Synthesis, crystal and electronic structure, and optical properties of two new chalcogenide-iodides: Ba3Q4I2 (Q = S, Se)†
Jian
Wang
,
Kathleen
Lee
and
Kirill
Kovnir
*
Department of Chemistry, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA. E-mail: kkovnir@ucdavis.edu
First published on 11th December 2015
Abstract
Two new ternary chalcogenide-iodides, Ba3S4I2 and Ba3Se4I2, were synthesized through high-temperature solid-state reactions, and their structures were determined via single-crystal X-ray diffraction. Both compounds are isostructural and crystallize in the monoclinic space group C2/c (no. 15) with unit cell parameters of a = 14.507(4)/15.080(7) Å, b = 10.104(3)/10.400(5) Å, c = 8.206(2)/8.383(4) Å, β = 101.847(4)/103.206(8)°, and Z = 4 for Ba3S4I2/Ba3Se4I2, respectively. The crystal structure of Ba3Q4I2 (Q = S, Se) is constructed from Q22− dumbbells and isolated I1− anions, which are surrounded by Ba2+ cations. According to UV/Vis spectroscopy, Ba3S4I2 is a semiconductor with a bandgap of 2.45(5) eV. Quantum-chemical calculations predict that Ba3S4I2 and Ba3Se4I2 are wide bandgap semiconductors with bandgaps of 2.50 and 2.06 eV, respectively. Electron localization function analysis of chemical bonding indicates covalent interactions in the Q22− dumbbells.
Introduction
Metal chalcogenide-halides exhibit unique structural chemistry and properties that are different from both metal chalcogenides and metal halides. The recent discoveries of giant Rashba-type splitting in BiTeI,1,2 spin density waves in La2TeI2,3 high ionic conductivity and excellent thermoelectric properties in Ag–Te–X compounds (X = Cl, Br) are just few examples of the diversity in metal chalcogenide-halides.4–8 In contrast to the ternary rare-earth- or transition metal-chalcogenide-halides, no ternary alkaline-earth-containing sulfide- or selenide-halides have been reported.9–11 Only a few quaternary or higher order compounds have been reported in literature, such as Ba4Fe2S4I5,12 Ca2LaGeS4Cl3,13 and ABa3Ga5Se10Cl2 (A = Cs, Rb, K).14 Systematic investigations of the alkaline-earth metal–chalcogen–halogen systems may reveal new compounds and further rationalize the relationship between their structures and properties. In the current work, we report the first examples of two ternary compounds, Ba3S4I2 and Ba3Se4I2, which were synthesized through high-temperature reactions of elements. Detailed characterizations of the crystal structure, optical properties, and the electronic structure and bonding are reported.Experimental
Synthesis
All preparation processes were handled in an argon-filled glovebox with the O2 level <1 ppm. All starting materials were commercial grade and used as received: Ba (Sigma Aldrich, 99.9%), I2 (Alfa Aesar, resublimed crystals, 99.9985%), S (Alfa Aesar, pieces, 99.999%), and Se (Alfa Aesar, powder 99.999%).Polycrystalline Ba3Q4I2 (Q = S, Se) samples were synthesized via two-step high-temperature reactions. In the first step, the reactants were loaded in stoichiometric ratios Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Q
Q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = 3
I = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 into carbonized silica ampoules, evacuated, and flame-sealed. The ampoules were placed in muffle furnaces and heated from room temperature to 1073 K over a period of 20 hours, and then annealed at this temperature for 20 h, after which the furnace was turned off and the ampoules were cooled to room temperature. In the second step, the ampoules were opened in a glovebox, ground, resealed inside evacuated carbonized silica ampoules, and heated to 1073 K with the same heating rate, and annealed at 1073 K for an extended period of time, 144 h. The polycrystalline Ba3S4I2 sample has a light yellow color, while Ba3Se4I2 is dark red. The Ba3S4I2 sample was almost phase pure with a tiny admixture of BaS according to powder X-ray diffraction (Fig. S1†), while the Ba3Se4I2 samples always contained admixtures of mainly BaSe and BaSe2. All our attempts to synthesize single-phase samples of Ba3Se4I2 by varying the temperature profile were unsuccessful. Both Ba3Se4I2 and Ba3Se4I2 are highly air and moisture sensitive, and will decompose in a few minutes under exposure to ambient atmosphere. Long time (2–3 months) storage of Ba3S4I2 sample in the glovebox also resulted in partial sample decomposition.
