Open Access Article
Open Access ArticleMirror symmetry breaking in cubic phases and isotropic liquids driven by hydrogen bonding†
Mohamed
Alaasar
 *ab,
Silvio
Poppe
a,
Qingshu
Dong
c,
Feng
Liu
*c and
Carsten
Tschierske
*a
*ab,
Silvio
Poppe
a,
Qingshu
Dong
c,
Feng
Liu
*c and
Carsten
Tschierske
*a
aInstitute of Chemistry, Martin Luther University Halle-Wittenberg, Kurt-Mothes Str.2, D-06120 Halle, Germany. E-mail: carsten.tschierske@chemie.uni-halle.de
bDepartment of Chemistry, Faculty of Science, Cairo University, Giza, Egypt. E-mail: malaasar@sci.cu.edu.eg
cState Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049, P. R. China. E-mail: feng.liu@xjtu.edu.cn
First published on 7th November 2016
Abstract
Achiral supramolecular hydrogen bonded complexes between rod-like 4-(4-alkoxyphenylazo)pyridines and a taper shaped 4-substituted benzoic acid form achiral (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d) and chiral “Im
d) and chiral “Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-type” bicontinuous cubic (I432) phases and a chiral isotropic liquid mesophase (Iso1[*]). The chiral phases, resulting from spontaneous mirror symmetry breaking, represent conglomerates of macroscopic chiral domains eventually leading to uniform chirality.
m-type” bicontinuous cubic (I432) phases and a chiral isotropic liquid mesophase (Iso1[*]). The chiral phases, resulting from spontaneous mirror symmetry breaking, represent conglomerates of macroscopic chiral domains eventually leading to uniform chirality.
Mirror symmetry breaking in liquid crystalline (LC) and liquid phases of achiral molecules is of significant interest as it provides an efficient way to spontaneous chirogenesis in fluids, thus being of potential importance for the emergence of biochirality as well as providing a new way to produce chiral materials.1 For example conglomerates of chiral domains were formed by achiral bent-core molecules in the optically isotropic dark-conglomerate (DC) phases2,3 as well as in birefringent SmC phases4 and nematic phases.5 Twist bend nematic phases (NTB) represent another type of mirror symmetry broken fluids formed by bent-core mesogens,6 rod-like dimesogens,7 trimesogens8 and main chain polymers.9 Recently, mirror symmetry breaking with formation of chiral conglomerates was even observed in Im
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-type bicontinuous cubic phases10 and in isotropic liquids (Iso1[*])11 of achiral rod-like multi-chain (polycatenar) molecules.1,12 A twisted organization of the molecules in the column segments of the branched networks forming these cubic phases and in the local cybotactic domains of the isotropic liquids is assumed to couple cooperatively with helical conformers of the transiently chiral molecules, leading to the development of macroscopic chirality.1,10,11 This dynamic mode of mirror symmetry breaking in the liquid state retains high entropy and allows fast reversible chiral segregation in the presence of relatively weak intermolecular interactions. In order to minimize the unfavourable entropy of mixing, relatively large molecules are required for this process. An efficient way to achieve larger supramolecular units is provided by self assembly of smaller molecules by noncovalent interactions, such as hydrogen bonding, halogen bonding and π-stacking.
m-type bicontinuous cubic phases10 and in isotropic liquids (Iso1[*])11 of achiral rod-like multi-chain (polycatenar) molecules.1,12 A twisted organization of the molecules in the column segments of the branched networks forming these cubic phases and in the local cybotactic domains of the isotropic liquids is assumed to couple cooperatively with helical conformers of the transiently chiral molecules, leading to the development of macroscopic chirality.1,10,11 This dynamic mode of mirror symmetry breaking in the liquid state retains high entropy and allows fast reversible chiral segregation in the presence of relatively weak intermolecular interactions. In order to minimize the unfavourable entropy of mixing, relatively large molecules are required for this process. An efficient way to achieve larger supramolecular units is provided by self assembly of smaller molecules by noncovalent interactions, such as hydrogen bonding, halogen bonding and π-stacking.
