Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dye-sensitized nanoparticles for efficient solar hydrogen generation
Vasilis
Nikolaou
 a,
Emmanouil
Nikoloudakis
a,
Emmanouil
Nikoloudakis
 bc,
Georgios
Charalambidis
bc,
Georgios
Charalambidis
 *d and
Athanassios G.
Coutsolelos
*d and
Athanassios G.
Coutsolelos
 *bc
*bc
aChimie Et Interdisciplinarité, Synthèse, Analyse, Modélisation (CEISAM), CNRS UMR 6230, Nantes, France
bLaboratory of Bioinorganic Chemistry, Department of Chemistry, University of Crete, Heraklion, Crete, Greece. E-mail: acoutsol@uoc.gr
cInstitute of Electronic Structure and Laser (IESL), Foundation for Research and Technology – Hellas (FORTH), Heraklion, Greece
dTheoretical and Physical Chemistry Institute, National Hellenic Research Foundation, Athens, Greece. E-mail: gcharal@eie.gr
First published on 14th June 2025
Abstract
Dye-sensitized photocatalytic systems (DSPs) for hydrogen (H2) evolution have garnered significant attention due to their ability to harness solar energy for efficient fuel production. In this feature article review, we discuss our recent advancements in DSPs, focusing on TiO2-based systems and self-assembled nanostructures for H2 evolution. We explore the role of porphyrins as photosensitizers and catalysts in H2 evolving DSPs, highlighting strategies to enhance light absorption and charge transfer efficiency. In addition, we introduce our alternative approach, utilizing self-assembled porphyrin architectures to overcome the limitations of conventional DSPs, such as the instability of anchoring groups. Finally, we present our recent approach using a photosensitizer–catalyst (PS–CAT) dyad, which enables alcohol oxidation coupled with H2 evolution, eliminating the need for a classic sacrificial electron donor (SED). In the final section, we offer perspectives and future directions for DSPs, aiming to foster the development of greener and more economically sustainable solar-driven fuel and chemical synthesis.
1. Introduction
The global dependence on fossil fuels as primary energy sources has been a significant driver of environmental challenges and climate change. The combustion of fossil fuels releases greenhouse gases into the atmosphere, which are responsible for accelerating global warming.1 Hence, there is an urgent need to transition from fossil fuels to sustainable and renewable energy sources through the development and utilization of low-carbon technologies.2 Among the various renewable energy sources, such as wind and hydropower, solar energy stands out as one of the most promising due to its exceptional abundance and accessibility.3 Over the past decade, solar-driven hydrogen (H2) production has gained significant attention as an innovative and efficient approach to generating an energy-rich fuel by utilizing sunlight, a clean and inexhaustible energy source.4Recent advancements have shown that overall water splitting is feasible through dye-sensitized Z-scheme photocatalytic systems.5 These systems integrate multiple semiconductors and/or photosensitizers to facilitate both H2 and oxygen (O2) evolution under solar irradiation.6,7 Typically, the configuration involves non-TiO2-based photoanodes, such as BiVO4 or WO3, which function as oxidation photocatalysts.8,9 These are coupled with dye-sensitized photocathodes responsible for proton (H+) reduction to H2. The tandem arrangement enhances the versatility of dye-sensitized photocatalysis, paving the way toward unbiased water splitting. Notably, Bai et al. reported a system combining a linear conjugated polymer with BiVO4, achieving water splitting under visible light irradiation.10 Moreover, Ni and co-workers developed a Z-scheme catalyst based on an ultrathin nanosheet, resulting in highly efficient overall water splitting activity.11
The development of dye-sensitized photocatalytic systems (DSPs) is one of the most straightforward approaches for light-driven hydrogen (H2) evolution, primarily due to their tunability, stability, and efficiency.12,13 In a typical DSP (Fig. 1), nanoparticles (NPs) of a semiconductor, such as titanium dioxide (TiO2 NPs), are functionalized with a photosensitizer (PS) and a catalyst (CAT), forming a heterogeneous photocatalyst. Upon light irradiation, the PS is excited and injects electrons into the conduction band of TiO2. These electrons are then transferred to the catalyst, where they facilitate the reduction reaction (2H+ → H2). The final step in completing the catalytic cycle is the regeneration of the oxidized PS (PS+) through a sacrificial electron donor (SED).
 | ||
| Fig. 1 Typical configuration of dye-sensitized photocatalytic systems (DSPs) in the literature, and our approaches presented in this feature article. | ||
Porphyrins are widely used as photosensitizers (PSs) in DSPs due to their ease of preparation, structural versatility, and ability to fine-tune both photophysical and electrochemical properties.12,13 These characteristics have facilitated the development of highly efficient systems for sustainable H2 production.14 The rational design of porphyrins, in DSPs, is governed by three main criteria: (i) strong absorption in the visible region, (ii) suitable energy alignment with the conduction band of TiO2, and (iii) robust anchoring onto the TiO2 NPs. Through directed metalation of the porphyrin ring along with targeted substitution at the meso-positions with electron-donating or electron-withdrawing groups, porphyrins can be systematically modified to achieve optimal redox behavior and spectral response.15,16 Additionally, by adopting the donor–π–acceptor (D–π–A) concept the absorption of porphyrin sensitizers can be extended and the undesired charge recombination can be suppressed.17–21 In this feature article, we present our group's innovative approaches to developing porphyrin-based DSPs through two distinct strategies (Fig. 1). The first involves TiO2-based systems, where porphyrins serve as PSs anchored onto Pt-doped TiO2 nanoparticles (Pt–TiO2 NPs). Whereas, the second explores the self-assembly of porphyrin-based nanoparticles, taking advantage of their intrinsic supramolecular organization.