2 into carbonized silica ampoules, evacuated, and flame-sealed. The ampoules were placed in muffle furnaces and heated from room temperature to 1073 K over a period of 20 hours, and then annealed at this temperature for 20 h, after which the furnace was turned off and the ampoules were cooled to room temperature. In the second step, the ampoules were opened in a glovebox, ground, resealed inside evacuated carbonized silica ampoules, and heated to 1073 K with the same heating rate, and annealed at 1073 K for an extended period of time, 144 h. The polycrystalline Ba3S4I2 sample has a light yellow color, while Ba3Se4I2 is dark red. The Ba3S4I2 sample was almost phase pure with a tiny admixture of BaS according to powder X-ray diffraction (Fig. S1†), while the Ba3Se4I2 samples always contained admixtures of mainly BaSe and BaSe2. All our attempts to synthesize single-phase samples of Ba3Se4I2 by varying the temperature profile were unsuccessful. Both Ba3Se4I2 and Ba3Se4I2 are highly air and moisture sensitive, and will decompose in a few minutes under exposure to ambient atmosphere. Long time (2–3 months) storage of Ba3S4I2 sample in the glovebox also resulted in partial sample decomposition.
X-ray powder diffraction
Powder X-ray diffraction (XRD) was carried out on a Rigaku Miniflex 600 diffractometer employing Cu-Kα radiation. Air-sensitive holders with Be or Kapton windows were used to prevent the decomposition of the samples during data collection.Single crystal X-ray diffraction
Due to the air-sensitive nature of Ba3Q4I2 (Q = S, Se), crystal selection and cutting were performed inside the glove box. Suitable crystals were quickly transferred to the dry nitrogen flow in the single crystal X-ray diffractometer. The datasets were collected at 90 K under a N2 stream using a Bruker AXS SMART diffractometer with Mo-Kα radiation and an APEX-II CCD detector. The datasets were recorded as ω-scans with a 0.4° step width and integrated with the Bruker SAINT software package.15 Multi-scan absorption corrections were applied.15 The solutions and refinements of the crystal structures were carried out using the SHELX suite of programs.16 The final refinements were performed using anisotropic atomic displacement parameters for all atoms. A summary of pertinent information related to unit cell parameters, data collection, and refinements is provided in Table 1 and the atomic parameters and interatomic distances are provided in Tables 2 and 3. Further details of the crystal structure determination may be obtained from Fachinformations-zentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen, Germany, by quoting the depository numbers CSD-430355 (Ba3S4I2) and CSD-430356 (Ba3Se4I2).| Empirical formula | Ba3S4I2 | Ba3Se4I2 |
|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|; wR2 = [∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]]1/2, and w = 1/[σ2Fo2 + (A·P)2 + B·P], P = (Fo2 + 2Fc2)/3; A and B are weight coefficients. | ||
| Formula weight | 794.06 g mol−1 | 947.00 g mol−1 |
| CSD number | 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 355 355 |
430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 356 |
| Temperature | 90(2) K | |
| Radiation, wavelength | Mo-Kα, 0.71073 Å | |