Hydrogen-bonding, especially between pyridines and benzoic acids was previously used to design nematic, smectic and columnar mesomorphic materials13–16 whereas cubic LC phases formed by discrete self assembly between two or three components are rare.‡ The first examples of bicontinuous cubic phases formed through discrete intermolecular hydrogen bonding interaction is provided by the 4′-n-alkoxy-3′-nitrobiphenyl-4-carboxylic acids.17 Cubic phases were also reported for supramolecular systems constructed by self-assembly through intermolecular hydrogen-bond formation between 4,4′-bipyridines and 4-substituted benzoic acids with bulky siloxane moieties18a or branched perfluorinated chains.18b To the best of our knowledge there are no examples reported up to date for bicontinuous cubic phases formed by supramolecular hydrogen bonded polycatenar LCs12 and spontaneous symmetry breaking has not yet been reported for any supramolecular polycatenar mesogen.§
Herein we report for the first time how hydrogen bonding can be used to drive mirror-symmetry breaking in an isotropic liquid as well as in cubic phases of supramolecular tetracatenar complexes (AB8–AB14) between rod-like 4-phenylazopyridines Bn19,20 with one terminal alkoxy chain and the benzoic acid A, having three identical terminal alkoxy chains (Scheme 1).¶ The azopyridines Bn20b and the benzoic acid derivative A were synthesized through the synthetic pathway shown in Scheme 1. The detailed synthetic procedures and analytical data are reported in the ESI.†
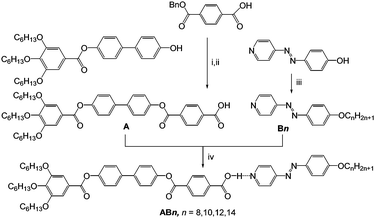 | ||
| Scheme 1 Synthetic route to the pyridines Bn20b and the benzoic acid A and formation of the polycatenar hydrogen-bonded complexes ABn. Reagents and conditions: (i) DCC, DMAP, DCM, stirring, rt, 48 h; (ii) 10%-Pd/C, H2, stirring, 45 °C, 48 h; (iii) BrCnH2n+1, KI, K2CO3, DMF, stirring, 50 °C, 48 h; (iv) melting with stirring. | ||
The 4-(4-alkyloxyphenylazo)pyridines Bn represent non-mesomorphic solids which directly melt to isotropic liquids between 66 and 74 °C (Table S1, ESI†).20b The benzoic acid A exhibits a hexagonal columnar LC phase (Colhex) between 162 and 246 °C as indicated by X-ray diffraction (XRD, ahex = 5.4 nm see Fig. S9 and Table S2, ESI†), in line with the birefringent fan-like texture observed under the polarizing microscope (PM, Fig. S6a, ESI†). The observation of a Colhex phase for A is attributed to dimer formation by intermolecular H-bonding between the COOH groups, leading to hexacatenar rod-like complexes which arrange side by side and on top of each other thus forming columns being rotationally disordered and arranged on a hexagonal lattice. In the columns the rod-like cores are aligned almost perpendicular to the column long axis, resulting in an optically negative Colhex phase (Fig. S6b and c, ESI†), as typical for hexacatenars.12 The supramolecular aggregates AB8–AB14 were prepared by mixing equimolar amounts of Bn and A and then melting them together in DSC pans (30 μl) with stirring. After crystallization the material was grinded, and this process was repeated to obtain a homogeneous mixture.
Homogenous melting and reproducible transition temperatures were observed for all supramolecular complexes ABn. The formation of the supramolecular 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
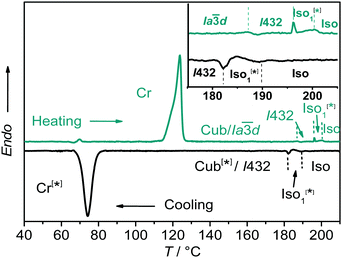
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between the benzoic acid A and the 4-phenylazopyridines Bn (for 1H-NMR, see Fig. S4, ESI†) leads to the suppression of the columnar phase and induction of broad cubic LC phase ranges for all hydrogen bonded complexes as determined by differential scanning calorimetry (DSC, see Fig. 1; the intense peaks of the individual components, see Fig. S5, are absent, ESI†), PM and XRD investigations (see Table 1).
