DOI:
10.1039/C2RA20785K
(Paper)
RSC Adv., 2012,
2, 11095-11103
Hydrogel based on an alginate–Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system
Received
26th April 2012
, Accepted 19th September 2012
First published on 20th September 2012
Abstract
This work reports on the formation and characterization of a novel hydrogel based on the polysaccharide alginate physically crosslinked by calcium ions (Ca2+) in the presence of chondroitin sulfate (CS). Swelling data and morphological analysis showed that the alginate–Ca2+/CS hydrogel has a clear pH-dependent behavior. Compression tests revealed a gradual decrease in the elastic moduli (E) as the pH of hydrogel-immersed solution is raised but a pronounced decrease for the samples swelled at pH 8 was observed. This fact was attributed to a complete disruption of the polymer network. On the other hand, the hydrogel matrix remains stable when immersed in acidic media. In vitro drug release studies were performed in simulated gastric and intestinal fluids and the collected data showed that the hydrogels formed in this work respond to external stimuli (changes in pH) and, thus, present great potential to be applied in a colon-specific drug delivery system.
1. Introduction
Hydrogels are three-dimensional hydrophilic polymer networks, chemically or physically crosslinked, which are able to absorb and retain large amounts of liquid or biological fluids.1 The chemical crosslinking is characterized by the formation of covalent bonds among the polymeric chains, producing irreversible hydrogels. On the other hand, the physical crosslinking is characterized by electrostatic interactions and/or hydrogen bonds among the polymeric chains that can be disrupted, at given conditions, enabling the production of a reversible hydrogel. Recently, hydrogels have been widely applied in the biomedical field as absorbing agents to remove water from edemas caused by burns,2 as devices for drug release,3etc. The hydrogels applied in these fields have to possess some particular features such as non-toxicity, biocompatibility, and biodegradability. In light of this, the hydrogels based on polymers from natural sources, for instance, sodium alginate,4 chitosan,5 arabic gum,6 and chondroitin sulfate7 fill all of those requested features. Sodium alginate (SA) is the sodium salt of the alginic acid. It is a non-toxic polysaccharide extracted from the marine algae Lessonia trabeculata (brown algae) possessing linear chains and a high molar mass.8,9 Alginate has been applied in the biomedical field due to its interesting and desirable features (e.g. biocompatibility, hydrophilicity and biodegradability).10,11 Another interesting feature of alginate is the ease with which it is crosslinked in the presence of divalent cations, such as calcium ions (Ca2+), forming physical but mechanically consistent hydrogels.12,13 Physically crosslinked hydrogels have some advantages over chemically crosslinked ones because no extra crosslinking agents are involved to form the network. Some criticisms have been made in the literature concerning the use of crosslinking agents to form hydrogels because it may decrease the biocompatibility, which might cause intoxication if administered in certain medical treatments.14 If the biocompatibility decreases due to the use of certain chemical crosslinking agents, the applicability of these hydrogels as biomaterials is restricted.
Besides the characteristics discussed above, it is consensual that to achieve applicability as a biomaterial, hydrogels have to present satisfactory mechanical properties.15 Hydrogels formed by the mixture of two different polymeric systems may result in new materials that, often, present entirely different properties from the original polymers.16 Therefore, the incorporation of others polymers, like chondroitin sulfate (CS), into the hydrogel network can improve the final properties of its 3D matrix.17 The CS is a natural polysaccharide classified as a glycosaminoglycan (GAG) and it has an important role as a constituent of the extracellular cartilage matrix (ECM).18 Due to the interesting features presented by both of these polysaccharides, alginate and CS, this work has an aim to form a hydrogel based on alginate crosslinked with Ca2+ ions in presence of CS. Furthermore, studies involving the in vitro release of indomethacin were performed to evaluate the potential of the alginate–Ca2+/CS hydrogel to be applied in a drug delivery system.
2. Materials and methods
2.1 Materials
SA [viscometric molar mass (Mv) of 3.46 × 105 g mol−1] was purchased from Aldrich (USA). The Mv of SA was determined by the intrinsic viscosity, [η], using the Mark–Houwink–Sakurada equation, [η] = K(MV)α. The parameters α = 0.97 and K = 2.0 × 10−5 cm3 g−1 were obtained from literature.19 CS (Mv of 22 × 103 g mol−1) was kindly supplied by Solabia (Brazil). The Mv of CS was determined according to a methodology proposed by Wasteson.20 The values for the Mark–Houwink–Sakurada constants, α and K, for CS are 1.1 and 5.0 × 10−5 cm3 g−1, respectively. It is important to note that the CS utilized in this work is a mixture of CS sulfate in the C4 and C6 forms of the N-acetylgalactosamine (GalNAc) moiety. Calcium chloride anhydrous (CaCl2) (≥93% purity) was purchased from Nuclear (Brazil) and commercial indomethacin (IND) was purchased from a local apothecary (99% purity). All reactants were used as received.