| Crystal system | Monoclinic | |
| Space group | C2/c (no. 15) | |
| Unit cell dimensions | a = 14.507(4) Å | a = 15.080(7) Å |
| b = 10.104(3) Å | b = 10.400(5) Å | |
| c = 8.206(2) Å | c = 8.383(4) Å | |
| β = 101.847(4)° | β = 103.206(8)° | |
| Unit cell volume | 1177.3(5) Å3 | 1280.0(11) Å3 |
| Z | 4 | |
| Density (calc.) | 4.48 g cm−3 | 5.09 g cm−3 |
| Absorption coefficient | 15.81 mm−1 | 25.27 mm−1 |
| Goodness-of-fit | 1.07 | 1.05 |
| Final R indicesa [I > 2σ(I)] | R 1 = 0.029 | R 1 = 0.040 |
| wR2 = 0.051 | wR2 = 0.075 | |
| Final R indicesa [all data] | R 1 = 0.040 | R 1 = 0.057 |
| wR2 = 0.056 | wR2 = 0.083 | |
| Atom | Wyckoff | x/a | y/b | z/c | S.O.F. |
U
eq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (Å2) a (Å2) |
|---|---|---|---|---|---|---|
| a U eq is defined as one third of the trace of the orthogonalized Uijtensor. | ||||||
| Ba 3 S 4 I 2 | ||||||
| Ba1 | 8f | 0.32778(2) | 0.08927(3) | 0.25914(3) | 1 | 0.00547(7) |
| Ba2 | 4e | 0 | 0.17704(4) | 1/4 | 1 | 0.00585(9) |
| I1 | 8f | 0.36677(2) | 0.40252(3) | 0.07541(4) | 1 | 0.00734(8) |
| S1 | 8f | 0.20128(8) | 0.29103(12) | 0.3998(2) | 1 | 0.0073(2) |
| S2 | 8f | 0.46625(8) | 0.0928(1) | 0.0035(2) | 1 | 0.0067(2) |
| Ba 3 Se 4 I 2 | ||||||
| Ba1 | 8f | 0.32777(5) | 0.09615(7) | 0.25626(9) | 1 | 0.0114(2) |
| Ba2 | 4e | 0 | 0.1639(1) | 1/4 | 1 | 0.0119(3) |
| I1 | 8f | 0.36657(6) | 0.40283(8) | 0.0708(1) | 1 | 0.0137(2) |
| Se1 | 8f | 0.19701(8) | 0.2952(1) | 0.3862(2) | 1 | 0.0115(3) |
| Se2 | 8f | 0.46253(8) | 0.1015(1) | −0.0002(2) | 1 | 0.0113(3) |
| Atom pair | Distance (Å) | Atom pair | Distance (Å) |
|---|---|---|---|
| Ba 3 S 4 I 2 | |||
| Ba1–S1 | 3.119(1) | Ba2–S1 × 2 | 3.145(1) |
| Ba1–S1 | 3.156(1) | S2 × 2 | 3.224(1) |
| Ba1–S1 | 3.276(1) | I1 × 2 | 3.5136(8) |
| Ba1–S2 | 3.128(1) | I1 × 2 | 3.6921(7) |
| Ba1–S2 | 3.188(1) | S1–S1 | 2.107(2) |
| Ba1–S2 | 3.215(1) | S2–S2 | 2.121(2) |
| Ba1–I1 | 3.5111(8) | ||
| Ba1–I1 | 3.6003(8) | ||
| Ba 3 Se 4 I 2 | |||
| Ba1–Se1 | 3.216(2) | Ba2–Se1 × 2 | 3.232(2) |
| Ba1–Se1 | 3.303(2) | Se2 × 2 | 3.347(2) |
| Ba1–Se1 | 3.341(2) | I1 × 2 | 3.506(2) |
| Ba1–Se2 | 3.260(2) | I1 × 2 | 3.773(2) |
| Ba1–Se2 | 3.279(2) | Se1–Se1 | 2.384(3) |
| Ba1–Se2 | 3.351(2) | Se2–Se2 | 2.394(3) |
| Ba1–I1 | 3.526(2) | ||
| Ba1–I1 | 3.654(2) | ||
Diffuse reflectance spectroscopy
UV-Vis diffuse reflectance spectra of Ba3S4I2 were recorded using a Thermo Scientific Evolution 220 Spectrometer equipped with an integrating sphere. The reflectance data were converted to the Kubelka–Munk function, f(R) = (1 − R)2(2R)−1. The sample was sealed inside a polypropylene bag under Ar atmosphere to prevent exposure to ambient conditions. An empty polypropylene bag was used for the baseline scan.Quantum-chemical calculations
The electronic structure calculations and bonding analyses were carried out using the tight binding, linear muffin-tin orbital, atomic sphere approximation (TB–LMTO–ASA) program.17 The Barth–Hedin exchange potential was employed for the LDA calculations. The radial scalar-relativistic Dirac equation was solved to obtain the partial waves. A basis set containing Ba(6s,5d,4f), I(5p), and S(3s,3p)/Se(4s,4p) orbitals with downfolded Ba(6p) and I(6s,5d) orbitals were used for Ba3S4I2 and Ba3Se4I2, respectively. The density of states (DOS), band structure, and electron localization function (ELF, η)18–21 were calculated after converging the total energies on a k-mesh grid with 12 × 12 × 12 points with 476 irreducible k-points. The Paraview program was used to visualize the ELF isosurfaces.22Results and discussion