1 complexes between the benzoic acid A and the 4-phenylazopyridines Bn (for 1H-NMR, see Fig. S4, ESI†) leads to the suppression of the columnar phase and induction of broad cubic LC phase ranges for all hydrogen bonded complexes as determined by differential scanning calorimetry (DSC, see Fig. 1; the intense peaks of the individual components, see Fig. S5, are absent, ESI†), PM and XRD investigations (see Table 1).
| No. | n | Phase sequence |
|---|---|---|
a Peak temperatures as determined from 1st heating (H) and 1st cooling (C) DSC scans with rate 10 K min−1; abbreviations: Cr = crystalline solid; I432 = chiral “Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-type” cubic LC phase with I432 symmetry; Ia m-type” cubic LC phase with I432 symmetry; Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d = achiral cubic LC phase with Ia d = achiral cubic LC phase with Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d symmetry; Cr[*] = chiral crystalline solid; Iso1[*] = chiral isotropic conglomerate liquid; Iso = achiral isotropic liquid. d symmetry; Cr[*] = chiral crystalline solid; Iso1[*] = chiral isotropic conglomerate liquid; Iso = achiral isotropic liquid.
|
||
| AB8 | 8 |
H: Cr 124 [28] Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d ∼187 [0.1] I432 196 [0.1] Iso1[*] 200 [0.1] Iso d ∼187 [0.1] I432 196 [0.1] Iso1[*] 200 [0.1] Iso
C: Iso 190 [0.1] Iso1[*] 183 [0.1] I432 75 [23] Cr[*] |
| AB10 | 10 |
H: Cr1 115 [11] Cr2 128 [41] I432 201 [0.8] Iso
C: Iso 195 [0.7] I432 75 [35] Cr |
| AB12 | 12 |
H: Cr 123 [48] I432 191 [1.2] Iso
C: Iso 182 [1.7] I432 87 [40] Cr |
| AB14 | 14 |
H: Cr 92 [27] I432 184 [1.7] Iso
C: Iso 177 [1.8] I432 |
Exclusively cubic phases were found for the complexes AB10–AB14, whereas for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
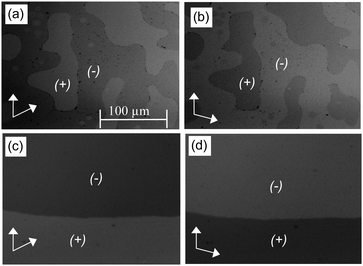
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex AB8 an addition liquid–liquid transition is indicated in the DSC traces by a broad feature in the isotropic liquid range (Iso–Iso1 transition, Fig. 1). Between crossed polarizers the liquid phases Iso as well as Iso1 appear uniformly dark. However, in the Iso1 phase range slightly rotating the analyzer by a few degrees (ca. −7°) out of the 90° orientation with respect to the polarizer leads to the appearance of dark and bright domains, which exchange their brightness after rotation of the analyzer by the same angle into the opposite direction (ca. +7°, see Fig. 2a and b). Rotating the sample between crossed polarizers does not lead to any change and these observations confirm that the distinct regions represent chiral domains. This is a clear indication for chirality synchronization in the Iso1 phase (Iso1[*]). No such domains can be observed in the Iso phase of AB8 at higher temperature or in the Iso phases of complexes AB10–AB14, which are achiral.
1 complex AB8 an addition liquid–liquid transition is indicated in the DSC traces by a broad feature in the isotropic liquid range (Iso–Iso1 transition, Fig. 1). Between crossed polarizers the liquid phases Iso as well as Iso1 appear uniformly dark. However, in the Iso1 phase range slightly rotating the analyzer by a few degrees (ca. −7°) out of the 90° orientation with respect to the polarizer leads to the appearance of dark and bright domains, which exchange their brightness after rotation of the analyzer by the same angle into the opposite direction (ca. +7°, see Fig. 2a and b). Rotating the sample between crossed polarizers does not lead to any change and these observations confirm that the distinct regions represent chiral domains. This is a clear indication for chirality synchronization in the Iso1 phase (Iso1[*]). No such domains can be observed in the Iso phase of AB8 at higher temperature or in the Iso phases of complexes AB10–AB14, which are achiral.