For the TiO2-based systems (Section 2), we employed porphyrins either as photosensitizer dyads (PS-dyads) or as single PS, fine-tuning their interactions with the TiO2 NPs to optimize light absorption and charge transfer.22–25 However, instead of relying solely on TiO2 as a redox mediator, we pursued a paradigm shift by designing self-assembled porphyrin nanostructures (Section 3).26–31 These architectures, with morphologies ranging from spheres and fibrils to flakes, exhibit tailored electronic properties and enhanced catalytic activity. Our strategy overcomes an essential drawback of typical DSPs, namely the need for anchoring groups to attach PS onto semiconductor surfaces. Such functionalization often hinders both their activity and stability, either due to the intrinsic low surface coverage, or because of their detachment over prolonged operation, respectively. By exploiting self-assembled porphyrin structures, we eliminate these limitations, enabling the development of more robust and high-performing photocatalytic systems.
Building on this concept, the future of DSPs lies in replacing SEDs with catalytic oxidation processes, such as water, alcohol, or amine oxidation. This shift is crucial because SEDs stand as significant challenges for scalability and commercialization, as they are often expensive, generate waste, and introduce sustainability concerns. The integration of CATs for alcohol oxidation instead of SEDs represents an innovative approach toward sustainable photocatalysis. In the final section of this article (Section 4), we will discuss our work, where we implemented a PS–CAT dyad to drive alcohol oxidation instead of relying on a SED. SpecificallFy, we developed a DSP for dual catalysis, enabling the simultaneous production of H2 (from Pt–TiO2 NPs) and value-added aldehydes (from PS–CAT) in water. This approach not only eliminates the limitations of SEDs but also enhances system efficiency and selectivity, paving the way for greener and more economically viable solar-driven fuel and chemical synthesis.
2. TiO2-based systems
In 1972, Honda and Fujishima established a new strategy for photocatalytic H2 production using semiconductor materials with their work on TiO2 catalyzed H2 generation.32 Titanium dioxide is the most extensively studied semiconductor for light-induced hydrogen generation due to its advantageous properties.33 Specifically, TiO2 is inexpensive, environmentally friendly, and highly resistant to photodegradation.34,35 Despite these benefits, TiO2 has several drawbacks, including its inability to absorb light in the visible region, its large band gap of 3.2–3.3 eV, and fast electron–hole recombination. All these characteristics inhibit its photocatalytic efficiency towards H2 production.36To address these challenges, dye sensitization has been employed to extend TiO2's light absorption in the visible spectrum.37 In recent years, researchers have explored various methods for utilizing dye-sensitized TiO2 nanoparticles (NPs) to enhance photocatalytic hydrogen evolution.38–40 A widely adopted technique involves anchoring a single dye molecule onto platinum-doped TiO2 nanoparticles (Pt–TiO2 NPs).41 These dye-sensitized photocatalytic systems (DSPs), were first introduced by Shimidzu and collaborators42 and later investigated by Abe et al.43 More recently, Reisner and his research group have made further advancements in DSPs for hydrogen production.44
Our group initialized its contribution in this field via the utilization of metallated tetracarboxy-porphyrins (PdTc3CP, PtTc3CP and ZnTc3CP, Fig. 2) adsorbed onto TiO2 nanoparticles.23 We demonstrated that TiO2 NPs play a dual role, acting both as an electron transport medium and as a scaffold that promotes the self-organization of porphyrins into H- and J-aggregates, which are important for catalytic activity. Notably, PtTc3CP forming J-aggregates demonstrated superior H2 evolution compared to PdTc3CP in H-aggregated form. The most efficient system was achieved by co-adsorbing both porphyrins onto TiO2, resulting in a production rate of 30.2 mmol(H2) g−1 and a turnover number of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 733 vs. PdTc3CP (167 vs. PtTc3CP). Time-resolved transient absorption spectroscopy confirmed that symmetry-breaking charge separation between the immobilized porphyrins initiates photocatalysis. This work highlights the potential of self-organized porphyrin aggregates for efficient solar-driven hydrogen production and offers a simple and scalable method for developing noble metal-free photocatalytic materials.23
733 vs. PdTc3CP (167 vs. PtTc3CP). Time-resolved transient absorption spectroscopy confirmed that symmetry-breaking charge separation between the immobilized porphyrins initiates photocatalysis. This work highlights the potential of self-organized porphyrin aggregates for efficient solar-driven hydrogen production and offers a simple and scalable method for developing noble metal-free photocatalytic materials.23
 | ||
| Fig. 2 Chemical structures of metallated porphyrins equipped with varying anchoring moieties which were applied in DSPs for H2 generation. | ||
