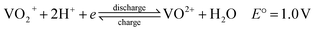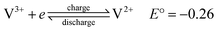Recent development of polymer membranes as separators for all-vanadium redox flow batteries
The Nam Long Doan
,
Tuan K. A. Hoang
and
P. Chen
*
Department of Chemical Engineering and Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L3G1, Canada. E-mail: p4chen@uwaterloo.ca; Fax: +1-519-888-4347; Tel: +1-519-888-4567 ext. 35586
First published on 20th August 2015
Abstract
The all-vanadium redox flow battery (VRFB) is one of the most promising energy storage systems to be associated with the grid. The system has been developed for almost 30 years. A key component for VRFBs is the membrane separator, which separates the positive and negative half-cells and prevents the cross-mixing of vanadium ions, while providing required ionic conductivity. In general, research is conducted to solve a multi-variable problem which requires optimization in both physical characteristics and electrochemical performance of the membrane. Nafion and its derivatives are still important materials thanks to their high chemical stability and ionic conductivity. However, weaknesses of these materials, such as high vanadium ion crossover and high cost, stimulate new approaches in materials design for VRFBs. New achievements in material sciences and polymer chemistry allow further development of other types of polymeric materials and composites as separators in VRFBs. This includes new cation exchange membranes, anion exchange membranes, amphoteric ion-exchange membranes, and non-ionic porous materials. Each type of material exhibits its advantages, accompanied by its weaknesses. Recent articles in polymer-containing membranes for use as separators in VRFBs are reviewed.
1. Introduction
Although the use of renewable energy sources, such as wind and solar power, is considered as “green”, their generated electric signals fluctuate over time. Therefore, it is impossible to dispatch the electricity effectively without the use of energy storage systems.1 For medium-to-large-scale energy storage (kW h to MW h), vanadium redox flow batteries have many benefits over other energy storage systems regarding low environmental impact, high energy efficiency and excellent cycle life,2 making them one of the most promising electrochemical energy storage systems which are suitable for integrating electricity from various renewable energy sources into the grids.1,3Redox flow batteries have been being developed during the last 40 years.4–6 A redox flow cell is an electrochemical system consists of fully soluble redox couples in two electrolyte solutions, two inert electrodes5 and an ion conducting separator. As a result, electrode side reactions are eliminated and the battery life is theoretically unlimited.5 Among various types of redox flow batteries, only the all-vanadium redox flow batteries (VRFBs), invented and developed by Prof. Skyllas-Kazacos and co-workers,5 are considered commercially successful thanks to their superior cyclability and high energy efficiency of more than 80% in large scale installations.1–3 The fundamental electrochemistry of the VRFBs could be found in details in a review written by Kear et al.2 Fig. 1 presents a simple schematic of an all-vanadium redox flow battery.6 The vanadium redox flow battery consists of the major components: the inert electrodes, the electrolytes (vanadium solutions in sulfuric acid) and a selective ion-exchange membrane. The membrane is used to separate the positive and negative half-cells and to prevent the cross mixing of the electrochemically active ions (vanadium ions) while providing the required ionic conductivity for the non-electrochemically active specific ions, such as H+. Each half-cell contains an inert electrode, which is normally made from highly porous carbon felt. The electrolytes, the anolyte and catholyte, are stored in external tanks and are pumped into their corresponding cells when the battery is in operation. During charge and discharge, the following electrode reactions happen between VO2+/VO2+ and V2+/V3+ redox couples:6,7
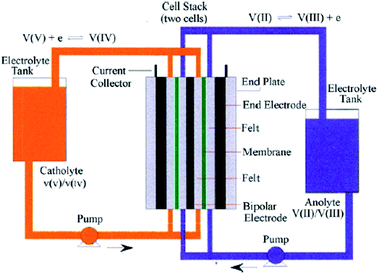 | ||
| Fig. 1 Schematic of a vanadium redox flow battery. Reprinted with permission from ref. 6. Copyright Elsevier, 2009. | ||
At the positive electrode:
At the negative electrode:
The membrane separator is the key material for commercialization of redox batteries because it decides the performance (both cyclability and efficiencies), and its cost can be up to 20% of the whole battery system.3 Thus, many research groups all over the world focus on investigating, finding and developing the right membrane for VRFBs, which provides good performance but low cost. Although there are excellent reviews about VRFBs1,2 and membranes for VRFBs,3,8,9 there are about 100 papers published in the last two years which were not covered in any reviews. More important, we can see a new research trends which focus on developing anion-exchange or other types of polymer membranes rather than only modifying Nafion or developing cation-exchange membranes, which dominated the literature in the previous years. Therefore, this present article is aimed to review the most recent development in polymer membranes as separators for VRFBs. For membrane classification, preparation and evaluation methods, a review of Prof. Skyllas-Kazacos and co-workers could be referred.3 Ions transport properties through membranes were well discussed by Xu and Zhao.10 Recently, the developments of anion exchange membranes for fuel cells and redox flow batteries were reviewed by Maurya et al.9
Most of the large scale VRFBs employ the expensive Nafion cation exchange membranes thanks to their excellent chemical stability and high ionic conductivity,8,11 except those installed by Sumitomo and Kashima-Kita Power Corporation (Japan),3,12 which used high performance anion exchange membrane. This membrane requires high purity vanadium electrolyte.
For other redox flow systems, cheaper membranes can be used due to the less corrosive environment.11 However, these systems are not in the scope of this review.
2. Modification of Nafion membranes
Nafion, a sulfonated tetrafluoroethylene based fluoropolymer-copolymer, is one of the best known ion conducting polymers.3–8 Since the immobilized sulfonic acid groups (–SO3H) are super acids, they interact strongly with water leading to hydration, thus enabling the mobility of the hydrated protons as well as other monovalent cations.8 Therefore, Nafion is a cation-exchange polymer. On the other hand, the incorporation of sulfonic acid groups and the stable Teflon backbone result in a high chemical stability.3Due to its high cation conductivity and excellent chemical stability, Nafion and Nafion's family have been used traditionally in redox-flow batteries.8 The problems of Nafion are its high cost and the crossover phenomenon of vanadium ions, which lead to a gradual decrease in energy efficiency. Therefore, modifications of Nafion membrane to improve its cation exchange selectivity have been an important research focus of many research groups for a long period of time. Although the main attention is shifted to anion exchange membranes and other types of cation exchange membranes recently, the research about Nafion based membranes for VRFBs is still important and a large number of papers were published.
The relationship between electrochemical performance of VRFBs using Nafion115 membrane and electrolyte compositions was investigated by Luo et al.13 It is well-known that in case of VRFBs with cation exchange membranes, there are transfers of both vanadium ions and water from the negative half-cell to the positive one up on cycling. The former one is due to the higher transfer rates of V2+ and V3+ vs. those of VO2+ and VO2+ when the later one is due to the sufficient amount of water carried by the hydration shells of V2+ and V3+.13,14 Thus, it can be concluded that the capacity fading is due to the imbalance of vanadium species in the positive and negative half-cells. However, it was found that the concentration of VO2+ increased and that of VO2+ decreased in the positive electrolyte, while the concentration of V2+ increased and that of V3+ decreased in the negative electrolyte upon cycling.13 These phenomena could be due to the self-discharge reactions between the transferred vanadium ions and the native vanadium ions.15,16 However, these self-discharge reactions are imbalanced, leading to the asymmetrical valent state of vanadium ions in positive and negative electrolytes. As a consequence, vanadium species in negative electrolyte are poorly utilized, capacity fading happens and higher overpotentials are created during the charge–discharge process.