The transition to the cubic phase is indicated by a small, but relatively sharp peak in the DSC traces (Fig. 1). The transition enthalpy of this transition rises with growing chain length from 0.1 to 1.8 J g−1 (Table 1). As typical for mesophases with long range 3D lattice there is a hysteresis of this transition to the cubic phase on cooling by ca. 6–9 K. At the Iso1[*]–Cub[*] transition of AB8 and the Iso–Cub[*] transitions of the complexes AB10–AB14 the samples remain optically isotropic, but these transitions are associated with a significant reduction of the fluidity leading to soft viscoelastic solids. The diffuse scattering in the wide angle range of the XRD patterns is retained (Fig. S10b, ESI†) indicating the absence of a long range positional order of the individual molecule as typical for LC phases. In the case of AB8 the chiral domains in the Iso1[*] phase grow to huge homogeneously chiral domains of either handedness, even across the original chiral domain boundaries (see Fig. 2c and d). The complexes AB10–AB14 form the chiral domains directly at the transition from the achiral Iso phase to the Cub[*] phases. Also for these complexes large chiral domains are formed (Fig. S8, ESI†) and on very slow cooling (<1 K min−1) it is even possible to achieve uniform chirality, indicating slow formation of the seeds of the cubic phase combined with a fast growth; the distribution of either chirality sense is stochastic.
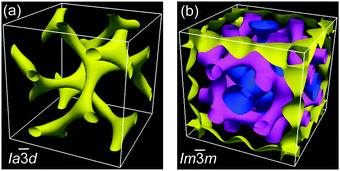
The powder XRD patterns of the cubic conglomerate phases of the supramolecules ABn (Fig. 3b and Fig. S10, ESI†) can be indexed to Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m lattices with nearly chain length independent lattice parameters (acub = 19.4–19.5 nm, see Tables S4–S6, ESI†). Based on this phase assignment the electron density map (EDM) of AB14 was reconstructed from the powder diffraction pattern, showing a tricontinuous structure of this cubic phase (Fig. 4b). It should be noted here that due to the chirality the actual space group is a chiral one, that with the highest symmetry being I432. However as the phase angle can represent any value between 0 and ±π in this non-centrosymmetric lattice we assume the centrosymmetric Im
m lattices with nearly chain length independent lattice parameters (acub = 19.4–19.5 nm, see Tables S4–S6, ESI†). Based on this phase assignment the electron density map (EDM) of AB14 was reconstructed from the powder diffraction pattern, showing a tricontinuous structure of this cubic phase (Fig. 4b). It should be noted here that due to the chirality the actual space group is a chiral one, that with the highest symmetry being I432. However as the phase angle can represent any value between 0 and ±π in this non-centrosymmetric lattice we assume the centrosymmetric Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure as a close approximate for electron density reconstruction, limiting the phase choices to 0 and ±π. The three interwoven but not connected high electron density networks (yellow, purple and blue in Fig. 4b, respectively) involve the hydrogen bonded aromatic cores arranged with their long axes perpendicular to the directions of the column segments forming the labyrinths. Due to the steric crowding of the alkyl chains at the ends the organization of the rods is not exactly parallel, but with a slight angle leading to a helical twist along the networks.10,21 The network structure leads to a long range transmission of the helix sense once formed and exciton coupling between the twisted π-systems is assumed to mainly contribute to optical rotation.22 As there are three networks, chirality cannot be cancelled even if the helix sense would be opposite in adjacent networks. The space between the networks is filled by the disordered alkyl chains.
m structure as a close approximate for electron density reconstruction, limiting the phase choices to 0 and ±π. The three interwoven but not connected high electron density networks (yellow, purple and blue in Fig. 4b, respectively) involve the hydrogen bonded aromatic cores arranged with their long axes perpendicular to the directions of the column segments forming the labyrinths. Due to the steric crowding of the alkyl chains at the ends the organization of the rods is not exactly parallel, but with a slight angle leading to a helical twist along the networks.10,21 The network structure leads to a long range transmission of the helix sense once formed and exciton coupling between the twisted π-systems is assumed to mainly contribute to optical rotation.22 As there are three networks, chirality cannot be cancelled even if the helix sense would be opposite in adjacent networks. The space between the networks is filled by the disordered alkyl chains.