In an effort to further improve this activity, our research group presented a novel approach to DSPs for H2 evolution, utilizing porphyrin derivatives as both photosensitizers and catalysts.24 By anchoring metalated porphyrins (MTc3CP and MTCP, Fig. 2) onto Pt–TiO2 NPs, we achieved highly efficient and stable hydrogen production in an aqueous medium. Notably, the platinum-porphyrin derivative Pt–Tc3CP, demonstrated unprecedented stability (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TONs) and a record-high H2 evolution rate (707 mmol g−1 h−1), outperforming previously reported systems. Based on the geometry of their chemical structure, it can be assumed that the M-TCP porphyrins use at most two –COOH moieties to bind onto the NPs, whereas the chemisorption of M-Tc3CP can be achieved via four carboxylic groups.45 Our study highlighted the crucial role of the position of the carboxyl anchoring groups, where the multisided anchoring mode M-Tc3CP exhibited superior organization and performance compared to M-TCP. Additionally, Pt–Tc3CP and Pd–Tc3CP functioned as both PS and CAT, eliminating the need for separate components, while Zn-based derivatives served only as photosensitizers.24
500 TONs) and a record-high H2 evolution rate (707 mmol g−1 h−1), outperforming previously reported systems. Based on the geometry of their chemical structure, it can be assumed that the M-TCP porphyrins use at most two –COOH moieties to bind onto the NPs, whereas the chemisorption of M-Tc3CP can be achieved via four carboxylic groups.45 Our study highlighted the crucial role of the position of the carboxyl anchoring groups, where the multisided anchoring mode M-Tc3CP exhibited superior organization and performance compared to M-TCP. Additionally, Pt–Tc3CP and Pd–Tc3CP functioned as both PS and CAT, eliminating the need for separate components, while Zn-based derivatives served only as photosensitizers.24
Having this knowledge in hand, we continued our investigation on the influence of the photosensitizer anchoring group via studying a series of zinc-trimesityl porphyrin carboxylic acid derivatives as PS in DSPs for H2 evolution.25 In this work the authors synthesized and studied ZnTM(c3COOH)P, ZnTM(pCOOH)P and ZnTM(oCOOH)P (Fig. 2) and adsorbed them onto Pt–TiO2 nanoparticles for photocatalytic testing under white LED irradiation. Among the tested derivatives, Zn-TM(pCOOH)P, with the carboxylic acid in the para-phenyl position, exhibited the highest H2 evolution rate (1959 mmol g−1 h−1) and remarkable stability (2514 TONs), outperforming its ortho-functionalized counterpart, which suffered from steric hindrance preventing effective adsorption. Further comparison with a flexible three-carbon alkyl-chain carboxylated derivative (Zn-TM(c3COOH)P) revealed that the rigid para-positioned carboxyl group enabled superior dye loading and electron injection, leading to enhanced photocatalytic performance. These results underlined the significant role of the PS anchoring group positioning in optimizing porphyrin-based DSPs for solar-driven hydrogen evolution.25
Moving one step forward, our research group employed two chromophore units (BODIPY and porphyrin) as hybrid PS in photocatalytic H2 production DSPs in order to further improve visible light capture.22 Two different structural approaches were investigated: covalent attachment (BDP-ZnP, Fig. 3) and axial coordination (BDP(Im)-ZnP) of the BODIPY moiety to the Zn–porphyrin. The covalently linked dyad (BDP-ZnP) exhibited superior catalytic performance (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TONs) compared to the axially coordinated system (13
500 TONs) compared to the axially coordinated system (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 TONs), highlighting the significance of connectivity mode in optimizing charge transfer and light absorption. Further enhancement was achieved by introducing an additional BDP(Im) moiety to form BDP-ZnP-BDP(Im), which demonstrated the highest efficiency with 18
700 TONs), highlighting the significance of connectivity mode in optimizing charge transfer and light absorption. Further enhancement was achieved by introducing an additional BDP(Im) moiety to form BDP-ZnP-BDP(Im), which demonstrated the highest efficiency with 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 TONs and H2 production rate of 225 mmol g−1 h−1 (Table 1). The photocatalytic performance was consistent with photoelectrochemical measurements, confirming a direct correlation between photocurrent density and H2 evolution. This study established the development of noble-metal-free chromophore dyads in DSPs, mimicking the energy transfer processes observed in natural photosynthesis and paving the way for the design of advanced photocatalytic systems with broader spectral coverage.22
600 TONs and H2 production rate of 225 mmol g−1 h−1 (Table 1). The photocatalytic performance was consistent with photoelectrochemical measurements, confirming a direct correlation between photocurrent density and H2 evolution. This study established the development of noble-metal-free chromophore dyads in DSPs, mimicking the energy transfer processes observed in natural photosynthesis and paving the way for the design of advanced photocatalytic systems with broader spectral coverage.22
 | ||
| Fig. 3 Chemical structures of hybrid PS molecules based on BODIPY and porphyrin units which were applied in photocatalytic H2 production DSPs. | ||
| PS | Activity (mmol g−1 h−1) | TONs vs. PS | Irr. Time (h) Activity/TONs | Publication |
|---|---|---|---|---|
| Pt–Tc3CP | 707 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
24/48 | 24 |
| Pd–Tc3CP | 593 | 7722 | 24 | |
| Zn–Tc3CP | 421 | 1031 | 24 | |
| Pt–TCP | 378 | 2525 | 24 | |
| Pd–TCP | 256 | 1147 | 24 | |
| Zn–TCP | 360 | 1192 | 24 | |