2.2 Alginate–Ca2+/CS hydrogel formation
The alginate–Ca2+/CS hydrogel was formed by mixing equal volumes (25 ml) of aqueous solutions of SA (28.9 μmol l−1) and CS (909.1 μmol l−1). The resultant solution was kept under magnetic stirring for 1 h at room temperature. The solution was deposited in a small cubic container and then, CaCl2 solution (450 mmol l−1) was gently sprayed upon the solution and a thin film was formed in the top. After, this small container was immersed into a larger container containing CaCl2 solution (300 mmol l−1). After 24 h at room temperature, a mechanically consistent hydrogel was obtained. The as-formed hydrogel was removed from the small cubic container and then purified in distilled water at room temperature for 12 h. The alginate–Ca2+/CS hydrogel was dried at room temperature for 48 h.
2.3 Hydrogel characterization
One fraction of dry hydrogel was crushed and characterized by Fourier transform infrared spectroscopy (FTIR) (Shimadzu Scientific Instruments - model 8300, Japan), by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Netzsch - model STA 409 PG/4/G Luxx, USA) and by wide-angle X-ray scattering (WAXS) (Shimadzu - model XRD-600, Japan).
2.3.1 SEM analysis.
Another fraction of dry hydrogel was re-swelled in distilled water and cut into cubes (edges about 1.5 cm). After swelling in distilled water and in buffer solutions (pH 2 and 8) for 6 h the samples were collected and frozen in N2(l) for 5 min, fractured and freeze-dried for 48 h. Their morphologies were investigated by scanning electron microscopy (SEM) in a Shimadzu microscope (model SS 550, Japan). The buffer solutions at constant ionic strength (0.1 mol l−1, by addition of KCl), in which the hydrogels were immersed, were produced according to the National Book of Formulas – United States Pharmacopoeia.21
2.3.2 Swelling degree.
For this test, the as-formed alginate–Ca2+/CS swollen hydrogels were cut into small samples (cubic shape - edges of about 1.5 cm) that were dried at room temperature. Therefore, the dry samples were weighed and then immersed in distilled water and in buffer solutions (pH 2, 4, 6, 8, and 10) for up to 10 h, at room temperature (ca. 25 °C). At desired time intervals, each sample was collected and weighed, in order to determine the value of St. For each condition three samples (n = 3) were used, so the St data refer to the average of triplicates. The swelling degree parameter (St), which evaluates the liquid uptake capacity of the hydrogels, was determined from the following equation: | |  | (1) |
where Wt is the weight of the swollen hydrogel at time t and Wo is its dry weight.
2.3.3 Mechanical properties.
The elastic moduli (E) were calculated for the swollen hydrogel samples (cubic shape - edges about 1.5 cm) after compression tests performed in a Texturometer (Stable Micro System, Model TA.TXT2, United Kingdom). The hydrogel samples were previously swollen at pH 2 or 8. The Texturometer apparatus was equipped with a loading cell of 5 N and a cylindrical probe with 12.7 mm diameter. The experimental parameters were adjusted for a maximum deformation equal to 10% and (probe depth of 3 mm) at a test speed of 1 mm s−1. The compression tests were performed at room temperature (25 °C) and constant humidity (RH ≈ 80%). Data generated by the equipment were the force necessary for a given compression, which were subsequently converted to the compressive stress (σ) as a function of the relative strain (λ). The values of σ and the elastic modulus, E, were determined from the following equation:22| |  | (2) |
where A is the probe sectional area (126 mm2). Thus, the E value of each sample was determined from the slope of the straight line obtained according to equation (2) [σ plotted against (λ–λ−2)]. In all cases, the relative strain (λ) was determined from the following equation: | |  | (3) |
where ΔL is the deformation of the sample and L0 is initial sample length. The values of E calculated for the swollen hydrogel samples were a simple average of 5 samples (n = 5), respectively.