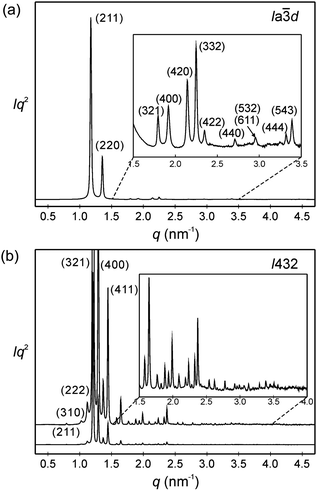 | ||
Fig. 3 SAXS diffractograms of (a) the Cub/Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase (acub = 13.10 nm) of AB8, recorded at T = 150 °C on heating (for wide angle scattering see Fig. S10b, ESI†); (b) the Cub[*]/I432 phase (acub = 19.46 nm) of AB14 at T = 140 °C, the curve on top is enhanced by a factor of 7 and only some diffractions at lower angles are labelled; see also Tables S3–S6 (ESI†). d phase (acub = 13.10 nm) of AB8, recorded at T = 150 °C on heating (for wide angle scattering see Fig. S10b, ESI†); (b) the Cub[*]/I432 phase (acub = 19.46 nm) of AB14 at T = 140 °C, the curve on top is enhanced by a factor of 7 and only some diffractions at lower angles are labelled; see also Tables S3–S6 (ESI†). | ||
Only the supramolecular complex AB8 exhibits an additional cubic phase which is achiral based on optical investigations (uncrossing the polarizers by a small angle in clockwise or anticlockwise direction does not lead to any change). The diffraction pattern in the achiral cubic phase (Fig. 3a) can be indexed to a cubic lattice with Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d symmetry (acub = 13.10 nm, Table S3, ESI†) and represents a gyroid-type bicontinuous double-network phase as shown in the reconstructed EDM in Fig. 4a. The two interwoven high electron density networks (yellow) are filled with the hydrogen bonded cores, being arranged perpendicular to the column segments of the networks and slightly twisted with respect to each other, thus forming only two helically twisted networks with opposite helix sense, cancelling each other to give an achiral structure.10 This achiral Ia
d symmetry (acub = 13.10 nm, Table S3, ESI†) and represents a gyroid-type bicontinuous double-network phase as shown in the reconstructed EDM in Fig. 4a. The two interwoven high electron density networks (yellow) are filled with the hydrogen bonded cores, being arranged perpendicular to the column segments of the networks and slightly twisted with respect to each other, thus forming only two helically twisted networks with opposite helix sense, cancelling each other to give an achiral structure.10 This achiral Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase is formed on melting the crystalline phase (Cr) at T = 124 °C and on heating transforms into the chiral I432 phase at T = 180–187 °C (acub = 19.53 nm) as indicated by optical investigations (appearance of chiral domains), DSC (small enthalpy of 0.1 J g−1) and from temperature dependent XRD studies by a change of the diffraction pattern (Table S4, ESI†). This chiral cubic phase melts at T = 196 °C with the formation of the chiral Iso1[*] phase which transforms into the achiral Iso phase at T = 200 °C. On cooling AB8 from the Iso state Iso1[*] is formed at T = 190 °C, followed by the transition to the chiral Cub[*] phase (I432) at T = 183 °C (Fig. 1 and Table 1). The chiral domains and the typical diffraction pattern of the I432 phase are retained till the crystallization at T = 75 °C. Interestingly, the crystalline state of AB8 exhibits also chiral domains as indicated by the textural observations under PM (see Fig. S7, ESI†), thus indicating crystallization in a chiral space group (Cr[*]). Heating this Cr[*] phase (which melts at T = 114 °C) leads directly to the chiral I432 phase without intermediate formation of the achiral Ia
d phase is formed on melting the crystalline phase (Cr) at T = 124 °C and on heating transforms into the chiral I432 phase at T = 180–187 °C (acub = 19.53 nm) as indicated by optical investigations (appearance of chiral domains), DSC (small enthalpy of 0.1 J g−1) and from temperature dependent XRD studies by a change of the diffraction pattern (Table S4, ESI†). This chiral cubic phase melts at T = 196 °C with the formation of the chiral Iso1[*] phase which transforms into the achiral Iso phase at T = 200 °C. On cooling AB8 from the Iso state Iso1[*] is formed at T = 190 °C, followed by the transition to the chiral Cub[*] phase (I432) at T = 183 °C (Fig. 1 and Table 1). The chiral domains and the typical diffraction pattern of the I432 phase are retained till the crystallization at T = 75 °C. Interestingly, the crystalline state of AB8 exhibits also chiral domains as indicated by the textural observations under PM (see Fig. S7, ESI†), thus indicating crystallization in a chiral space group (Cr[*]). Heating this Cr[*] phase (which melts at T = 114 °C) leads directly to the chiral I432 phase without intermediate formation of the achiral Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase. Thus, the chirality once achieved is retained on crystallization and the formation of the Ia