Tang et al. pointed out that the concentrations of acid or VO2+ has strong effects on ionic conductivity of Nafion.17 Sulfuric acid when entering the membrane can enhance membrane conductivity by increasing proton concentrations. However, water loss due to acid presence lead to significant decrease of the proton mobility. Thus, acid and water contents in the membrane are crucial parameters which define ionic conductivity of the membrane. When VO2+ is in the membrane equilibrated in diluted acid, it can compete and reduce proton concentration and proton mobility, leading to the decrease in membrane conductivity. Jeong et al. investigated the effect of Nafion membrane thickness on the performance of VRFBs.18 When membrane thickness increases, coulombic efficiency (CE) increases due to the decrease of the vanadium crossover rate, but power efficiency (PE) decreases because of the enhancement in ohmic resistance. Nevertheless, thicker membranes result in higher energy efficiency (EE) indicating that the crossover effect of vanadium ions is more important than ohmic resistance in deciding the electrochemical performance of VRFBs.
Teng et al.19,20 developed polytetrafluoroethylene (PTFE)/Nafion composite membranes by different methods, including the impregnation of porous PTFE membrane with Nafion solution,19 and the solution casting method.20 By impregnation method, Nafion resin can be uniformly filled into the micropores of the PTFE membrane. Due to the intrinsic hydrophobicity of PTFE, the obtained composite membrane shows water uptake of 19.5% lower than that of the Nafion212 membrane, as shown in Fig. 2.19 As a consequence, the swelling ratio, ion exchange capacity (IEC) and proton conductivity of the composite membrane are also lower than characteristic data of the Nafion212 membrane. However, lower IEC leads to lower water and vanadium ions transfer, resulting in higher CE, voltage efficiency (VE) and energy efficiency (EE) of the VRFBs containing the composite membranes compared with the performance of the VRFBs using the pristine Nafion212. Moreover, the batteries containing the composite membranes have better cyclability and lower self-discharge rates in comparison with the cells containing the pure Nafion212 membrane. On the other hand, Nafion/PTFE membranes prepared by solution casting method have higher crystallinity and thermal stability than Nafion membrane while the chemical stability of both types is on the same magnitude.20 Moreover, with addition of PTFE, the vanadium crossover and swelling ratio of the composite membrane are reduced, and thus VRFBs performance is improved, specifically for EE and self-discharge rate. Since PTFE is less expensive than Nafion, the composite membrane is a promising cost-effective candidate for large scale application of VRFBs. In addition to the good effect of PTFE, silica could be blended into the Nafion/PTFE membrane using tetraethoxysilane (TEOS) precursor via an in situ sol–gel preparation method. Water uptake and proton conductivity of the membranes increase proportionally with the silica content. It is commonly accepted that high water contents will improve the proton conductivity.3,8 Silica in composite membrane is highly hydrophilic, which secure the excellent water retention capability of the membrane materials. Because of the enhanced proton conductivity and the reduction in VO2+ permeability, electrochemical performance of VRFBs containing silica/Nafion/PTFE membrane is much better than VRFBs employing the Nafion/PTFE one. The reason for the reduction of VO2+ permeability is still elusive.
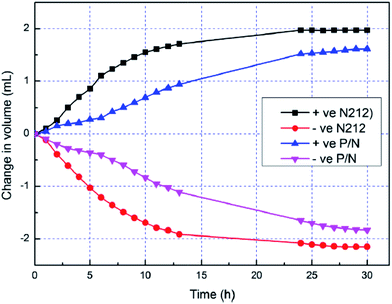 | ||
| Fig. 2 Water transfer behavior of the PTFE/Nafion (P/N) composite and Nafion212 (N212) membranes. Reprinted with permission from ref. 19. Copyright Elsevier, 2013. | ||
Ma et al. incorporated a cationic charge layer of dimethyl-aminoethyl methacrylate (DMA-EMA) onto Nafion membrane by radiation-induced graft copolymerization.21 The initial intention is to reduce vanadium crossover by exploiting the Donnan exclusion effect between –R3NH+ groups of protonated DMAEMA unit and vanadium ions. As a result, the VO2+ ion permeability is reduced with the increase of grafting yield (GY), and the VO2+ ion permeability is less than one tenth of that of the uncoated Nafion membrane when grafting yield (GY) exceeds 20%. However, the proton conductivity of the coated Nafion membrane is much lower than that of uncoated one and diminishes with the increment of GY. The reason may be due to the poorer proton conductivity of –NH+(CH3)2 group in DMAEMA unit and the restrained mobility of –SO3H group of the based Nafion membrane.
Fluorocarbon surfactant (FC) was introduced into the Nafion membrane by the solution casting method in a study of Teng et al.22 The introduction of FC intensifies the water uptake thanks to the broader water channels and thus the higher hydrophilicity. Therefore, proton conductivity rises slightly with the accruement of FC contents. However, ion selectivity also increases (the highest value is 2.1 times higher than that of pure Nafion membrane). The underlying reason is still under investigation by the authors. It may be a unique result since in most of the researches about modified Nafion membranes; the modified membranes have higher vanadium ion selectivity but lower proton conductivities than the pristine one. Nevertheless, electrochemical parameters, such as CE, VE and self-discharge rates, are significantly enhanced in comparison with performance of the pure Nafion membrane.
Recently in 2014, Lu et al. developed a Nafion-[polycation chitosan-phosphotungstic acid] composite membrane (Nafion-[CS-PWA]n, n is the number of bilayers) using the layer-by-layer self-assembly technique.23 Proton conductivity of the membrane quickly falls when the first bilayer is applied, and then decreases gradually versus the increase of n. In similarity with most of other studies about modified Nafion membranes, vanadium permeability also decreases drastically with the accumulation of n but becomes stable when n ≥ 3. As a result, a single cell of VRFBs showed higher CE and EE and lower self-discharge rate than the characteristics of the cell using pristine Nafion212 membrane.
The strong development of graphene-based products has influenced some of the Nafion modifications.24,25 Lee and Chu investigated the preparation of graphene oxide (GO)/Nafion composite membranes for VRFBs by casting the mixtures of the GO suspension solution and Nafion ionomer solution.24 The process was assisted by ultrasonic waves to disperse GO in the mixture solution. The obtained composite membranes exhibit much lower proton conductivity than that of Nafion117 (less than three times). This may be due to the shrinkage of the inter-planar space, calculated from X-ray diffraction (XRD) data. The smaller inter-planar space minimizes the water uptake and thus reduces the proton conductivity. As a consequence, VO2+ permeability drops dramatically. In fact, proton conductivity and VO2+ permeability are not dependable on the existence of GO phase, thus it is reasonable to conclude that the preparation method leads to the desired changes in parameters, not because of the introduction of GO phase. On the other hand, Kim et al. investigated the properties of sulfonated graphene oxide (sGO)/Nafion composite membranes in VRFBs.25 In order to promote miscibility with Nafion, sGO was treated with phenyl isocyanate to form isGO. This composite membrane shows comparable proton conductivity with Nafion117 at room temperature, but lower vanadium permeability in the temperature range of 25 to 85 °C. The vanadium permeability difference between two compared membranes intensifies disproportionally with the operating temperature, indicating that this composite membrane may be promising for further investigations in VRFBs.
