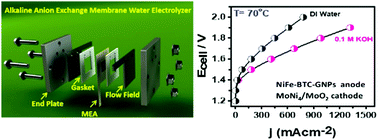
Practical hydrogen production using high-efficiency, low-cost, and stable oxygen electrodes is crucial for a sustainable clean energy future. Herein we report a graphene-nanoplatelets-supported (Ni,Fe) metal–organic framework (MOF) as a superior and ultra-durable (>1000 h) anode for alkaline water electrolysis. The MOF on carbon-fiber paper electrodes requires an overpotential η = 220 mV to achieve a current density j = 10 mA cm−2 (η = 180 mV on nickel foam for j = 20 mA cm−2) with a Tafel slope of 51 mV per decade, high turnover frequency (1.22 s−1), high faradaic efficiency (99.1%), and long-term durability of >1000 h in continuous electrolysis. In an alkaline anion exchange membrane water electrolyzer (AAEMWE), it exhibits a record current density of 540 mA cm−2 at 1.85 V at 70 °C, outperforming the state-of-the-art Pt/C//IrO2. A breakthrough strategy introduced in membrane electrode assembly fabrication by extending the electrical contact with an aqueous electrolyte offers an additional OH− transport pathway to regenerate the original conductivity of the AAEMWE in continuous electrolysis, without any significant change in the pH of the electrolyte. These findings open up durable, high-performance AAEMWE and direct solar-to-fuel conversion, especially to replace high-cost proton exchange membrane water electrolysis that already works with ultra-pure water.