Structure description
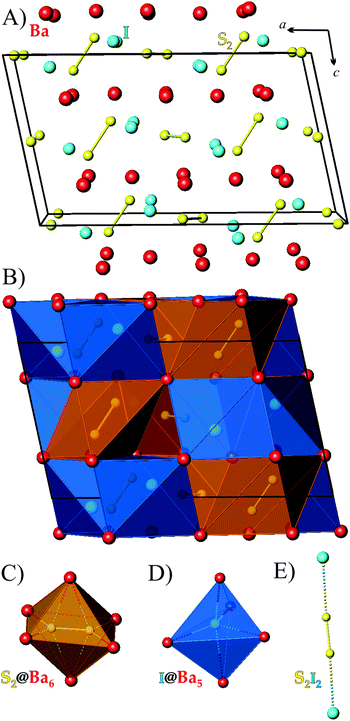
Both title compounds are isostructural and crystallize in the monoclinic space group C2/c (no. 15). For clarity only the crystal structure of Ba3S4I2 is discussed (Fig. 1).In the asymmetric unit of Ba3S4I2 there are two Ba, one I, and two S sites that are crystallographically independent. All of the sulfur atoms are paired forming S22− dumbbells (Fig. 1). Each sulfur atom is additionally coordinated by four Ba atoms forming a distorted tetrahedron. Two such tetrahedra share a common edge in such a way that the S22− dumbbells reside inside distorted Ba6 octahedra (orange polyhedra in Fig. 1B and C). Each iodine atom is surrounded by five Ba atoms forming a distorted trigonal bipyramid (blue polyhedra in Fig. 1B and D). Both types of Ba atoms are surrounded by a distorted capped trigonal prisms composed of eight or nine atoms: Ba1 is surrounded by six sulfur atoms and three I atoms and Ba2 is coordinated to four sulfur atoms and four I atoms (Fig. S2†).
In Ba3S4I2, the S–S distances are 2.107(2) and 2.121(2) Å, which are close to the sum of the corresponding Pauling radius of S (1.05 Å)23 and comparable to S–S distances in barium polysulfides, such as BaS2 (2.12 Å),24 BaS3 (2.08 Å),25 Ba3Sb2S7 (2.09 Å),26 and Sr6Sb6S17 (2.09 Å).27 The Ba–S distances in Ba3S4I2 (3.11–3.28 Å) are similar to the distances found in Ba3Sb2S7 (3.14–3.35 Å),26 BaSb2S4 (3.13–3.35 Å),28 and Ba4Fe2S4I5 (3.20–3.30 Å).12 The Ba–I distances (3.51–3.69 Å) are similar to the Ba–I distances in Ba4Fe2S4I5, which contains I atoms that are coordinated by four or five Ba atoms (3.51–3.73 Å).12
The bond distances in Ba3Se4I2 exhibit similar trends as those in Ba3S4I2 (Table 3). The Se–Se bonds in the Se22− dimers, 2.384(3) and 2.394(3) Å, are much longer than the S–S bonds. These distances are close to the sum of the corresponding Pauling radius of Se (1.20 Å)23 and comparable to Se–Se distances in elemental selenium and other reported compounds: 2.45 Å in Se1,29 2.36 Å in Se6,30 2.33–2.35 Å in Se8,31 2.37 Å in Ba3Sb2Se7,26 and 2.37–2.42 Å in Ba4Sb4Se11.32 The Ba–Se distances in Ba3Se4I2, 3.22–3.35 Å, are similar to the distances in various Ba selenides, such as 3.29 Å in BaSe,33 3.30–3.56 Å in Ba2Sb2Se5,34 3.29–3.41 Å in Ba4Sb4Se11,32 and 3.26–3.60 Å in BaSb2Se4.35 The Ba–I distances in Ba3Se4I2, 3.51–3.77 Å, are longer than those in Ba3S4I2, but are comparable to distances found in Ba3SiI2 (3.54–3.81 Å),36 Ba3GeI2 (3.55–3.81 Å),36 and Ba4Fe2S4I5 (3.51–3.73 Å).12
The shortest Q–I distances are nearly identical in both compounds with distances of 3.55 Å (Q = S) and 3.56 Å (Q = Se). Half of the Q22− dumbbells are coordinated by iodine atoms forming almost linear Q2I2 fragments with angles of ∠I–Q–Q of 172.0° (Q = S) and 170.4° (Q = Se) (Fig. 1E), while the remaining Q22− dumbbells are coordinated by Ba atoms only.