Kim et al. developed a sandwiched-type Nafion/layered silicate AMH-3 (Nafion/AMH-3) membrane for VRFBs.26 Thanks to its 3-D ordered microporous structure, the AMH-3 layer acts as a permselective barrier for VO2+ using tortuous pathway effect. As a result, vanadium crossover is significantly minimized, leading to the enhancement in the CE and the cyclability. However, adding the AMH-3 layer magnifies the membrane resistance, thus reducing both VE and EE at 40 mA cm−2. At lower current density of 20 mA cm−2, EE of the sandwiched membrane is higher.
The purpose of modifying Nafion membrane is to maintain the excellent proton conductivity and chemical stability of Nafion while alleviating the VO2+ permeability. However, these two parameters often reverse. In most cases, lower VO2+ permeability (or higher ion selectivity) could only be achieved by reducing the proton conductivity. Thus, the balance between proton conductivity and VO2+ permeability is very important in deciding what modification may be chosen for further investigation and development.
3. Other polymeric cation exchange membranes
Despite the excellent proton conductivity and chemical stability, Nafion-based membranes have high vanadium permeability and high cost. This triggered the search for alternative non-Nafion-based membranes for VRFBs. Chen and Hickner studied the degradation of the sulfonated Radel (s-Radel) membrane in 1.7 M V5+ + 3.3 M H2SO4 solution at 40 °C for 3 days.27 Although the membrane exhibits good cell performance at room temperature, the ductile sample becomes brittle after the degradation test, probably due to the three-dimensional pores which are formed inside the membrane. After the degradation test, the intrinsic viscosity decreases by almost 50%. The degradation happens to other types of non-Nafion cation exchange membranes, reported by the same group of authors, indicating that the cation exchange membrane better than Nafion family is yet to be found up to date. In addition, Hickner and co-workers investigated and compared the vanadium crossover mechanisms in Nafion117 and s-Radel membrane.28 While the dominant mode of vanadium transport in Nafion is diffusion, convection dominates the vanadium transport through s-Radel membrane type. It was found by simulation that vanadium crossover in s-Radel changes direction during charge and discharge as shown in Fig. 3, leading to the lower “net” vanadium crossover in s-Radel in comparison with that in Nafion.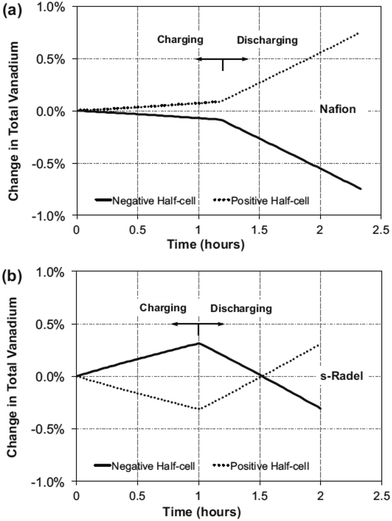 | ||
| Fig. 3 Predicted change in total vanadium during charge and discharge at both half-cells at the 10th cycle for (a) Nafion and (b) s-Radel membrane. Reprinted with permission from ref. 28. Copyright Elsevier, 2013. | ||
Winardi et al. prepared and optimized sulfonated poly (ether ether ketone) (SPEEK) membranes for VRFBs.29 In order to achieve the proton conductivity, and the ion exchange capacity (IEC) in the same magnitude of Nafion117, the optimized SPEEK has 2.5 times higher in water uptake and 2 times higher in swelling ratio comparing with characteristics of Nafion117. It was reported by the same authors that the cells containing SPEEK exhibit slightly higher CE, VE, and EE than the cells use Nafion117. In addition, OCV of the cells containing SPEEK drops more slowly than OCV of the cells employing Nafion117, indicating a smaller self-discharge rate. However, the chemical stability of SPEEK is much worse than that of Nafion117, even at room temperature. Further research is required to fix this problem before large scale exploitation of SPEEK can be made.
In attempts to improve the performance of SPEEK in VRFBs, several blend membrane materials of SPEEK and other polymers have been prepared by Li et al.30–32 and Liu et al.33 The second polymer could be polyacrylonitrile (PAN),33 poly(vinylidene fluoride) (PVDF),31 poly(vinylidene-co-hexafluoropropylene) (P(VDF-co-HFP)),32 polyetherimide (PEI).33 All of the blend membranes show enhanced CE and EE and better cyclability compared with Nafion117 and the pristine SPEEK, thanks to the smaller vanadium ion permeability. However, all obtained membranes also possess inferior proton conductivity and chemical stability (if tested) than those of Nafion117. On the other hand, composite membranes of SPEEK and various materials such as graphene,34 graphene oxide,35 mesoporous silica,36 and zirconium phosphate sulfophenylphosphonates (ZrPSPP)37 were prepared. All composite membranes show higher CE, higher EE, lower proton conductivity, and lower vanadium permeability comparing with Nafion117 performance.
Hickner and co-workers investigated the effect of the thickness of the sulfonated fluorinated poly(arylene ether) (SFPAE) in VRFBs.38 The membrane's thickness is optimized by controlling two parameters: the ohmic loss, and electrolyte crossover loss in the VRFBs. Thicker membranes generally deliver higher cell resistance while thinner ones exhibit higher vanadium ion crossover rates, leading to poor cell performance. Researchers from the same group prepared blends of SFPAE and P(VDF-co-HFP) for use as composite membranes in VRFBs.39 The mechanical strength of the membranes amplifies proportionally with the content of P(VDF-co-HFP), while vanadium ion permeability diminishes. Although the CE, VE and EE of VRFBs assembled with SFPAE/10%P(VDF-co-HFP) and SFPAE are similar, the cell lifetime of the composite membrane is improved by 44% comparing to pristine SFPAE. This may be due to the reinforcement effect of the P(VDF-co-HFP) in the blend.
Wang et al. fabricated the composite membrane consisted of sulfonated poly(phthalazinone ether ketone) (SPPEK) and tungstophosphoric acid (TPA) by solution casting method.40 Introduction of TPA lessens the swelling ratio and vanadium ion permeability as well as strengthens the tensile in comparison with the pristine SPPEK. As a result, EE of the composite membrane after 100 cycles at 60 mA cm−2 is comparable with that of Nafion117. However, the increment of TPA leads to reduction of the chemical stability of SPPEK, which is already lower than that of Nafion117. Wang et al. synthesized an amphiphilic block copolymer composed of hydrophobic polyaryletherketone (PAEK) and hydrophilic sulfonated polyaryletherketone (SPAEK) blocks.41 Then, a membrane was prepared from this block copolymer as used as separator in VRFBs. The membrane has five times higher in tensile strength and ten times higher in Young's modulus than those of Nafion117. In addition, the vanadium permeability of the block copolymer membrane is about 20 times lower than that of Nafion117, while ionic conductivity is only a half lower. As a result, CE of the block copolymer is almost 100%, while EE is comparable to that of Nafion117.
