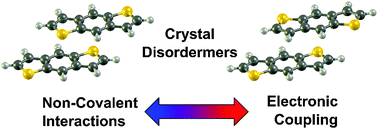
Many high performing organic semiconductor materials contain heteroaromatic rings in order to control the molecular packing and material electronic properties. Here we use a combination of density functional theory and symmetry-adapted perturbation theory calculations to explore the intermolecular noncovalent interactions, which guide solid-state molecular packing, and electronic couplings in a series of benzodithiophene-based dimer models. A novel concept, termed the disordermer, is introduced to delineate how the reduced molecular symmetry of benzodithiophene, when compared to the more highly symmetric anthracene molecule, can present intermolecular isomerism in the solid state that results in a wide range of available molecular packing arrangements that in turn influence the magnitudes of the electronic couplings. The insight developed through the investigation of these disordermers is demonstrated to hold important implications in the design of new generations of organic semiconductor materials.