Ba3Q4I2 (Q = S, Se) are the first reported ternary mixed anions alkaline-earth sulfide- or selenide-halides. The isolated I1− anions combined with Q22− dumbbells, which have intrinsic covalent interactions, compensate the positive charge of the Ba2+ cations. Ba3Q4I2 compounds exhibit two distict types of chemical bonding: covalent bonding in the Q22− dumbbells and ionic bonding between cations and anions. Assuming +2 and −1 formal oxidation states for Ba and I, respectively, and a −1 oxidation state for all chalcogen atoms in the Q2 dumbbells, total electroneutrality is achieved: (Ba2+)3(Q22−)2(I1−)2. The ionic interactions among the Ba2+, I−, and S22− ions result in a charge-balanced compound making Ba3S4I2 a large band gap semiconductor, which was predicted from electronic structure calculations. Optical measurements and the translucent yellow color of Ba3S4I2 further support such a description. Mercury-thallium chalcogenides with compositions similar to the title compounds, Hg3Q4Tl2, also crystallize in C2/c space group. In these compounds, the chalcogen atoms are well separated from each other as Q2− anions and instead of I1− anions the Tl1+ cations are present, maintaining total electroneutrality as (Hg2+)3(Q2−)4(Tl1+)2.37
UV–Vis diffuse-reflectance spectroscopy
Solid-state UV-Visible Kubelka–Munk (KM) diffuse reflectance spectroscopy was employed to determine the bandgap of Ba3S4I2. The UV–Vis spectrum exhibits apparent absorption edges around 490 nm (Fig. 2). According to the Tauc plots, the indirect and direct band gaps of Ba3S4I2 are 2.45(5) and 2.6(1) eV, respectively (Fig. 2, bottom). The calculated value of the direct bandgap depends on the range selected for the linear fit, which resulted in high standard deviations. This result is in good agreement with the observed yellow-translucent color and calculated direct bandgap of 2.50 eV (vide infra). The combination of spectroscopic and computational approaches indicates that Ba3S4I2 is a wide bandgap semiconductor.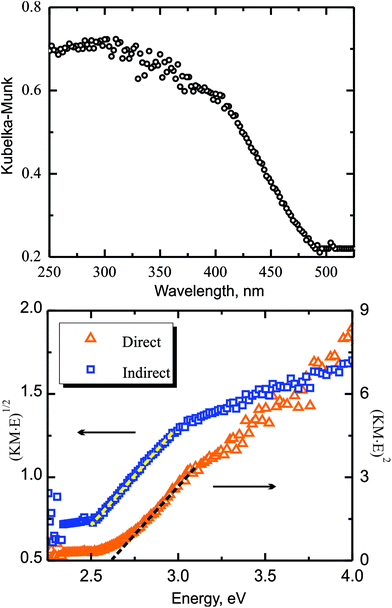 | ||
| Fig. 2 (Top) Solid-state UV-Visible Kubelka–Munk diffuse reflectance spectrum of Ba3S4I2. (Bottom) Tauc plots for allowed direct (orange triangles) and indirect (blue squares) transitions. | ||
Electronic structure