By blending boehmite (AlOOH) with sulfonated polyimide (SPI) at different compositions, Yang et al. improves the chemical stability of the SPI-based membranes when in contact with VRFBs electrolyte.42 A combination of a high proton conductive SPI membrane with a highly chemically stable AlOOH inorganic filter results in CPI/AlOOH composite membranes, which signify water uptake quantities (2–3 times) and obstruct VO2+ permeability (5–15 times smaller than Nafion117). However, the proton conductivity values of CPI CPI/AlOOH are about 50% comparing with the performance of Nafion117. The optimized CPI/AlOOH membrane contains 10% AlOOH. Such membrane exhibits higher CE and EE than Nafion117 membrane in a range of current density of 20–70 mA 2cm−2. Moreover, the self-discharge of batteries using CPI/AlOOH membrane is successfully suppressed. Authors from the same group also reported a preparation of sulfonated polyimide/chitosan (SPI/CS) composite membrane.43 With only 5.6 wt% of CS content, the water uptake decreases a half in comparison with that of pure SPI, while still double that of Nafion117. Nevertheless, its proton conductivity is less than two third of that of Nafion117, and the VO2+ permeability coefficient of the SPI/CS membrane is about 13 times smaller. Surprisingly, the CE and EE are only slightly higher than that of Nafion117. Considering its lower cost, the composite membrane still possesses some advantages over Nafion117 membrane. In addition, the same authors also added TiO2 and ZrO2 to form the composite membranes with SPI.44,45 Their results indicate that adding TiO2 and ZrO2 leads to increase in proton conductivity.
Semiz et al. studied the effect of the degree of disulfonation on the performance of the disulfonated poly(arylene ether sulfone) copolymer membranes in VRFBs.46 The best performance is achieved with the membrane which is disulfonated at 35 molar percent. The optimized membrane has three times higher in IEC, double in proton conductivity and about 10 times lower in vanadium ion permeability in comparison with those of Nafion212.47 Thus, the EE of the synthesized membrane is significantly higher than that of Nafion212 in current density range of 20–80 mA cm−2. However, the chemical stability and cyclability data for this very promising membrane are not yet reported.
Development of other types of CEMs is very attractive for a long period of time due to their superior ionic conductivity and chemical stability in comparison with AEMs, as well as their lower cost, and smaller vanadium ion permeability compared with the Nafion family.
4. Anion exchange membranes
In principal, anion exchange membranes (AEMs) are preferred for VRFBs since they prevent vanadium cations from entering the membrane owing to the Donnan exclusion effect. Thus, the vanadium permeability and the reaction rate with highly oxidizing VO2+ are diminished.3,8 However, AEMs normally suffer from the limited chemical stability as well as insufficient ionic conductivity (anions have lower mobility than proton), leading to the decrease of both cyclability and voltage efficiency.3 Therefore, there were limited publications about AEMs for VRFBs before 2012 as indicated by Prifti et al.3 However, a new trend arises and many papers have been published in the last two years about the development of AEMs for use in VRFBs. Typical publications related to AEMs are discussed here.Choi et al. evaluated the chemical stability of three commercial AEMs (aminated polysulphone anion exchange membrane (APS) from Asahi Glass Co., and ammonium-type anion exchange membrane (AHA), antifouling anion exchange membrane (AFN) from ASTOM Co.).48 Membrane resistance values of APS and AFN are relatively small and slightly change when soaking in VO2+ solution. On the other hand, use of AHA leads to larger membrane resistance and significant change of membrane resistance during the soaking process. VRFBs using AFN membrane have constant cell resistances with different states of charge (SOC) and different states of discharge (SOD), respectively. Compared with cells containing Nafion117, cells using AFN membrane have higher CE (better vanadium selectivity), but lower VE (lower ionic conductivity), and resulting in similar EE.
Zhang et al. prepared the poly(phthalazinone ether ketone ketone) AEMs with pyridinium as ion exchange groups (PyPPEKK).49 The PPEKK base membrane was prepared by the solution casting method, and then soaked in pyridine solution. Because the pyridinium ions hold positive charge, the optimized membrane has lower vanadium ion permeability compared with Nafion117. However, similar area resistances could be obtained for both types of membranes. As a result, cells using PyPPEKK membrane have higher CE but similar VE compared with cells containing Nafion117. Thus, EE of cells using PyPPEKK membrane is higher than cells using Nafion177 while the rate capabilities are of the same magnitude. In addition, the PyPPEKK membrane shows good durability after immersion in VO2+ solution for 60 days at room temperature. Researchers from the same group also investigated the performance of the quaternized poly(phthalazinone ether ketone) (QBPPEK) anion exchange membranes for VRFBs.50 The obtained membrane performs comparable water uptake and similar area resistance to the Nafion117. Since the quaternary groups hold positive charges and prevent vanadium ions from entering the membrane, the mass transfer coefficient of vanadium ions in QBPPEK is about 5 times smaller compared with Nafion117. As a result, the CE, VE and EE values of QBPPEK are similar to those of PyPPEKK, as well as the high chemical stability and cyclability.
Yun et al. synthesized the cardo-polyetherketone (PEK-C) based AEMs by chloromethylation of PEK-C, followed by quaternization using trimethylamine (TMA).51,52 The VO2+ permeability of the obtained membrane is 35 times lower compared with Nafion212 membrane. The significantly low VO2+ permeability could be explained by the Donnan exclusion effect initiated by quaternary ammonium groups and the presence of narrow and loosely connected hydrophilic channels which strongly confine water molecules. The AEMs exhibit comparable ultimate tensile strength and Young's modulus compared with Nafion212. However, the elongation at break of the membrane is at least 10 times smaller than that of Nafion and this leads to the possibility of cracking during VRFBs operation. Moreover, the membranes partly lose their ionic conductivity and ultimate tensile strength after prolong cycling for 100 h. Chemical stability of this membrane during VRFBs operation should be improved for practical application.
Seo et al. prepared the AEMs (PE/VBC) for VRFBs using a porous polyethylene substrate (PE) filled with poly(4-vinylbenzyl chloride) (VBC), and then amination was conducted to deliver membranes containing pyridyl functional groups.53 At a comparable electrical area resistance, the PE/VBC membrane has similar value of water uptake but more than 7 times smaller in swelling ratio in comparison with Nafion117 thanks to the strength of the porous PE film. Moreover, vanadium permeability of the PE/VBC membrane is about 10 times lower than that of Nafion117, which is mainly due to the positively charged pyridyl functional groups on the polymer backbone and the dense polymer structure. Thus, the CE, VE and EE of the optimized PE/VBC membrane are 3.1%, 2.6% and 5.4% higher compared with Nafion117, respectively. Chemical stability test should be conducted to verify further development of this promising membrane.
