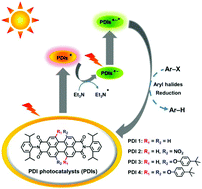
The consecutive photoexcitation of perylene diimide (PDI) produces the excited doublet state of its radical anion (PDI˙−*), which is demonstrated to be a strong photoreductant for difficult-to-reduce substrates, such as aryl halides. Reported herein is a molecular structural modification of perylene diimides (PDIs) by the introduction of two electron-withdrawing nitro groups (PDI 2) as well as two and four electron-donating 4-tert-butylphenoxyl groups (PDI 3 and PDI 4) at the bay positions to explore the substituent effects on their photocatalytic performances. Similar to bay-unsubstituted PDI 1, all bay-substituted PDIs are easily photoreduced to their radical anions (PDIs˙−). However, the subsequent photoexcitation of these obtained PDIs˙− leads to remarkable substituent-dependent activities for the reductive dehalogenations of aryl halides: higher conversion efficiencies for PDI 3 with electron-donating substituents at the bay positions (e.g., from 74% for PDI 1 to 95% for PDI 3) but almost no conversions for PDI 2 with electron-withdrawing groups, which are supposed to be controlled by their excited-state reduction potentials and SOMO−1 energies. The structure–property relationships established here will guide the applications of bay-substituted PDIs for photoredox aryl halide reduction and other small molecule transformations.