Quantum chemical calculations show that both compounds are wide bandgap semiconductors with bandgaps of 2.50 and 2.06 eV for Ba3S4I2 and Ba3Se4I2, respectively (Fig. 3, left). Calculations suggest that Ba3S4I2 is a direct bandgap semiconductor, while Ba3Se4I2 is an indirect bandgap semiconductor. Ba3S4I2 has direct bandgaps of 2.50 eV at both the L and Z points of the Brillouin zone. Unlike Ba3S4I2, Ba3Se4I2 has an indirect bandgap of 2.06 eV with a direct bandgap of slightly higher energy (2.18 eV) at the V point. The direct transitions for Ba3Se4I2 at the L, Z, and Γ points of 2.20, 2.27, and 2.26 eV, respectively, are very similar in energy to the energy difference at the V point.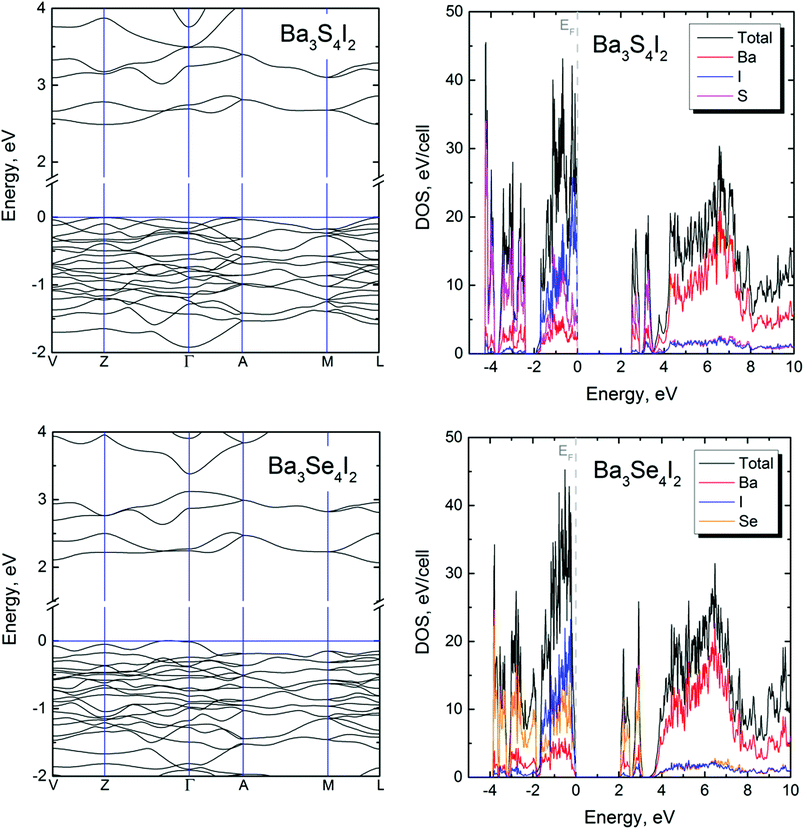 | ||
| Fig. 3 Band structures (left) and density of states (DOS) (right) for Ba3S4I2 (top) and Ba3Se4I2 (bottom). | ||
Elemental projections of the density of states diagram (Fig. 3, right) indicate that for both compounds, the states near the top of the valence band are composed from orbital contributions from all three elements, with dominating contributions from S (or Se) and I. Contributions from Ba orbitals dominate in the states at the conduction band at energies >+4 eV. The bottom of the conduction band is composed of two sharp peaks, which are essentially S (or Se) orbitals with minimal contributions from Ba and I.
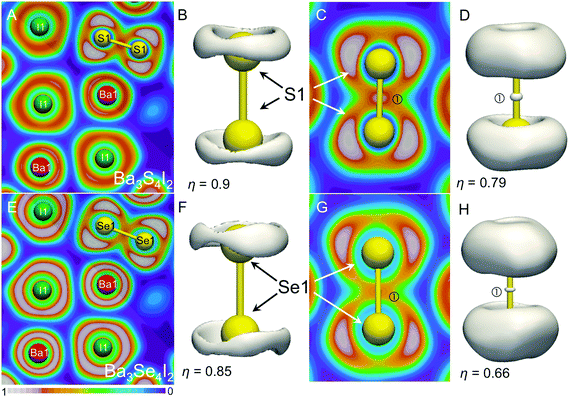
Chemical bonding in Ba3Q4I2 was analyzed with the help of electron localization function (ELF) analysis. The bonding analysis confirmed the expected covalent Q–Q bonding for the Q2 dumbbells (attractor ①) with torus-like attractors on the terminal Q atoms (Fig. 4). Similar torus-like distributions for ELF were found for the S22− units in Ba3Sb2S7.26 According to Zintl or Lewis descriptions the Q1− atoms in the isolated Q22− dumbbells should have three electron lone pairs, and the spatial arrangements of those lone pairs should conform with D∞h local symmetry. The observed torus-like arrangement is in accordance with those expectations. Similar ELF distributions were observed for the three lone-pairs located on O atoms in the OH1− hydroxyl anions with C∞h symmetry.38 For the linear fragments with two terminal electron lone pairs, such as C34−, different, an umbrella-like ELF distribution was reported.39 In turn, for linear Sb37− and isoelectronic ClF21− where three electron lone pairs are expected on the central atoms, the ELF distribution is also torus-like.20,40
The ELF slices show deep minima surrounding the Ba and I atoms indicating no covalent bonds between the ions. However, significant structuring of both iodine and barium cores were observed, indicating the participation of 5th shell electrons of Ba and I in bonding interactions.