Mallinson et al. investigated the effect of other amine-functionalized groups (dimethylamine (DMA), trimethylamine (TMA) or diazabicyclo(2,2,2)octane (DABCO)) on the application of AEMS in VRFBs.54 The AEMs were prepared by radiation grafting of vinylbenzyl chloride (VBC) onto ethylene tetrafluoroethylene (ETFE) co-polymer film, and then amine-functionalized. While the ion exchange capacities of the synthesized membranes are all higher than that of Nafion115, there is a tight relationship between the water uptake and the vanadium(IV) ion permeability of the membranes, as shown in Fig. 4. Controlling water transport through the membrane is important for limiting vanadium ion crossover. Further thermos-oxidative stability test indicated that the least hydrophilic anion-exchange membrane (with DMA functional groups) is stable toward oxidation, owning to its hydrophobic nature so that the aqueous vanadium solution cannot interact effectively with it. Other two AEMs show significant degradation.
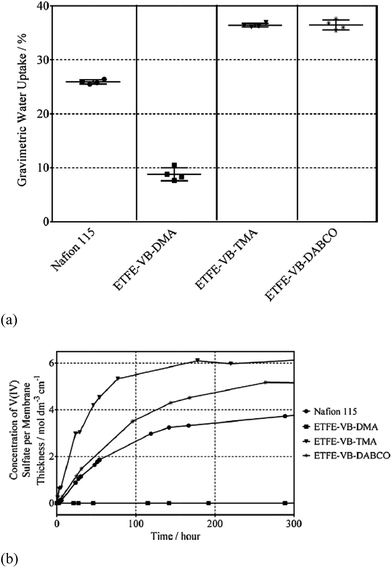 | ||
| Fig. 4 (a) Gravimetric water uptake and (b) vanadium(IV) ion permeability of aminated AEMs in comparison to those of Nafion115. Reprinted with permission from ref. 54. Copyright Elsevier, 2014. | ||
Jung et al. reported the polysulfone-based AEMs with quaternary benzyl trimethylammonium functional groups (PSF-TMA) in VRFBs.55 The membranes are characterised by a 40-fold reduction in VO2+ permeability when compared to Nafion212 membrane. After a stability test of about 30 days in VO2+ solution, the PSF-TMA membrane becomes very brittle. The authors implied that mechanical stresses are the main causes of brittleness, not chemical degradation. In addition, the PSF-TMA membrane has lower ionic conductivity than Nafion membrane. As a result, at 30 mA cm−2, the PSF-TMA membranes offer higher CE, but smaller VE comparing to Nafion during about 10 charge–discharge cycles.
Fang et al. developed an anion exchange membrane based on the copolymer of N-vinylimidazole(VI) and 2,2,2-trifluoroethyl methacrylate (TFEMA).56 This is an anion exchange membrane which possesses ionic conductivity and VO2+ permeability lower compared to Nafion117 about 5 and 30 times, respectively. Thus, the membrane has an excellent CE of 99.5% but low VE of 75.3% at 50 mA g−2. As a consequence, EE of the membrane is slightly higher than that of Nafion117. In addition, the tensile stress and elongation of the membrane slightly change after 350 cycles of VRFBs operation. Hwang et al. prepared the AEMs by the solution polymerization of VI, TFEMA and divinylbenzene (DVB), then quaternized with bromoethane (QVDT) and finally the films are cast.57 Water uptake and IEC rise proportionally with the VI content, while the electrical resistance decreases. These phenomena are explained by the high hydrophilicity of VI, and the quaternization of VI promotes efficient ion exchanges. The AEMs have stable CE, VE and EE of 94.6%, 79.6%, and 75.3%, leading to discharge capacity retention of 47% after 150 cycles at a current density of 40 mA cm−2.
Chen et al. investigated the effect of IEC on the performance of quaternary ammonium functionalised Radel (QA-Radel) AEMs in VRFBs.58 Ionic conductivity, VO2+ permeability, and VE magnify with the increase of IEC, while CE decreases. Thus, material parameters must be optimized to achieve the maximum cell performance. The optimized QA-Radel demonstrates comparable water uptake, swelling ratio and ionic conductivity with Nafion212, but exhibits two orders of magnitude smaller in VO2+ permeability. EE of all QA-Radel membranes are significantly higher than that of Nafion212 at various current densities in the 20–80 mA cm−2 range. However, the chemical stabilities of these membranes were not mentioned in this work. This group also reported the effect of the degree of functionalisation (DF) by the chloromethylation and crosslinking on the properties of the cross-linked quaternary ammonium functionalised Radel membranes.59 DF could be easily controlled by monitoring the chloromethylation time. Ionic conductivity enhances with the increment of DF, along with the decrease of vanadium ion permeability. On the other hand, crosslinking hinders the membranes from swelling in water but reduces the ionic conductivity.
Chen et al. characterised their quaternary ammonium functionalised poly(fluorenyl ether) (QA-PFE) membrane in VRFBs and found there is no detectable VO2+ crossover after one month of vanadium permeability test.60 On the other hand, the ionic conductivity of QA-PFE is about a half of the Nafion212. As a result, the CE of QA-PFE is 100%, or close to, indicating that there is no vanadium crossover or side reaction during controlled conditions (using sealed and N2 purged electrolyte tank and keep the upper limit potential of 1.7 V to avoid side reactions of vanadium ions and electrode corrosion, respectively). Within a current density range of 20–80 mA cm−2, CE of QA-PFE is 100% and the VE is smaller than that of Nafion212 due to lower ionic conductivity, but it exhibits higher EE than Nafion212 when the current density is smaller than 60 mA cm−2. There is no measurable capacity fading over 15 cycles in VRFBs containing QA-PFE, which correlates well with the 100% CE of the battery. This membrane is highly desirable for moderate current density VRFB operation if it is mechanically and chemically stable.
Mai et al. prepared the quaternized poly(tetramethyl diphenyl ether sulfone) (QAPES) through the bromination synthesis.61 QAPES membranes show much lower vanadium permeability rates (about 2 orders of magnitude) than that of Nafion115 thanks to the Donnan exclusion effect of the –NR3+ group and the loosely connected hydrophilic domains. In addition, the self-discharge time of QAPES membranes is 4 times higher than that of Nafion215. Furthermore, the QAPES membranes could endure more than 250 h immersed in VO2+ solution with a slight increase in VO2+, indicating high chemical stability in the tested condition. The optimized QAPES membrane has higher CE and EE but comparable VE to Nafion115, and these variables are stable during 100 charge–discharge cycles at 80 mA cm−2.
In their study about quaternary ammonium functionalised Diels–Alder poly(phenylene)s (QDAPPs) with different ion exchange capacities (IECs), Sun et al. emphasized the trade-off between rate capability, indicated by cell voltage loss at a given current density, and vanadium crossover rate.62 This trade-off can be applied to all mentioned AEMs above, since lower vanadium ion permeability is corresponded to lower IECs as well as lower ionic conductivity, leading to the reduction of VE and rate capability. In addition, all studied AEMs cannot be compared with Nafion membranes in chemical stability, especially at elevated temperature. Nevertheless, the low vanadium ion permeability is promising, since VRFBs using AEMs normally have good cyclability and low self-discharge rate, and some of them achieved almost 100% of CE in controlled experimental conditions.