| Zn-TM(pCOOH)P | 1959 | 2514 | 24/6 | 25 |
| Zn-TM(c3COOH)P | 468 | 659 | 24/6 | |
| BDP-ZnP-BDP(Im) | 225 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
72 | 22 |
| BDP-ZnP | 115 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
72 | |
| BDP(Im)-ZnP | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
72 |
3. Self-assembled schemes
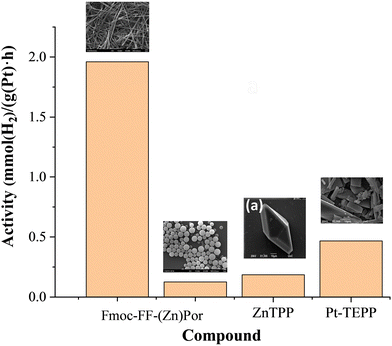
As discussed above, photocatalytic H2 evolution is a promising approach to address both energy and environmental challenges, while porphyrin-based systems play a pivotal role in advancing this field. This section discusses the contribution of our research group in investigating the morphological factors affecting the photocatalytic performance of porphyrin derivatives.The influence of self-assembled porphyrin nanostructures on photocatalytic H2 generation was clearly demonstrated in the work of Nikolaou et al.26 To this end, the authors prepared a peptide-porphyrin hybrid (Fmoc-FF-(Zn)Por) and employed the self-assembled morphologies in photocatalytic HER in combination with Pt nanoparticles as catalytic moieties. The hybrid formed spheres and fibrils under different solvent conditions utilising the “good” and “bad” solvent self-assembly protocol, with fibrils demonstrating superior catalytic performance compared to spheres (Fig. 4a and 5). In detail the fibrils achieved a hydrogen production rate of 1.96 mmol g−1 h−1 and a TON of 155, with remarkable stability over 400 hours. This work highlights the critical influence of nanoscale morphology on photocatalytic activity, showing that the fibrillar structure facilitates improved electron transfer and light-harvesting compared to spherical structure.26 Following the same approach, namely peptide-induced self-assembly of porphyrinoid chromophores, our team employed a peptide nucleic acid (PNA) covalently linked to meso-tetraphenylporphyrin (PNA–TPP) and a boron-dipyrromethene (BODIPY) molecule (PNA–BDP) in order to convey self-assembling properties to the chromophores.31 These hybrid molecules successfully self-assembled into spherical nanostructures with improved light-harvesting ability (Fig. 4b). The resulting nanospheres were applied into a photocatalytic system in combination with Pt nanoparticles as photocatalysts and ascorbic acid as sacrificial electron donor achieving 135.64 nmol H2 generation after 4.5 h of irradiation.31
 | ||
| Fig. 4 Chemical structures and self-assembly motif of (a) Fmoc-FF-(Zn)Por (b) PNA–TPP, (c) Pt-TEPP and (d) SnPy3P-FF. | ||
Continuing the morphology-dependent hydrogen production systems, our research group moved one step forward by eliminating the peptide moiety as a necessity to create porphyrin based self-assemblies.28 In another work Zn(II) tetra-phenyl porphyrin (ZnTPP) was self-assembled into “flower,” octahedral, and “manta ray” structures utilizing the same “good–bad” solvent protocol. Once again, we demonstrated that the photocatalytic activity varied significantly among the different self-assembled morphologies. In detail, octahedral assemblies achieved the highest hydrogen production rate (185.5 μmol g−1 h−1) overcoming the other morphologies by a factor of four. Moreover, the recyclability of the chromophores in these systems paves the way for cost-effective and efficient photocatalytic devices.28 Since the self-assembling morphology derives mainly from the molecular structure design, we explored the role of gadolinium porphyrin double-decker complexes and their mono-porphyrinate counterparts in photocatalytic HER.29 By systematically modifying the meso- and beta-positions of the porphyrin macrocycle, we highlighted how the structural design impacts hydrogen production. The double-decker configuration was found to be essential for photocatalytic activity, with GdH(TPyP)2 demonstrating a TON of 166 over 48 hours under visible light irradiation. Interestingly, the self-assembly of these porphyrins into various morphologies such as flakes, nanospheres and octahedral prisms, did not significantly affect their photocatalytic activity, demonstrating that peripheral substitution is the dominant factor.29
The above reports had one major common aspect, namely the utilisation of Pt nanoparticles as catalytic moiety in combination with the porphyrin nanostructures. Our research team studied another approach, where Pt ions are inserted into the porphyrin macrocycle instead of forming nanoparticles. Towards this aim we investigated Pt-metalated porphyrins (Fig. 4c) for hydrogen evolution in aqueous solutions under visible light with Pt-TEPP being the most efficient photocatalysts.27 The system achieved a hydrogen production rate of 467.3 μmol g−1 h−1 and maintained the porphyrin structural integrity over 25 hours of continuous irradiation. Notably, the Pt-functionalized porphyrin outperformed its free-base counterpart and systems incorporating Pt nanoparticles, owing to enhanced exciton generation, effective charge separation, and optimized morphology. This study demonstrates the potential of simple, molecular-level photocatalysts that combine high efficiency with recyclability.27 Comparison between the best Pt based photocatalytic systems is visualized in Fig. 5. These results clearly indicate that the morphology of the porphyrin photosensitizer as well as the state of the platinum catalytic moiety (Pt2+ in the porphyrin core or Pt nanoparticles decorated on the porphyrin nanostructures) affect significantly the overall catalytic performance.