Conclusions
Two new ternary chalcogenide iodides, Ba3S4I2 and Ba3Se4I2, have been synthesized and structurally characterized. Both compounds crystallize in the monoclinic space group C2/c (no. 15). The crystal structure of these compounds is comprised of Q22− dumbbells and isolated I1− anions surrounded by Ba2+ cations. Ba3S4I2 is a wide bandgap semiconductor with an optical bandgap of 2.45(5) eV, which is supported by electronic band structure calculations. ELF analyses indicate covalent bonding within the Q22− dumbbells as well as polarization of the barium and iodine 5th electronic shells.Acknowledgements
The authors would like to thank Prof. F. Osterloh for access to the UV-visible diffuse reflectance spectrometer. K. L. acknowledges the GAANN and ARCS fellowships. This research is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE-SC0008931.References
- K. Ishizaka, M. S. Bahramy, H. Murakawa, M. Sakano, T. Shimojima, T. Sonobe, K. Koizumi, S. Shin, H. Miyahara, A. Kimura, K. Miyamoto, T. Okuda, H. Namatame, M. Taniguchi, R. Arita, N. Nagaosa, K. Kobayashi, Y. Murakami, R. Kumai, Y. Kaneko, Y. Onose and Y. Tokura, Nat. Mater., 2011, 10, 521–526 CrossRef CAS PubMed.
- J. S. Lee, G. A. H. Schober, M. S. Bahramy, H. Murakawa, Y. Onose, R. Arita, N. Nagaosa and Y. Tokura, Phys. Rev. Lett., 2011, 107, 117401 CrossRef CAS PubMed.
- M. Ryazanov, A. Simon and H. Mattausch, Inorg. Chem., 2006, 45, 10728–10733 CrossRef CAS PubMed.
- T. Nilges, S. Nilsen, A. Pfitzner, T. Doert and P. Böettcher, Chem. Mater., 2004, 16, 806–812 CrossRef CAS.
- T. Nilges, C. Dreher and A. Hezinger, Solid State Sci., 2005, 7, 79–88 CrossRef CAS.
- S. Lange and T. Nilges, Chem. Mater., 2006, 18, 2538–2544 CrossRef CAS.
- S. Lange, M. Bawohl, D. Wilmer, H. W. Meyer, H. D. Wiemhöefer and T. Nilges, Chem. Mater., 2007, 19, 1401–1410 CrossRef CAS.
- T. Nilges, O. Osters, M. Bawohl, J. L. Bobet, B. Chevalier, R. Decourt and R. Weihrich, Chem. Mater., 2010, 22, 2946–2954 CrossRef CAS.
- P. M. Carkner and H. M. Haendler, J. Solid State Chem., 1976, 18, 183–189 CrossRef CAS.
- C. M. Schurz, S. Frunder and T. Schleid, Eur. J. Inorg. Chem., 2013, 2923–2929 CrossRef CAS.
- M. Larres, I. Pantenburg and G. Meyer, Z. Anorg. Allg. Chem., 2013, 639, 2744–2747 CrossRef CAS.
- D. L. Gray, G.-J. Long, F. Grandjean, R. P. Hermann and J. A. Ibers, Inorg. Chem., 2008, 47, 94–100 CrossRef CAS PubMed.
- R. L. Gitzendanner and F. J. DiSalvo, Inorg. Chem., 1996, 35, 2623–2626 CrossRef CAS PubMed.