5. Amphoteric ion exchange membranes
Cation exchange membranes (CEMs) possess high ionic conductivity, good chemical stability but seriously high vanadium crossover rates, which lead to low CE and poor cyclability. On the other hand, AEMs have much lower vanadium ion permeability owing to the Donnan exclusion effect, leading to higher CE and better cyclability. However, the use of AEMs is restricted by their lower ionic conductivity, resulting in the reduction in VE and rate capability of the VRFBs. Amphoteric ion exchange membranes (AIEMs) have both cation and anion exchange capabilities, thus have both low vanadium ion permeability and high ionic conductivity. Surprisingly, there are only a few papers about AIEMs for VRFBs have been published recently.An AIEM was prepared by Yuan et al. by radiation grafting of sodium styrene sulfonate (SSS) and N,N-dimethylaminoethyl methacrylate (DMAEMA) simultaneously into poly(vinylidene difluoride) (PVDF) film.63 At similar conductivity level, water uptake of the AIEM is less than a half of that of Nafion117, while vanadium ion permeability of the AIEM is about 15 times lower compared with Nafion117. As a result, OCV of the AIEM is maintained above 1.4 V for 85 h which is six-fold longer than that of Nafion117, indicating a minimal self-discharge rate.
Wang et al. modified the sulfonated poly(fluorenyl ether ketone) (SPFEK) membranes by layer-by-layer assembly of positive charged poly(diallyl dimethyl ammonium chloride) (PDDA) and negative charged poly(sodium styrene sulfonate) (PSS).64 The obtained membranes (PDDA/PSS-SPFEK) demonstrate smaller proton conductivity as well as vanadium ion permeability in comparison with the pristine SPFEK membrane and the Nafion117. Specifically, the proton conductivity and vanadium ion permeability of SPFEK membranes with two self-assembly bilayers of PDDA/PSS are of two third and one tenth of these of Nafion117. Increment of the number of bilayers slightly changes these values. While the PDDA/PSS-SPFEK membrane possesses significantly higher CE than those of SPFEK and Nafion117 in a range of current densities of 20–60 mA cm−2, its self-discharge performance is slightly better than those of the other two. The same group of researchers also synthesized a sulfonated poly(fluorenyl ether ketone) with pendant quaternary ammonium groups (SPFEKA) by one-pot copolymerization method.65 The copolymers were dissolved in aprotic solvents, cast onto membranes, immersed into 1 M sulphuric acid and used in VRFBs. While the proton conductivity of the optimized SPFEKA membrane is one third of that of Nafion115, the vanadium ion permeability of the AIEMs is less than 5% of the Nafion membrane. As a result, SPFEKA membranes possess higher discharge capacity and much higher CE compared with Nafion115 at the current density of 50 mA cm−2.
Li et al. prepared the AIEMs by attaching 2-aminoethanesulfonic acid (taurine), as both anion and cation exchange groups, to the brominated fluorinated poly(aryl ether oxadiazole) main polymer chain.66 The final membranes were prepared via solution casting method. The optimized membrane outperforms the Nafion115 thanks to the significantly lower vanadium permeability and much better self-discharge performance.
All reported AIEMs show smaller vanadium ion permeability, higher CE, longer self-discharge periods, and smaller (but acceptable) proton conductivity. However, cyclability and rate capability of the VRFBs containing the AIEMs were not mentioned in these papers, indicating that the AIEMs may not meet the requirements of these criteria yet to be considered for practical application in VRFBs.
6. Nonionic porous membranes
Besides ion exchange membranes (IEMs) such as CEMs, AEMs and AIEMs, nonionic porous (micro or nanoporous) membranes have been developed for application in VRFBs. The porous membranes obstruct vanadium ion transport, but allow proton transfer via pore size exclusion effect. In comparison with the commercial IEMs such as Nafion membranes, porous membranes are of remarkably lower cost, thus they may be excellent alternatives to potentially replace the highly expensive IEMs.67–69Wei et al. investigated the application of polyvinylidene fluoride (PVDF) ultrafiltration membranes in VRFBs.68 PVDF porous membranes with different cross section morphologies could be prepared by applying the phase inversion method via changing the polymer solution concentration. The smallest attainable pore size is around 50 nm. Although with these relatively large pore sizes, the PVDF membranes have about 35% lower vanadium ion permeability in comparison with the Nafion membrane with similar thicknesses. Possible reasons can be the hydrophobic pore walls and the long and tortuous path, which could increase the resistance of vanadium ion transport through the membranes. The proton is much smaller in size and its mobility is less likely to be affected by the hydrophobicity and tortuosity. As a result, CE and EE of such porous PVDF are of the same magnitude with Nafion115. Moreover, the PVDF membranes are stable after over 1000 charge–discharge cycles at 80 mA cm−2, almost unchanged in CE, VE, EE and morphology.
Wei et al. prepared a nanoporous polytetrafluoroethylene (PTFE)/silica composite membrane.69 The membrane was prepared by blending amorphous silica particles in PTFE dispersion, delivering a nanostructure with an average pore size of 38 nm and 48% of porosity. The nanoporous membrane possesses an H/V selectivity of 7.7, and slightly smaller CE, VE and EE compared with Nafion115. However, Fig. 5 shows that VRFBs using PTFE/silica membrane could maintain stable capacity and energy retention upon 50 charge–discharge cycles (50 mA cm−2), which is contrary to the poor cyclability of Nafion115. This may be due to the difference in the capacity fading mechanism.
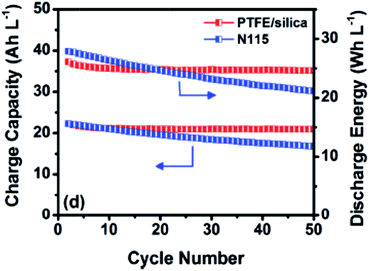 | ||
| Fig. 5 Charge capacity and discharge energy over cycling of the VRFBs containing nanoporous polytetrafluoroethylene/silica (PTFE/silica) composite membrane and Nafion115 (N115) membrane at the current density of 50 mA cm−2. Reprinted with permission from ref. 69. Copyright John Wiley & Sons, 2013. | ||
Zhou et al. prepared a porous polybenzimidazole (PBI) membrane by immersing a commercial PBI membrane in 4 M H2SO4 for 7 days.70 The ionic conductivity of the PBI membrane is one third of that of Nafion211, which is much higher than the one of the pristine PBI. On the other hand, vanadium ion permeability of the PBI membrane is two orders of magnitude lower than that of Nafion211. As a result, CE of the PBI membrane is up to 99% at current density range of 20–80 mA cm−2, and the cyclability is improved. In addition, PBI membrane shows decent chemical stability in 1 M V5+ solution at room temperature for 120 days.
In VRFBs, high vanadium ion permeability through Nafion membranes is caused by the sulfonic acid functional groups, while the non-ionic porous membranes contains none of these groups.70 Therefore, ion transport mechanisms of non-ionic porous membranes and Nafion membranes are different.69 However, ion selection mechanisms in non-ionic porous membranes are explained in slightly different ways among research groups. Wei et al. indicated that the non-ionic porous membranes select vanadium ions from protons due to their difference in radius, charge density and specific interactions with electrolyte and membranes.68 Therefore, larger vanadium ions may be excluded from the well-tuned pores. On the other hand, Wang's group reported that high H/V selectivity (7.7) was achieved simply because of the much larger diffusion rate of H+ comparing with that of VO2+ (as well as other vanadium ions), due to the significant differences in Stokes radii and molecular weight.69 On the contrary, PBI membranes prepared by Zhou et al. have the pore size in a range from 0.5 to 2 nm, which is much smaller than that of Nafion (typically 4 nm).70 Thus, PBI membranes allow proton transport and limit the transport of vanadium ions only due to size-exclusion effect.