d phase. Thus, the chirality once achieved is retained on crystallization and the formation of the Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase is suppressed. The Ia
d phase is suppressed. The Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase is only obtained after heating the crystalline sample after prolonged storage. It appears that the (metastable) Cr[*] phase slowly transforms into an achiral crystalline phase (Cr, m.p. = 124 °C) from which the achiral Ia
d phase is only obtained after heating the crystalline sample after prolonged storage. It appears that the (metastable) Cr[*] phase slowly transforms into an achiral crystalline phase (Cr, m.p. = 124 °C) from which the achiral Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d phase is formed on heating. This means that the cubic I432 phase is metastable below 187 °C, but once formed seems to be persistent. The transition Ia
d phase is formed on heating. This means that the cubic I432 phase is metastable below 187 °C, but once formed seems to be persistent. The transition Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d–I432 observed in the series ABn on chain elongation and with rising temperature (AB8) is in line with the recently proposed helical model, as the effective chain volume increases with rising temperature and growing alkyl chain length and this reduces the helical pitch becoming incompatible with the Ia
d–I432 observed in the series ABn on chain elongation and with rising temperature (AB8) is in line with the recently proposed helical model, as the effective chain volume increases with rising temperature and growing alkyl chain length and this reduces the helical pitch becoming incompatible with the Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d structure and leading to formation of the I432 cubic phase.10
d structure and leading to formation of the I432 cubic phase.10
In summary, we report herein the design and synthesis of the first examples of hydrogen bonded supramolecular complexes with polycatenar structure showing dynamic mirror-symmetry breaking by chirality synchronization in a liquid conglomerate (Iso1[*]) at the liquid–liquid transition as well as in chiral “Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-type” cubic phases (Cub[*]/I432). The liquid conglomerate is obviously only formed if the alkyl chains are short and nano-segregation between alkyl chain and core unit is sufficiently weak to prevent formation of a long range cubic lattice. Overall, this work could initiate further work on using hydrogen bonding for symmetry breaking in fluids. Moreover, the possibilities provided by the photosensitive azobenzene units23 could lead to interesting perspectives for chirality switching and phase modulation by interaction with non-polarized and (linear or circular) polarized light.24
m-type” cubic phases (Cub[*]/I432). The liquid conglomerate is obviously only formed if the alkyl chains are short and nano-segregation between alkyl chain and core unit is sufficiently weak to prevent formation of a long range cubic lattice. Overall, this work could initiate further work on using hydrogen bonding for symmetry breaking in fluids. Moreover, the possibilities provided by the photosensitive azobenzene units23 could lead to interesting perspectives for chirality switching and phase modulation by interaction with non-polarized and (linear or circular) polarized light.24
The work was funded by the DFG (Grant Ts 39/24-1) and the National Natural Science Foundation of China (No. 21374086). We thank Beamline BL16B1 at SSRF (Shanghai Synchrotron Radiation Facility, China) for providing the beamtimes.