- P. Yu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227–2235 CrossRef CAS PubMed.
- Bruker APEX2, Bruker AXS Inc., Madison, WI, 2005 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- O. Jepsen, A. Burkhardt and O. K. Andersen, The Program TB-LMTO-ASA, Version 4.7, Max-Planck-Institut für Festkörperforschung, Stuttgart, Germany, 1999 Search PubMed.
- A. D. Becke and K. E. Edgecombe, J. Chem. Phys., 1990, 92, 5397–5403 CrossRef CAS.
- A. Savin, O. Jepsen, J. Flad, O. K. Andersen, H. Preuss and H. G. Von Schnering, Angew. Chem., 1992, 104, 186–188 ( Angew. Chem., Int. Ed. Engl. , 1992 , 31 , 187–188 ) CrossRef CAS.
- A. Savin, R. Nesper, S. Wengert and T. F. Fässler, Angew. Chem., 1997, 109, 1892–1918 ( Angew. Chem., Int. Ed. Engl. , 1997 , 36 , 1808–1832 ) CrossRef.
- Yu. Grin, A. Savin and B. Silvi, in The Chemical Bond : Fundamental Aspects of Chemical Bonding, ed. G. Frenking and S. Shaik, Wiley-VCH, Weinheim, 2014, pp. 345–382 Search PubMed.
- (a) Paraview: Parallel visualization application, version 4.1.0 64 bit, Sandia National Labs, Kitware Inc, Los Alamos National Labs, USA; http://paraview.org Search PubMed; (b) A. I. Baranov, Visualization plugin for ParaView, Version 4.1.0, 2014 Search PubMed.
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 3rd edn, 1960 Search PubMed.
- I. Kawada, K. Kato and S. Yamaoka, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 2905–2906 CrossRef.
- S. Yamaoka, J. T. Lemley, J. M. Jenks and H. Steinfink, Inorg. Chem., 1975, 14, 129–131 CrossRef CAS.
- J. Wang, K. Lee and K. Kovnir, Z. Anorg. Allg. Chem., 2015, 641, 1087–1092 CrossRef CAS.
- K.-S. Choi and M. G. Kanatzidis, Inorg. Chem., 2000, 39, 5655–5662 CrossRef CAS PubMed.
- G. Cordier, C. Schwidetzky and H. Schaefer, J. Solid State Chem., 1984, 54, 84–88 CrossRef CAS.
- Y. Akahama, M. Kobayashi and H. Kawamura, Phys. Rev. B: Condens. Matter, 1993, 47, 20–26 CrossRef CAS.
- Y. Miyamoto, J. Appl. Phys., 1980, 19, 1813–1819 CrossRef CAS.
- P. Cherin and P. Unger, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 313–317 CrossRef CAS.
- G. Cordier, R. Cook and H. Schäfer, Angew. Chem., 1980, 92, 310 ( Angew. Chem., Int. Ed. Engl. , 1980 , 19 , 324–325 ) CrossRef CAS.
- Y. B. Lyskova and A. V. Vakhobov, Inorg. Mater., 1975, 11, 1784–1785 Search PubMed.
- J. Wang, K. Lee and K. Kovnir, J. Mater. Chem. C, 2015, 3, 9811–9818 RSC.
- G. Cordier and H. Schäfer, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1979, 34, 1053–1056 Search PubMed.
- S. Wengert and R. Nesper, J. Solid State Chem., 2000, 152, 460–465 CrossRef CAS.
- S. Johnsen, S. C. Peter, S. L. Nguyen, J.-H. Song, H. Jin, A. J. Freeman and M. G. Kanatzidis, Chem. Mater., 2011, 23, 4375–4383 CrossRef CAS.
- M. E. Tuckerman, D. Marx and M. Parrinello, Nature, 2002, 417, 925–929 CrossRef CAS PubMed.
- D. A. Lang, J. V. Zaikina, D. D. Lovingood, T. E. Gedris and S. E. Latturner, J. Am. Chem. Soc., 2010, 132, 17523–17530 CrossRef CAS PubMed.
- Y. Hu, J. Wang, A. Kawamura, K. Kovnir and S. M. Kauzlarich, Chem. Mater., 2015, 27, 343–351 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qi00225g |
| This journal is © the Partner Organisations 2016 |