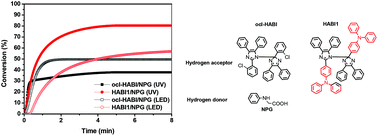
In this study, three triphenylamine-based hexaarylbiimidazole (HABI) derivatives featuring different numbers of methoxy groups (none for HABI1, two for HABI2, and four for HABI3) have been synthesized. For comparison, a common hydrogen acceptor photoinitiator, ocl-HABI (2-chlorohexaarylbiimidazole), was also applied to compare the corresponding properties. The new HABIs displayed a more red-shifted and higher molar extinction coefficient than ocl-HABI with the absorption region extended to visible light. A larger free radical concentration of HABIs was also achieved, which was confirmed via electron paramagnetic resonance (ESR) spectroscopy. These visible light-sensitive HABIs or ocl-HABI in combination with a suitable hydrogen donor, i.e., N-phenyl glycine (NPG) were utilized as photoinitation systems for conventional radical polymerization (RDRP). All the packages exhibited good electron-transfer ability by the calculation of the free energy changes (ΔGET). The HABI1/NPG system exhibited the best double bond conversion efficiency and outperformed the corresponding ocl-HABI/NPG-based formulation under similar UV light source testing conditions. We then employed the HABI1/NPG and ocl-HABI/NPG systems for LED light source testing. Again, HABI1/NPG had better photocuring conversion efficiency and shorter time at maximum heat flow than ocl-HABI/NPG, indicating that the HABI1/NPG system can be applied to light curing applications under different light sources, such as UV and LED.