Notes and references
- C. Tschierske and G. Ungar, ChemPhysChem, 2016, 17, 9 CrossRef CAS PubMed.
- (a) J. Thisayukta, Y. Nakayama, S. Kawauchi, H. Takezoe and J. Watanabe, J. Am. Chem. Soc., 2000, 122, 7441 CrossRef CAS; (b) G. Dantlgraber, A. Eremin, S. Diele, A. Hauser, H. Kresse, G. Pelzl and C. Tschierske, Angew. Chem., Int. Ed., 2002, 41, 2408 CrossRef CAS; (c) L. E. Hough, M. Spannuth, M. Nakata, D. A. Coleman, C. D. Jones, G. Dantlgraber, C. Tschierske, J. Watanabe, E. Körblova, D. M. Walba, J. E. Maclennan, M. A. Glaser and N. A. Clark, Science, 2009, 325, 452 CrossRef CAS PubMed; (d) H. Sasaki, Y. Takanishi, J. Yamamoto and A. Yoshizawa, J. Phys. Chem. B, 2015, 119, 4531 CrossRef CAS PubMed.
- H. Takezoe, Top. Curr. Chem., 2012, 318, 303 CrossRef CAS PubMed.
- (a) M. Alaasar, M. Prehm, M. Nagaraj, J. K. Vij and C. Tschierske, Adv. Mater., 2013, 25, 2186 CrossRef CAS PubMed; (b) M. Alaasar, M. Prehm, K. May, A. Eremin and C. Tschierske, Adv. Funct. Mater., 2014, 24, 1703 CrossRef CAS.
- V. Görtz, Liq. Cryst. Today, 2010, 19, 37 CrossRef.
- D. Chen, J. H. Porada, J. B. Hooper, A. Klittnick, Y. Shen, M. R. Tuchband, E. Körblova, D. Bedrov, D. M. Walba, M. A. Glaser, J. E. Maclennan and N. A. Clark, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15931 CrossRef CAS PubMed.
- (a) V. Borshch, Y.-K. Kim, J. Xiang, M. Gao, A. Jakli, V. P. Panov, J. K. Vij, C. T. Imrie, M. G. Tamba, G. H. Mehl and O. D. Lavrentovich, Nat. Commun., 2013, 4, 2635 CAS; (b) M. Cestari, S. Diez-Berart, D. A. Dunmur, A. Ferrarini, M. R. de la Fuente, D. J. B. Jackson, D. O. Lopez, G. R. Luckhurst, M. A. Perez-Jubindo, R. M. Richardson, J. Salud, B. A. Timimi and H. Zimmermann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031704 CrossRef CAS PubMed; (c) R. J. Mandle, Soft Matter, 2016, 12, 7883 RSC.
- (a) R. J. Mandle and J. W. Goodby, ChemPhysChem, 2016, 17, 967 CrossRef CAS PubMed; (b) Y. Wang, Z. Zheng, H. K. Bisoyi, K. G. Gutierrez-Cuevas, L. Wang, R. S. Zolab and Q. Li, Mater. Horiz., 2016, 3, 442 RSC.
- G. Ungar, V. Percec and M. Zuber, Macromolecules, 1992, 25, 75 CrossRef CAS.
- C. Dressel, F. Liu, M. Prehm, X. Zeng, G. Ungar and C. Tschierske, Angew. Chem., Int. Ed., 2014, 53, 13115 CrossRef CAS PubMed.
- (a) C. Dressel, T. Reppe, M. Prehm, M. Brautzsch and C. Tschierske, Nat. Chem., 2014, 6, 971 CrossRef CAS PubMed; (b) M. Alaasar, M. Prehm, Y. Cao, F. Liu and C. Tschierske, Angew. Chem., Int. Ed., 2016, 55, 312 CrossRef CAS PubMed; (c) C. Dressel, W. Weissflog and C. Tschierske, Chem. Commun., 2015, 51, 15850 RSC.