Nonionic porous membranes may be attracted in term of cost, however, their proton/vanadium selectivity is much less than AEMs, and their proton conductivity is inferior compared with CEMs. Since there are just a few research groups working on porous membranes, more investigations are required to prove the feasibility of these membranes in industrial VRFB applications. Difficulty in controlling the heterogeneous morphology may be a challenge for applications of porous separators in large-scale VRFBs.33
Six categories of polymer membranes for used in VRFBs are compared and presented in Table 1.
7. Conclusions
While the Nafion family of membranes is still in at interests, research progress has been made to mitigate the disadvantage properties. The best reported modified Nafion membrane (Nafion/FC) has ion selectivity 2.1 times higher than the pristine Nafion while remaining comparable proton conductivity.22 The maturing of other cation exchange materials and the evolution of anion exchange materials make crucial contributions. The CEMs prepared from the block copolymer of PAEK and SPAEK have superior mechanical strength and 20 times lower in vanadium ion permeability versus those of Nafion117, while ionic conductivity is only a half lower.47 This leads to CE of almost 100%. On the other hand, there are reports of AEMs having vanadium ion permeability two orders of magnitude lower than that of Nafion membranes.58,61 However, ionic conductivity and chemical stability of those AEMs are normally not acceptable. It is predicted that more works related to new membranes will be reported. The exploitations of amphoteric membranes and non-ion exchange membranes in VRFBs are relatively new and promising research trends thanks to the advantage of novel outputs from materials science and polymer chemistry to further the design and targeted synthesis of membrane materials. The development of membranes for VRFBs is a trade-off between ionic conductivity and vanadium ion selectivity. Thus, it depends on the requirements of each specific battery system, suitable candidates can be screened and selected.Acknowledgements
This research was financially supported by Positec, Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Foundation for Innovation (CFI), the Canada Research Chairs (CRC) program, and Mitacs (IT04444).Notes and references
- A. Parasuraman, T. M. Lim, C. Menictas and M. Skyllas-Kazacos, Electrochim. Acta, 2013, 101, 27–40 CrossRef CAS PubMed.
- G. Kear, A. A. Shah and F. C. Walsh, Int. J. Energy Res., 2012, 36, 1105–1120 CrossRef CAS PubMed.
- H. Prifti, A. Parasuraman, S. Winardi, T. M. Lim and M. Skyllas-Kazacos, Membranes, 2012, 2, 275–306 CrossRef CAS PubMed.
- L. H. Thaller, Proceedings of the 9th Intersociety Energy Conversion Engineering Conference, 1974 Search PubMed.
- M. Rychcik and M. Skyllas-Kazacos, J. Power Sources, 1988, 22, 59–67 CrossRef CAS.
- F. Rahman and M. Skyllas-Kazacos, J. Power Sources, 2009, 189, 1212–1219 CrossRef CAS PubMed.
- P. K. Leung, Q. Xu, T. S. Zhao, L. Zeng and C. Zhang, Electrochim. Acta, 2013, 105, 584–592 CrossRef CAS PubMed.
- K.-D. Kreuer, Chem. Mater., 2014, 26, 361–380 CrossRef CAS.
- S. Maurya, S.-H. Shin, Y. Kim and S.-H. Moon, RSC Adv., 2015, 5, 37206–37230 RSC.
- Q. Xu and T. S. Zhao, Prog. Energy Combust. Sci., 2015, 49, 40–58 CrossRef PubMed.
- V. Viswanathan, A. Crawford, D. Stephenson, S. Kim, W. Wang, B. Li, G. Coffey, E. Thomsen, G. Graff, P. Balducci, M. Kintner-Meyer and V. Sprenkle, J. Power Sources, 2014, 247, 1040–1051 CrossRef CAS PubMed.
- M. Skyllas-Kazacos, M. H. Chakrabarti, S. A. Hajimolana, F. S. Mjalli and M. Saleem, J. Electrochem. Soc., 2011, 158, R55–R79 CrossRef CAS PubMed.
- Q. Luo, L. Li, W. Wang, Z. Nie, X. Wei, B. Li, B. Chen, Z. Yang and V. Sprenkle, ChemSusChem, 2013, 6, 268–274 CrossRef CAS PubMed.
- T. Mohammadi, S. C. Chieng and M. Skyllas-Kazacos, J. Membr. Sci., 1997, 133, 151–159 CrossRef CAS.
- C. Sun, J. Chen, H. Zhang, X. Han and Q. Luo, J. Power Sources, 2010, 195, 890–897 CrossRef CAS PubMed.
- A. Tang, J. Bao and M. Skyllas-Kazacos, J. Power Sources, 2011, 196, 10737–10747 CrossRef CAS PubMed.
- Z. Tang, R. Svoboda, J. S. Lawton, D. S. Aaron, A. B. Papandrew and T. A. Zawodzinski, J. Electrochem. Soc., 2013, 160, F1040–F1047 CrossRef CAS PubMed.
- S. Jeong, L.-H. Kim, Y. Kwon and S. Kim, Korean J. Chem. Eng., 2014, 31, 2081–2087 CrossRef CAS.
- X. Teng, J. Dai, J. Su, Y. Zhu, H. Liu and Z. Song, J. Power Sources, 2013, 240, 131–139 CrossRef CAS PubMed.
- X. Teng, C. Sun, J. Dai, H. Liu, J. Su and F. Li, Electrochim. Acta, 2013, 88, 725–734 CrossRef CAS PubMed.
- J. Ma, S. Wang, J. Peng, J. Yuan, C. Yu, J. Li, X. Ju and M. Zhai, Eur. Polym. J., 2013, 49, 832–1840 Search PubMed.
- X. Teng, J. Dai, J. Su and G. Yin, J. Membr. Sci., 2015, 476, 20–29 CrossRef CAS PubMed.
- S. Lu, C. Wu, D. Liang, Q. Tan and Y. Xiang, RSC Adv., 2014, 4, 24831–24837 RSC.
- K. J. Lee and Y. H. Chu, Vacuum, 2014, 107, 269–276 CrossRef CAS PubMed.
- B. G. Kim, T. H. Han and C. G. Cho, J. Nanosci. Nanotechnol., 2014, 14, 9073–9077 CrossRef CAS PubMed.
- J. Kim, J.-D. Jeon and S.-Y. Kwak, Electrochem. Commun., 2014, 38, 68–70 CrossRef PubMed.
- D. Chen and M. A. Hickner, Phys. Chem. Chem. Phys., 2013, 15, 11299–11305 RSC.
- E. Agar, K. W. Knehr, D. Chen, M. A. Hickner and E. C. Kumbur, Electrochim. Acta, 2013, 98, 66–74 CrossRef CAS PubMed.
- S. Winardi, S. C. Raghu, M. O. Oo, Q. Yan, N. Wai, T. M. Lim and M. Skyllas-Kazacos, J. Membr. Sci., 2014, 450, 313–322 CrossRef CAS PubMed.
- Z. Li, W. Dai, L. Yu, L. Liu, J. Xi, X. Qiu and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 18885–18893 CAS.
