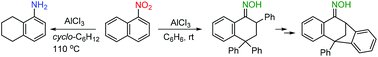Zhongwei Zhu, Alexander M. Genaev, George E. Salnikov and Konstantin Yu. Koltunov
Org. Biomol. Chem., 2018,16, 9129-9132
DOI:
10.1039/C8OB02653J,
Communication
1-Nitronaphthalene smoothly reacts with benzene and undergoes selective reduction with cyclohexane in the presence of aluminum chloride to give 2,4,4-triphenyl-3,4-dihydronaphthalen-1(2H)-one oxime and 5,6,7,8-tetrahydro-1-naphthylamine, respectively. The mechanistic aspects of these and related reactions are discussed on the basis of DFT, providing insight into the protonation behavior of 1-nitronaphthalene coordinated to AlCl3.