It is widely recognized that sustainable hydrogen generation requires systems free of noble metals. Our research team moved one step forward by eliminating the necessity of Pt. We integrated the known diphenylalanine dipeptide onto a tripyridyl porphyrin macrocycle and produced a hybrid molecule, SnPy3P-FF, with self-assembling properties towards enhanced photocatalytic hydrogen evolution.30 Metallation with tin proved crucial, enabling the formation of spherical nanostructures (Fig. 4d) which were combined with a known cobaloxime catalyst and demonstrated improved H2 production, that outperformed amorphous aggregates. This work mimics natural photosynthetic systems by employing self-assembling chromophores to harvest and store solar energy efficiently, offering a biomimetic route to advanced photocatalytic applications.30
The main theme across these studies is the integration of molecular design, self-assembly, and metalation strategies to enhance photocatalytic hydrogen evolution. The consistent finding that self-assembly, the metal center and the peripheral modifications can independently or synergistically influence catalytic performance provides a roadmap for developing next-generation photocatalysts. Moreover, the ability to recycle and reconfigure these systems ensures their practical viability for long-term applications. One of the major drawbacks of the above systems is the utilization of a sacrificial reagent for the regeneration of the photosensitizer so future steps should address this issue by eliminating the necessity of a SED. Additionally, other future directions could explore the integration of these systems into device architectures, aiming for enhanced solar-to-fuel conversion efficiencies.
4. Perspectives and future directions
Conventional DSPs typically rely on SEDs such as triethanolamine, BIH, or ascorbic acid to regenerate the oxidized PS (Fig. 6). While this approach is effective, these SEDs raise significant sustainability and scalability concerns due to their cost, toxicity, and the generation of undesirable by-products. Within this context, alcohols have emerged as a mechanistically distinct subclass of sacrificial donors, capable of serving a dual function by enabling both H2 evolution and the formation of value-added oxidation products, such as aldehydes. However, it is worth noting that alcohols are still considered as sacrificial donors in such systems, and their use does not circumvent the thermodynamic limitations inherent to water splitting. Moreover, their sustainability depends on whether they are derived from biomass or fossil sources. Although alcohol oxidation does not constitute an alternative to sacrificial pathways, it represents a mechanistically distinct strategy that enhances atom economy and broadens system utility by enabling the concurrent generation of solar fuel and value-added chemicals within a single photocatalytic platform.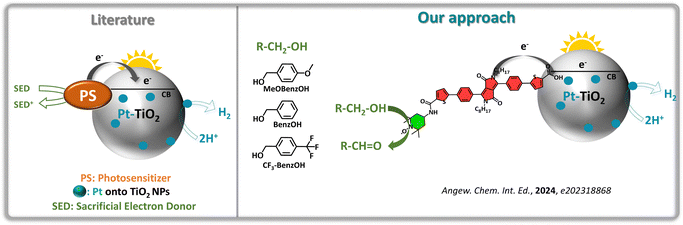 | ||
| Fig. 6 Typical configuration of dye-sensitized photocatalytic systems (DSPs) in the literature, and our approach using a TEMPO-catalyst for alcohol oxidation without the utilization of SED. | ||
Following such an approach, a photosensitizer-catalyst (PS–CAT) dyad is utilized, which enables alcohol oxidation without requiring an external SED for PS regeneration. This strategy not only enhances the sustainability of DSPs but also improves overall catalytic efficiency. Developing DSPs for dual catalysis, simultaneously driving H2 evolution and alcohol oxidation, provides a more sustainable alternative by eliminating toxic SEDs while producing valuable carbonyl compounds as co-products. This strategy enhances both the efficiency and applicability of DSPs, provided that careful consideration is given to substrate selection and system design.
Several studies have already demonstrated the feasibility of incorporating alcohol oxidation into dye-sensitized photoelectrochemical cells (DSPECs) to drive solar fuel production. For instance, Sun and co-workers designed a hybrid system composed of graphitic carbon nitride (g-C3N4), a ruthenium-based oxidation catalyst, and platinum for H2 evolution, achieving selective benzyl alcohol oxidation with enhanced efficiency.46 In addition, a cyanamide-functionalized carbon nitride paired with a nickel(II) bis(diphosphine) catalyst was developed by Reisner and co-workers.47 This system exhibited remarkable activity for both H2 evolution and aldehyde formation, reaching a quantum efficiency of 15%. More recently, Reek and colleagues developed a DSPEC that enables simultaneous H2 production and glycerol oxidation. The mesoporous TiO2 electrode used in their study was functionalized with a thienopyrroledione-derived organic dye as the photosensitizer and a TEMPO catalyst to facilitate glycerol oxidation.48 All the above-mentioned studies highlight the increasing interest in replacing conventional oxidation reactions with value-added transformations that not only boost photocatalytic performance but also promote sustainable chemical synthesis.
While this concept has been explored in DSPECs, its application in DSPs had remained unexplored until our recent work. In collaboration with the research groups of Odobel and Vauthey, we designed a diketopyrrolopyrrole (DPP) dye covalently linked to a TEMPO-based catalyst. This PS–CAT dyad was then anchored onto Pt–TiO2 NPs to achieve simultaneous H2 production and alcohol oxidation to aldehydes (Fig. 6).49 We investigated the conversion of three different alcohols to their corresponding aldehyde counterparts: p-methoxybenzyl alcohol (MeOBenzOH), benzyl alcohol (BenzOH), and p-trifluoromethylbenzyl alcohol (CF3BenzOH). By optimizing key parameters such as dye loading, Pt concentration, and pH, we achieved a maximum H2 evolution rate of 200 μmol h−1 g−1 TiO2 using MeOBenzOH as substrate. However, the DPP dye exhibited gradual photodegradation over time, highlighting the need for more photostable dye structures. Despite this challenge, the sustained H2 evolution under simulated sunlight confirms the potential of our approach for practical solar energy conversion applications.
This feature article presents our advancements in DSPs by introducing three distinct approaches that contribute to a deeper understanding of the field and the rational design of new photocatalytic systems. Specifically, we explored TiO2-based DSPs with porphyrin-based dyads as photosensitizers (PSs), which enhance light absorption and charge transfer efficiency, thereby improving the overall photocatalytic performance for H2 evolution and other reactions. We also explored self-assembled porphyrin nanostructures, which eliminate the need for anchoring groups to attach to semiconductor surfaces. These nanostructures self-organize, enhancing both stability and catalytic activity without the limitations typically associated with conventional anchoring methods. Finally, we introduced a novel PS–CAT dyad that enables the simultaneous oxidation of alcohols and evolution of H2, all without the need for SEDs.
By employing these approaches, we have successfully developed DSPs that not only enhance light absorption and charge transfer efficiency but also provide viable pathways for sustainable fuel and chemical production. Our findings demonstrate that by eliminating the need for SEDs and integrating alternative strategies for alcohol oxidation, we can overcome the limitations of traditional DSPs. This dual approach sets the stage for next-generation photocatalytic systems that combine high efficiency, stability, and real-world applicability in solar fuel and chemical production.
Beyond alcohol oxidation, DSPs are also gaining attention in the field of light-driven carbon dioxide (CO2) reduction, particularly for the conversion of CO2 into valuable C1 and C2+ fuels and several chemicals. The integration of porphyrin-based PSs and CATs for CO2-to-CO and/or CO2-to-formate conversion offers a promising method to broaden the applicability of DSPs. Nevertheless, key challenges still remain, namely limited selectivity, catalyst degradation, and competition from H+ reduction pathways. Our current research focuses on developing stable, selective, and robust porphyrin-based DSPs capable of promoting multi-electron CO2 reduction under solar irradiation, with the long-term goal of enabling efficient and sustainable artificial photosynthetic schemes.
Future efforts should focus on improving the anchoring of the PS–CAT dyad by utilizing more effective anchoring groups, such as phosphonates or silanes, which perform better in basic pH environments.50 Additionally, rather than relying on PS–CAT dyads attached to TiO2 nanoparticles, an alternative approach could involve exploring the self-assembly of the PS–CAT dyad into nanoparticles. This strategy may offer more robust and efficient systems, potentially improving the overall performance and stability of the photocatalytic systems.
Conflicts of interest
There are no conflicts to declare.Data availability
No new data were created or analyzed in this study. All data presented in the figures are derived from previously published sources, which are cited in the manuscript.Acknowledgements
Dr Emmanouil Nikoloudakis gratefully acknowledges the Bodossaki Foundation for financial support. This work was partially supported by the “Hubert Curien 2023 Partnership” programme (DISPHORED).References
- J. Du, D. Xiang, K. Zhou, L. Wang, J. Yu, H. Xia, L. Zhao, H. Liu and W. Zhou, Nano Energy, 2022, 104, 107875 CrossRef CAS.