- Z. Li, J. Xi, H. Zhou, L. Liu, Z. Wu, X. Qiu and L. Chen, J. Power Sources, 2013, 237, 132–140 CrossRef CAS PubMed.
- Z. Li, L. Liu, L. Yu, L. Wang, J. Xi, X. Qiu and L. Chen, J. Power Sources, 2014, 272, 427–435 CrossRef CAS PubMed.
- S. Liu, L. Wang, Y. Ding, B. Liu, X. Han and Y. Song, Electrochim. Acta, 2014, 130, 90–96 CrossRef CAS PubMed.
- W. Dai, L. Yu, Z. Li, J. Yan, L. Liu, J. Xi and X. Qiu, Electrochim. Acta, 2014, 132, 200–207 CrossRef CAS PubMed.
- W. Dai, Y. Shen, Z. Li, L. Yu, J. Xi and X. Qiu, J. Mater. Chem. A, 2014, 2, 12423–12432 CAS.
- Z. Li, W. Dai, L. Yu, J. Xi, X. Qiu and L. Chen, J. Power Sources, 2014, 257, 221–229 CrossRef CAS PubMed.
- J. Pan, S. Wang, M. Xiao, M. Hickner and Y. Meng, J. Membr. Sci., 2013, 443, 19–27 CrossRef CAS PubMed.
- D. Chen, M. A. Hickner, E. Agar and E. C. Kumbur, J. Membr. Sci., 2013, 437, 108–113 CrossRef CAS PubMed.
- D. Chen, S. Kim, V. Sprenkle and M. A. Hickner, J. Power Sources, 2013, 231, 301–306 CrossRef CAS PubMed.
- N. Wang, J. Yu, Z. Zhou, D. Fang, S. Liu and Y. Liu, J. Membr. Sci., 2013, 437, 114–121 CrossRef CAS PubMed.
- F. Wang, J. M. Sylvia, M. M. Jacob and D. Peramunage, J. Power Sources, 2013, 242, 575–580 CrossRef CAS PubMed.
- Y. Zhang, J. Li, L. Wang and S. Zhang, J. Solid State Electrochem., 2014, 18, 3479–3490 CrossRef CAS.
- M. Yue, Y. Zhang and L. Wang, J. Appl. Polym. Sci., 2013, 127, 4150–4159 CrossRef CAS PubMed.
- J. Li, Y. Zhang and L. Wang, J. Solid State Electrochem., 2014, 18, 729–737 CrossRef CAS.
- J. Li, Y. Zhang, S. Zhang, X. Huang and L. Wang, Polym. Adv. Technol., 2014, 25, 1610–1615 CrossRef CAS PubMed.
- L. Semiz, N. D. Sankir and M. Sankir, J. Membr. Sci., 2014, 468, 209–215 CrossRef CAS PubMed.
- L. Semiz, N. D. Sankir and M. Sankir, Int. J. Electrochem. Sci., 2014, 9, 3060–3067 Search PubMed.
- H.-S. Choi, Y.-H. Oh, C.-H. Ryu and G.-J. Hwang, J. Taiwan Inst. Chem. Eng., 2014, 45, 2920–2925 CrossRef CAS PubMed.
- S. Zhang, B. Zhang, D. Xing and X. Jian, J. Mater. Chem. A, 2013, 1, 12246–12254 CAS.
- S. Zhang, B. Zhang, G. Zhao and X. Jian, J. Mater. Chem. A, 2014, 2, 3083–3091 CAS.
- S. Yun, J. Parrondo and V. Ramani, ECS Trans., 2014, 58, 55–64 CrossRef PubMed.
- S. Yun, J. Parrondo and V. Ramani, J. Mater. Chem. A, 2014, 2, 6605–6615 CAS.
- S.-J. Seo, B.-C. Kim, K.-W. Sung, J. Shim, J.-D. Jeon, K.-H. Shin, S.-H. Shin, S.-H. Yun, J.-Y. Lee and S.-H. Moon, J. Membr. Sci., 2013, 628, 17–23 CrossRef PubMed.
- S. L. Mallinson, J. R. Varcoe and R. C. T. Slade, Electrochim. Acta, 2014, 140, 145–151 CrossRef CAS PubMed.
- M.-S. J. Jung, J. Parrondo, C. G. Arges and V. Ramani, J. Mater. Chem. A, 2013, 1, 10458–10464 CAS.
- J. Fang, H. Xu, X. Wei, M. Guo, X. Lu, C. Lan, Y. Zhang, Y. Liu and T. Peng, Polym. Adv. Technol., 2013, 24, 168–173 CrossRef CAS PubMed.
- C. W. Hwang, H.-M. Park, C. M. Oh, T. S. Hwang, J. Shim and C.-S. Jin, J. Membr. Sci., 2014, 468, 98–106 CrossRef CAS PubMed.
- D. Chen, M. A. Hickner, E. Agar and E. C. Kumbur, ACS Appl. Mater. Interfaces, 2013, 5, 7559–7566 CAS.
- D. Chen, M. A. Hickner, E. Agar and E. C. Kumbur, ECS Trans., 2013, 53, 83–89 CrossRef PubMed.
- D. Chen, M. A. Hickner, E. Agar and E. C. Kumbur, Electrochem. Commun., 2013, 26, 37–40 CrossRef CAS PubMed.
- Z. Mai, H. Zhang, H. Zhang, W. Xu, W. Wei, H. Na and X. Li, ChemSusChem, 2013, 6, 328–335 CrossRef CAS PubMed.
- C.-N. Sun, Z. Tang, C. Belcher, T. A. Zawodzinski and C. Fujimoto, Electrochem. Commun., 2014, 43, 63–66 CrossRef CAS PubMed.
- J. Yuan, C. Yu, J. Peng, Y. Wang, J. Ma, J. Qiu, J. Li and M. Zhai, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 5194–5202 CrossRef CAS PubMed.
- Y. Wang, S. Wang, M. Xiao, D. Han, M. A. Hickner and Y. Meng, RSC Adv., 2013, 3, 15467–15474 RSC.
- Y. Wang, S. Wang, M. Xiao, S. Song, D. Han, M. A. Hickner and Y. Meng, Int. J. Hydrogen Energy, 2014, 39, 16123–16131 CrossRef CAS PubMed.
- C.-P. Li, S.-M. Zhang, S.-B. Wang, X.-F. Xie and C.-S. Deng, ECS Electrochem. Lett., 2014, 3, A102–A104 CrossRef CAS PubMed.
- B. Li, Q. Luo, X. Wei, Z. Nie, E. Thomsen, B. Chen, V. Sprenkle and W. Wang, ChemSusChem, 2014, 7, 577–584 CrossRef CAS PubMed.
- W. Wei, H. Zhang, X. Li, H. Zhang, Y. Li and I. Vankelecom, Phys. Chem. Chem. Phys., 2013, 15, 1766–1771 RSC.
- X. Wei, Z. Nie, Q. Luo, B. Li, B. Chen, K. Simmons, V. Sprenkle and W. Wang, Adv. Energy Mater., 2013, 3, 1215–1220 CrossRef CAS PubMed.
- X. L. Zhou, T. S. Zhao, L. An, L. Wei and C. Zhang, Electrochim. Acta, 2015, 153, 492–498 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |