Yang Hu, Xuguang Yin, Ziyi Chen, Xiu-Qin Dong and Xumu Zhang
Org. Chem. Front., 2018,5, 2000-2003
DOI:
10.1039/C8QO00307F,
Research Article
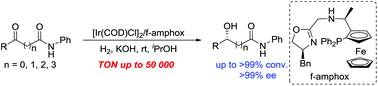
Asymmetric synthesis of chiral hydroxy amides has been successfully accomplished by enantioselective catalytic hydrogenation of various prochiral α-, β-, γ-, δ-keto amides in the presence of an Ir/f-amphox catalyst with excellent results (up to >99% conversion and >99% ee). Furthermore, the asymmetric hydrogenation of 5-(4-fluorophenyl)-5-oxo-N-phenylpentanamide was found to proceed smoothly with only 0.002 mol% (S/C = 50 000) catalyst. The hydrogenation product was obtained with >99% conversion and >99% ee, and which is an important structural framework of the anti-hyperlipidemic drug Ezetimibe.