Jian-Xing Xu, Fei Ye, Xing-Feng Bai, Jin Zhang, Zheng Xu, Zhan-Jiang Zheng and Li-Wen Xu
RSC Adv., 2016,6, 45495-45502
DOI:
10.1039/C6RA09657C,
Paper
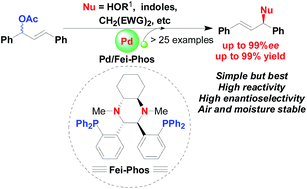
To shed light on the scope and limitations of palladium-catalyzed allylic alkylation in the presence of chiral trans-1,2-diaminocyclohexane-derived Fei-Phos as an effective phosphine ligand, the asymmetric palladium-catalysed alkylation of structurally diverse hard/soft nucleophiles, including allylic etherification of alcohols and the allylic alkylation of activated methylene compounds, indoles, and aromatic amines were investigated in this study; the corresponding products with various functional groups were achieved in good yield and with high enantioselectivity (up to 99% ee).