2.7
In vitro IND release studies
The in vitro IND release studies were performed in simulated intestinal fluid (SIF, 6.8 g KH2PO4 and 77 ml aqueous NaOH 0.2 M in 1000 ml of water – final pH = 6.8) and simulated gastric fluid (SGF, 2.0 g NaCl and 7.0 ml HCl 0.1 M in 1000 ml of water – final pH = 1.2), both without the presence of enzymes. Two sets of IND loaded samples were prepared for these tests. The samples tested were labeled according to the fluid utilized and to the amount of IND loaded in the sample. The samples were labeled as: SIF1 and SGF1 (samples loaded with 1.2 mg of IND); SIF2 and SGF2 (samples loaded with 2.4 mg of IND); SIF3 and SGF3 (samples loaded with 4.8 mg of IND); and SIF4 and SGF4 (samples loaded with 7.2 mg of IND). In situ loading (addition of the drug during hydrogel formation) was used because the desired drug amount could be loaded into the hydrogel. The IND loaded alginate–Ca2+/CS hydrogel samples were formed according to the methodology described above except that during the final solution different amounts of IND were added to the system. The amounts of IND in the hydrogel forming solutions were adjusted to 1.2, 2.4, 4.8, and 7.2 mg, respectively. After the formation of IND loaded hydrogels , they were removed from the container, washed with distilled water (2 × 50 ml) and then dried at room temperature. For all the cases, the distilled water utilized to wash the IND loaded samples were collected and analyzed by a UV spectroscopy technique (UV-Vis Femto - model 800Xi, Brazil) at λ = 320 nm, to determinate the loading efficiency. The analytical curve, necessary to quantify the amount of IND that remained in the supernatant, was built using standard IND solutions with concentrations varying from 0.374 to 60 mg l−1 having water–ethanol (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent. The linear correlation coefficient (R2) value was higher than 0.999. The loading efficiency was determined from the following equation:
1) as the solvent. The linear correlation coefficient (R2) value was higher than 0.999. The loading efficiency was determined from the following equation: | |  | (4) |
where [IND0] refers to the amount of IND used for each hydrogel preparation and [INDs] is the amount of IND that remained in the solution after the hydrogel preparation.
The release studies were carried out in a dissolution apparatus with individual sealed vessels. Each sealed vessel was filled with 350 ml of SIF or SGF and then, 1.0 g of hydrogel with loaded-IND was added to each fluid. Each run was performed three times (n = 3). After the dipping of each sample in a desired fluid, they were kept under constant stirring (75 rpm) at 37 °C over the whole test period. At desired time intervals, an aliquot (ca. 3 ml) was removed from each vessel to quantify the amount of IND released through UV measurements (UV-spectrophotometric method at λ = 320 nm). For the determination of the released IND, analytical curves for SGF and SIF, containing IND, were built in the range of 0.375 to 60 mg l−1. For both simulated fluids the R2 values were higher than 0.999.
3. Results and discussion
3.1 Characterization of the hydrogel structure
FTIR spectra of alginate–Ca2+ (a), alginate–Ca2+/CS hydrogel (b) and raw CS (c) are shown in Fig. 1. The spectrum of alginate–Ca2+ (1a) showed a broad band assigned to the –OH stretching at 3410 cm−1, absorption bands assigned to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) asymmetric and symmetric stretching at 1618 and 1425 cm−1, and the C–O–C stretching at 1037 cm−1.23,24 The spectrum of CS (1c) showed a broad band assigned to the –OH and to the N–H stretching, in which the –OH stretching overlaps with the N–H one, with a maximum at 3436 cm−1. In addition, the absorption bands assigned to the bending vibrations of the N–H (N-acetylated residues, amide band), C–O–C and C–O stretching, and O–H angular coupling, indicating the existence of free carboxyl groups, were observed at 1645, 1419, and 1037 cm−1, respectively. The absorption band assigned to the S
O) asymmetric and symmetric stretching at 1618 and 1425 cm−1, and the C–O–C stretching at 1037 cm−1.23,24 The spectrum of CS (1c) showed a broad band assigned to the –OH and to the N–H stretching, in which the –OH stretching overlaps with the N–H one, with a maximum at 3436 cm−1. In addition, the absorption bands assigned to the bending vibrations of the N–H (N-acetylated residues, amide band), C–O–C and C–O stretching, and O–H angular coupling, indicating the existence of free carboxyl groups, were observed at 1645, 1419, and 1037 cm−1, respectively. The absorption band assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching related to the sulfate groups on CS, appeared at 1238 cm−1 and the absorption band assigned to the α-(1,4) glycoside bond is observed at 925 cm−1.25–27 The spectrum of the alginate–Ca2+/CS hydrogel (1b) showed the characteristic absorption bands of the alginate–Ca2+ hydrogel. After the addition of CS it is possible to realize that some characteristic absorption bands proceeding from CS are present in the hydrogel spectrum. The spectrum of the alginate–Ca2+/CS hydrogel absorption shows the band assigned to the S
O stretching related to the sulfate groups on CS, appeared at 1238 cm−1 and the absorption band assigned to the α-(1,4) glycoside bond is observed at 925 cm−1.25–27 The spectrum of the alginate–Ca2+/CS hydrogel (1b) showed the characteristic absorption bands of the alginate–Ca2+ hydrogel. After the addition of CS it is possible to realize that some characteristic absorption bands proceeding from CS are present in the hydrogel spectrum. The spectrum of the alginate–Ca2+/CS hydrogel absorption shows the band assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching related to the sulfate groups on CS, at 1238 cm−1, which confirms that the CS was successfully entrapped into the alginate–Ca2+ matrix.