- A. Rahman, O. Farrok and M. M. Haque, Renewable Sustainable Energy Rev., 2022, 161, 112279 CrossRef.
- S. Martinez-Villarreal, M. Kammoun and A. Richel, Curr. Opin. Green Sustainable Chem., 2023, 39, 100716 CrossRef CAS.
- J. Radhakrishnan, S. Ratna and K. Biswas, Inorg. Chem. Commun., 2022, 145, 109971 CrossRef CAS.
- B.-J. Ng, L. K. Putri, X. Y. Kong, Y. W. Teh, P. Pasbakhsh and S.-P. Chai, Adv. Sci., 2020, 7, 1903171 CrossRef CAS PubMed.
- K. Hojo, S. Nishioka, Y. Miseki, Y. Kamakura, T. Oshima, K. Sayama, T. E. Mallouk and K. Maeda, ACS Appl. Energy Mater., 2021, 4, 10145–10152 CrossRef CAS.
- S. Nishioka, K. Hojo, L. Xiao, T. Gao, Y. Miseki, S. Yasuda, T. Yokoi, K. Sayama, T. E. Mallouk and K. Maeda, Sci. Adv., 2025, 8, eadc9115 CrossRef PubMed.
- L. Thirumalaisamy, Z. Wei, K. R. Davies, M. G. Allan, J. McGettrick, T. Watson, M. F. Kuehnel and S. Pitchaimuthu, ACS Sustainable Chem. Eng., 2024, 12, 3044–3060 CrossRef CAS PubMed.
- J. Abdul Nasir, A. Munir, N. Ahmad, T. Ul Haq, Z. Khan and Z. Rehman, Adv. Mater., 2021, 33, 2105195 CrossRef CAS PubMed.
- Y. Bai, K. Nakagawa, A. J. Cowan, C. M. Aitchison, Y. Yamaguchi, M. A. Zwijnenburg, A. Kudo, R. S. Sprick and A. I. Cooper, J. Mater. Chem. A, 2020, 8, 16283–16290 RSC.
- D. Luo, L. Peng, Y. Wang, X. Lu, C. Yang, X. Xu, Y. Huang and Y. Ni, J. Mater. Chem. A, 2021, 9, 908–914 RSC.
- J. Willkomm, K. L. Orchard, A. Reynal, E. Pastor, J. R. Durrant and E. Reisner, Chem. Soc. Rev., 2016, 45, 9–23 RSC.
- G. Reginato, L. Zani, M. Calamante, A. Mordini and A. Dessì, Eur. J. Inorg. Chem., 2020, 899–917 CrossRef CAS.
- E. Nikoloudakis, I. López-Duarte, G. Charalambidis, K. Ladomenou, M. Ince and A. G. Coutsolelos, Chem. Soc. Rev., 2022, 51, 6965–7045 RSC.
- Y. Zhang, T. Higashino and H. Imahori, J. Mater. Chem. A, 2023, 11, 12659–12680 RSC.
- J. Min Park, J. H. Lee and W.-D. Jang, Coord. Chem. Rev., 2020, 407, 213157 CrossRef.
- Y. Zhang, T. Higashino and H. Imahori, J. Mater. Chem. A, 2023, 11, 12659–12680 RSC.
- P. S. Gangadhar, S. Dasgupta, P. R. Bangal, T. H. Chowdhury, A. Islam and L. Giribabu, ACS Appl. Energy Mater., 2024, 7, 3309–3320 CrossRef CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- J. Zou, Y. Tang, G. Baryshnikov, Z. Yang, R. Mao, W. Feng, J. Guan, C. Li and Y. Xie, J. Mater. Chem. A, 2022, 10, 1320–1328 RSC.
- N. Chanda, M. Naga Rajesh, P. Siva Gangadhar, S. Sk, S. Bojja, L. Giribabu and U. Pal, Fuel, 2024, 378, 132854 CrossRef CAS.
- V. Nikolaou, G. Charalambidis, G. Landrou, E. Nikoloudakis, A. Planchat, R. Tsalameni, K. Junghans, A. Kahnt, F. Odobel and A. G. Coutsolelos, ACS Appl. Energy Mater., 2021, 4, 10042–10049 CrossRef CAS.
- V. Nikolaou, G. Charalambidis, K. Ladomenou, E. Nikoloudakis, C. Drivas, I. Vamvasakis, S. Panagiotakis, G. Landrou, E. Agapaki, C. Stangel, C. Henkel, J. Joseph, G. Armatas, M. Vasilopoulou, S. Kennou, D. M. Guldi and A. G. Coutsolelos, ChemSusChem, 2021, 14, 961–970 CrossRef CAS PubMed.
- V. Nikolaou, E. Agapaki, E. Nikoloudakis, K. Achilleos, K. Ladomenou, G. Charalambidis, E. Triantafyllou and A. G. Coutsolelos, Chem. Commun., 2023, 59, 11256–11259 RSC.
