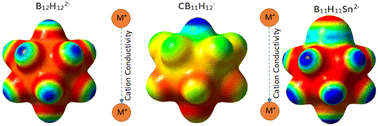
Metal substituted dodecaborate anions can be coupled with alkali metal cations to have great potential as solid-state ion conductors for battery applications. A tin atom can replace a B–H unit within an unsubstituted dodecaborate cage to produce a stable, polar divalent anion. The chemical and structural change in forming a stannaborate results in a modified crystal structure of respective group 1 metal salts, and as a result, improves the material's ion conductivity. Li2B11H11Sn shows high ion conductivity of ∼8 mS cm−1 at 130 °C, similar to the state-of-the-art LiCB11H12 at these temperatures, however, obtaining high ion conductivity at room temperature is not possible with pristine alkali metal stannaborates.