O stretching related to the sulfate groups on CS, at 1238 cm−1, which confirms that the CS was successfully entrapped into the alginate–Ca2+ matrix.
 |
| | Fig. 1 FTIR spectra of (a) alginate–Ca2+, (b) alginate–Ca2+/CS hydrogel and (c) pure CS. | |
The FTIR data suggest CS is entrapped inside the alginate–Ca2+ matrix and not interacting with it neither chemically nor physically. An aqueous solution of CS does not jellify on contact with Ca2+ ions. Thus, the CS remains as an inert filler inside the hydrogel matrix. The crosslinking of alginate chains quickly occurs when in contact with a Ca2+ solution forming a consistent structure, as described in the literature by the “egg box” model.28
DSC curves (Fig. 2) of pure CS and SA showed exothermic events at 245 and 239 °C, respectively, which are related to the degradation process of these polysaccharides. However, the degradation temperature of alginate–Ca2+ was slightly higher than pure SA suggesting that when crosslinked with Ca2+ the alginate–Ca2+ matrix is thermally more stable than neat alginate. The DSC curve of the alginate–Ca2+/CS hydrogel showed a degradation peak at 261 °C, slightly higher than the degradation temperature observed for the alginate–Ca2+ hydrogel (crosslinked without CS). Therefore, the DSC data suggest that the incorporation of CS into the alginate–Ca2+ matrix did not increase the thermal stability considerably because the CS does not interact effectively with the crosslinked network. In addition, the data provided by the TGA curves (Fig. 3) are in agreement with the DSC curves and also show that the incorporation of CS into the alginate–Ca2+ matrix did not affect considerably the thermal stability.
 |
| | Fig. 2 DSC curves for pure CS and SA, alginate–Ca2+ and alginate–Ca2+/CS hydrogel. | |
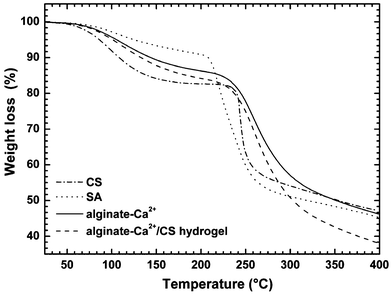 |
| | Fig. 3 TGA curves for pure CS and SA, alginate–Ca2+ and alginate–Ca2+/CS hydrogel. | |
Fig. 4 shows the WAXS pattern of raw CS and SA, and also of alginate–Ca2+ and alginate–Ca2+/CS hydrogels. Pure CS does not exhibit sharp peaks but rather a broad halo with a maximum intensity at 2θ = 23.3° suggesting a lack of crystallinity. The diffraction pattern of pure SA and alginate–Ca2+ exhibited a low intense peak at 2θ = 12.2° that indicates low crystallinity. The diffraction pattern of the alginate–Ca2+/CS hydrogel presented intense and very sharp peaks at 2θ = 43.9° and at 2θ = 64.2°. The periodic distances among the ordered regions were calculated by Bragg's law. The periodic distances calculated were 2.06 Å for 2θ = 64.2° and 1.46 Å for 2θ = 43.9°, respectively. The data provided by WAXS analyses suggest that the CS chains, which are entrapped into the hydrogel matrix, are confined in such a way that hydrogen bond interactions between them are possible. Han et al.,29 discuss the formation of hydrogen bonds and physical entanglements among the CS chains when they are in the presence of Ca2+ and/or other divalent cations. This happens because the carboxyl and sulfate groups from CS are able to interact with the Ca2+ ions by ion binding.
