra and K. Parida, Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction, J. Mater. Chem. A Mater., 2015, 3, 18622–18635 Search PubMed.
- N. A. M. Barakat and H. Y. Kim, Effect of silver-doping on the crystal structure, morphology and photocatalytic activity of TiO2 nanofibers, IOP Conf. Ser. Mater. Sci. Eng., 2012, 40, 012003 Search PubMed.
- H. Tian, C. Wan, X. Xue, X. Hu and X. Wang, Effective electron transfer pathway of the ternary TiO2/RGO/Ag nanocomposite with enhanced photocatalytic activity under visible light, Catalysts, 2017, 7, 156 Search PubMed.
- W. Sun, Q. Meng, L. Jing, L. He and X. Fu, Synthesis of long-lived photogenerated charge carriers of Si-modified α-Fe2O3 and its enhanced visible photocatalytic activity, Mater. Res. Bull., 2014, 49, 331–337 CAS.
- G. T. S. How, A. Pandikumar, H. N. Ming and L. H. Ngee, Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing, Sci. Rep., 2014, 4, 2–9 Search PubMed.
- S. Dai, Y. Wu, T. Sakai, Z. Du, H. Sakai and M. Abe, Preparation of highly crystalline TiO2 nanostructures by acid-assisted hydrothermal treatment of hexagonal-structured nanocrystalline titania/cetyltrimethyammonium bromide nanoskeleton, Nanoscale Res. Lett., 2010, 5, 1829–1835 CrossRef CAS.
- R. Geng, J. Yin, J. Zhou, T. Jiao, Y. Feng, L. Zhang, Y. Chen, Z. Bai and Q. Peng, In situ construction of Ag/TiO2/g-C3N4 heterojunction nanocomposite based on hierarchical co-assembly with sustainable hydrogen evolution, Nanomaterials, 2020, 10, 1 Search PubMed.
- S. Athithya, V. S. Manikandan, S. K. Harish, K. Silambarasan, S. Gopalakrishnan, H. Ikeda, M. Navaneethan and J. Archana, Plasmon effect of Ag nanoparticles on TiO2/rGO nanostructures for enhanced energy harvesting and environmental remediation, Nanomaterials, 2023, 13, 1–16 Search PubMed.
- A. Bokare, S. Chinnusamy and F. Erogbogbo, TiO2-graphene quantum dots nanocomposites for photocatalysis in energy and biomedical applications, Catalysts, 2021, 11, 1–51 CrossRef.
- Y. Zhang, Q. Li, Q. Gao, S. Wan, P. Yao and X. Zhu, Preparation of Ag/β-cyclodextrin co-doped TiO2 floating photocatalytic membrane for dynamic adsorption and photoactivity under visible light, Appl. Catal., B, 2020, 267, 118715 CrossRef CAS.
- X. Zhan, C. Yan, Y. Zhang, G. Rinke, G. Rabsch, M. Klumpp, A. I. Schäfer and R. Dittmeyer, Investigation of the reaction kinetics of photocatalytic pollutant degradation under defined conditions with inkjet-printed TiO2 films – from batch to a novel continuous-flow microreactor, React. Chem. Eng., 2020, 5, 1658–1670 RSC.
- D. Chatterjee and A. Mahata, Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light, Appl. Catal., B, 2001, 33, 119–125 CrossRef CAS.
- I. Prabha and S. Lathasree, Effective photocatalytic demineralization of reactive red 198 utilizing nanocomposite particles under UV light irradiation, J. Ind. Chem. Soc., 2017, 94, 269–277 CAS.
- V. Lalan, V. P. Mahadevan Pillai and K. G. Gopchandran, Enhanced electron transfer due to rGO makes Ag–CaTiO3@rGO a promising plasmonic photocatalyst, J. Sci. Adv. Mater. Dev., 2022, 7, 100468 Search PubMed.
- V. Vaiano, O. Sacco, G. Di Capua, N. Femia and D. Sannino, Use of visible light modulation techniques in urea photocatalytic degradation, Water, 2019, 11, 1642 Search PubMed.
- X. Xu, Y. Sun, Z. Fan, D. Zhao, S. Xiong, B. Zhang, S. Zhou and G. Liu, Mechanisms for ˙O2− and ˙OH production on flowerlike BiVO4 photocatalysis based on electron spin resonance, Front. Chem., 2018, 6, 1–12 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00245a |
| This journal is © The Royal Society of Chemistry 2025 |