- W. Weissflog, in Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2014, vol. 5, p. 89 Search PubMed.
- T. Kato and J. M. J. Frechet, J. Am. Chem. Soc., 1989, 111, 8533 CrossRef CAS.
- B. Friot, D. Boyd, K. Willis, B. Donnio, G. Ungar and D. W. Bruce, Liq. Cryst., 2000, 27, 605 CrossRef CAS.
- (a) C. M. Paleos and D. Tsiourvas, Liq. Cryst., 2001, 28, 1127 CrossRef CAS; (b) T. Kato and Y. Kamikawa, in Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2014, vol. 5, 513 Search PubMed.
- S. M. Jansze, A. Martinez-Felipe, J. M. D. Storey, A. T. M. Marcelis and C. T. Imrie, Angew. Chem., Int. Ed., 2015, 54, 643 CAS.
- (a) G. W. Gray, B. Jones and F. Marson, J. Chem. Soc., 1957, 393 RSC; (b) D. Demus, G. Kunicke, J. Neelsen and H. Sackmann, Z. Naturforsch., A: Phys. Sci., 1968, 23, 84 CAS; (c) S. Kutsumizu, Isr. J. Chem., 2012, 52, 844 CrossRef CAS.
- (a) E. Nishikawa and E. T. Samulski, Liq. Cryst., 2000, 27, 1463 CrossRef CAS; (b) E. Nishikawa, J. Yamamoto and H. Yokoyama, Liq. Cryst., 2003, 30, 785 CrossRef CAS.
- (a) J. Mamiya, A. Yoshitake, M. Kondo, Y. Yu and T. Ikeda, J. Mater. Chem., 2008, 18, 63 RSC; (b) K. Aoki, M. Nakagawa and K. Ichimura, J. Am. Chem. Soc., 2000, 122, 10997 CrossRef CAS; (c) M. Pfletscher, C. Wölper, J. S. Gutmann, M. Mezger and M. Giese, Chem. Commun., 2016, 52, 8549 RSC.
- (a) W. Zhou, T. Kobayashi, H. Zhu and H. F. Yu, Chem. Commun., 2011, 47, 12768 RSC; (b) Y. J. Chen, H. F. Yu, L. Y. Zhang, H. Yang and Y. F. Lu, Chem. Commun., 2014, 50, 9647 RSC.
- K. Saito, Y. Yamamura, Y. Miwa and S. Kutsumizu, Phys. Chem. Chem. Phys., 2016, 18, 3280 RSC.
- G. Pescitelli, L. Di Bari and N. Berova, Chem. Soc. Rev., 2014, 43, 5211 RSC.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809 RSC.
- D. A. Paterson, J. Xiang, G. Singh, R. Walker, D. M. Agra-Kooijman, A. Martınez-Felipe, M. Gao, J. M. D. Storey, S. Kumar, O. D. Lavrentovich and C. T. Imrie, J. Am. Chem. Soc., 2016, 138, 5283 CrossRef CAS PubMed.
- K. Borisch, S. Diele, P. Göring, H. Kresse and C. Tschierske, Angew. Chem., Int. Ed., 1997, 36, 2087 CrossRef CAS.
- T. Kajitani, S. Kohmoto, M. Yamamoto and K. Kishikawa, Chem. Mater., 2005, 17, 3812 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, analytical data, additional data. See DOI: 10.1039/c6cc08226b |
| ‡ Multiple cooperative hydrogen bonding between amphiphilic glycerol-based or carbohydrate based molecules forming polymeric hydrogen bonding networks are more common.25 |
| § Mirror symmetry breaking by hydrogen bonding in soft matter was previously reported for the NTB phases of hydrogen bonded mesogenic trimers24 and the cubic phase of a bent molecule.26 |
| ¶ The azopyridine derivatives Bn have previously been used for the formation of supramolecular self-assembled hydrogen bonded19 and halogen-bonded LCs20 with light induced phase transitions. |
| This journal is © The Royal Society of Chemistry 2016 |