- E. Agapaki, K. Ladomenou, V. Nikolaou and A. G. Coutsolelos, J. Porphyrins Phthalocyanines, 2023, 27, 479–489 CrossRef CAS.
- V. Nikolaou, G. Charalambidis and A. G. Coutsolelos, Chem. Commun., 2021, 57, 4055–4058 RSC.
- E. Orfanos, K. Ladomenou, P. A. Angaridis, T. Papadopoulos, G. Charalambidis, M. Vasilopoulou and A. G. Coutsolelos, Sustainable Energy Fuels, 2022, 6, 5072–5076 RSC.
- E. Orfanos, K. Ladomenou, P. Angaridis and A. G. Coutsolelos, Dalton Trans., 2022, 51, 8009–8014 RSC.
- E. Nikoloudakis, G. Charalambidis, M. Vasila, E. Orfanos, P. Angaridis, G. A. Spyroulias and A. G. Coutsolelos, Polyhedron, 2021, 208, 115421 CrossRef CAS.
- E. Nikoloudakis, M. Pigiaki, M. N. Polychronaki, A. Margaritopoulou, G. Charalambidis, E. Serpetzoglou, A. Mitraki, P. A. Loukakos and A. G. Coutsolelos, ACS Sustainable Chem. Eng., 2021, 9, 7781–7791 CrossRef CAS.
- E. Nikoloudakis, K. Karikis, J. Han, C. Kokotidou, A. Charisiadis, F. Folias, A. M. Douvas, A. Mitraki, G. Charalambidis, X. Yan and A. G. Coutsolelos, Nanoscale, 2019, 11, 3557–3566 RSC.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- G. K. Naik and Y. Ahn, Int. J. Hydrogen Energy, 2022, 47, 32121–32132 CrossRef CAS.
- G. L. Chiarello, M. V. Dozzi and E. Selli, J. Energy Chem., 2017, 26, 250–258 CrossRef.
- F. Bhom and Y. M. Isa, Global Challenges, 2024, 8, 2400134 CrossRef PubMed.
- V. Etacheri, C. Di Valentin, J. Schneider, D. Bahnemann and S. C. Pillai, J. Photochem. Photobiol., C, 2015, 25, 1–29 CrossRef CAS.
- N. Manfredi, M. Monai, T. Montini, M. Salamone, R. Ruffo, P. Fornasiero and A. Abbotto, Sustainable Energy Fuels, 2017, 1, 694–698 RSC.
- M. A. Gross, A. Reynal, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2014, 136, 356–366 CrossRef CAS PubMed.
- S. Gonuguntla, R. Kamesh, U. Pal and D. Chatterjee, J. Photochem. Photobiol., C, 2023, 57, 100621 CrossRef CAS.
- M. Rafique, S. Hajra, M. Irshad, M. Usman, M. Imran, M. A. Assiri and W. M. Ashraf, ACS Omega, 2023, 8, 25640–25648 CrossRef CAS PubMed.
- J. Li, Y. E. L. Lian and W. Ma, Int. J. Hydrogen Energy, 2013, 38, 10746–10753 CrossRef CAS.
- T. Shimidzu, T. Iyoda and Y. Koide, J. Am. Chem. Soc., 1985, 107, 35–41 CrossRef CAS.
- R. Abe, K. Hara, K. Sayama, K. Domen and H. Arakawa, J. Photochem. Photobiol., A, 2000, 137, 63–69 CrossRef CAS.
- J. Willkomm, K. L. Orchard, A. Reynal, E. Pastor, J. R. Durrant and E. Reisner, Chem. Soc. Rev., 2016, 45, 9–23 RSC.
- A. S. Hart, C. B. Kc, H. B. Gobeze, L. R. Sequeira and F. D’Souza, ACS Appl. Mater. Interfaces, 2013, 5, 5314–5323 CrossRef CAS PubMed.
- F. Li, Y. Wang, J. Du, Y. Zhu, C. Xu and L. Sun, Appl. Catal., B, 2018, 225, 258–263 CrossRef CAS.
- H. Kasap, C. A. Caputo, B. C. M. Martindale, R. Godin, V. W. Lau, B. V. Lotsch, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2016, 138, 9183–9192 CrossRef CAS PubMed.
- D. F. Bruggeman, A. A. H. Laporte, R. J. Detz, S. Mathew and J. N. H. Reek, Angew. Chem., Int. Ed., 2022, 61, e202200175 CrossRef CAS PubMed.
- D. Romito, C. Govind, V. Nikolaou, R. J. Fernández-Terán, A. Stoumpidi, E. Agapaki, G. Charalambidis, S. Diring, E. Vauthey, A. G. Coutsolelos and F. Odobel, Angew. Chem., Int. Ed., 2024, 63, e202318868 CrossRef CAS PubMed.
- B. J. Brennan, M. J. Llansola Portolés, P. A. Liddell, T. A. Moore, A. L. Moore and D. Gust, Phys. Chem. Chem. Phys., 2013, 15, 16605–16614 RSC.
| This journal is © The Royal Society of Chemistry 2025 |