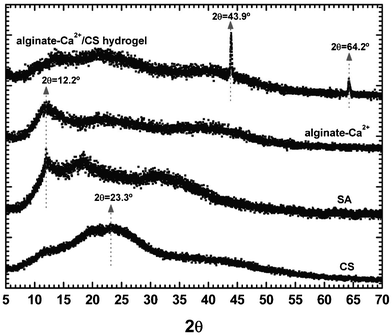 |
| | Fig. 4 WAXS profiles of pure CS and SA, alginate–Ca2+ and alginate–Ca2+/CS hydrogels. | |
3.2 Swelling degree
According to the swelling curves shown in Fig. 5, it is possible to verify that at pH 2 the alginate–Ca2+/CS hydrogel showed no significant value for St (<1). At this pH-condition, the hydrogel quickly achieves the equilibrium swelling, less than two hours after its immersion. This happens because all the functional groups from alginate and CS, in this pH condition, are in their neutral form (–COOH and –OSO3H), which makes the hydrogel network more hydrophobic and consequently reduces the liquid uptake capacity of the alginate–Ca2+/CS hydrogel. It is worthy to say that the pKa value of the sulfate groups from CS is close to 2.6 and the carboxylic groups from alginate could present a pKa between 3.38–3.65.30–32 On the other hand, at pH 4 and 6, the hydrogel showed higher St values (ca. 3.5 and 5.8, respectively) and their swelling curves did not reach the equilibrium swelling even after 10 h of immersion. In this pH range (4 ≤ pH ≤ 6) both functional groups of CS and alginate are in their anionic forms (–COO− and –OSO3−). The increase of negative charge density inside the hydrogel network positively affects the water uptake capacity of the hydrogel because it favors the increased distance between the polymeric chains (by anion–anion repulsion), which increases the amount of available space, allowing the hydrogel network to be more hydrophilic. Therefore, as a result, an increase in the St data evaluated at pH 4 and 6 was observed. At pH 8, the swelling curve increases up to 9 h and then tends to be constant, which suggests that the sample achieved the equilibrium swelling. However, after 10 h immersed at this pH-condition the hydrogel sample tends to disintegrate. At pH 8 the high concentration of hydroxy groups (OH−) increases, and they withdraw the Ca2+ ions, which, in turn, disintegrates the hydrogel network. The disruption of the hydrogel network due to the presence of hydroxy groups is enhanced at pH 10. At pH 10, the swelling curve showed an increase up to 4 h after the sample immersion. But, after this period, the St values decreased considerably due to the sample disintegration. A dashed line is used to clarify that after 4 h of immersion the hydrogel samples started a process of disruption due to the removal of Ca2+ ions , which supported the crosslinked network.
 |
| | Fig. 5 Time-dependent swelling curves of the alginate–Ca2+/CS hydrogel at different pH conditions. | |
Fig. 6 shows some photos taken of the alginate–Ca2+/CS hydrogels immersed in buffer solutions at pH 2 and 8 at different immersion times (in hours). In this experiment the hydrogel samples remain immersed in the buffer solutions without stirring. It is evident from the images that when the hydrogel is immersed at low pH conditions it maintains its consistency and morphological characteristics even after 10 h of immersion.
 |
| | Fig. 6 Photography images of the alginate–Ca2+/CS hydrogel immersed at pH 2 and 8 as a function of time. | |
Fig. 7 shows the SEM images of the dry alginate–Ca2+/CS hydrogel and of the alginate–Ca2+/CS hydrogels previously swelled up to equilibrium in distilled water and in buffer solutions with different pHs, after be lyophilized. The hydrogel sample previously swelled at pH 2 exhibited a compact morphology without pores, although some roughness can be observed. This fact agrees with the low liquid uptake capacity at pH 2. In addition, the incorporation of CS chains into the alginate–Ca2+ hydrogel enhanced the polymer-chain density and reduced the available spaces in the polymer network. As can be observed in Fig. 7, the alginate–Ca2+/CS hydrogel previously swelled in distilled water presents some cracks on the surface. Furthermore, the appearance of small pores is observed. A noteworthy change in the hydrogel morphology was observed in the SEM image of the hydrogel sample swollen at pH 8. The sample exhibited a highly porous and sponge-like morphology. According to the swelling data, when the hydrogel sample is swelled in alkaline conditions the hydrogel absorbs large amounts of liquid due to anion–anion repulsive forces existing between the chains; the polymeric matrix disintegrates due to the removal of the Ca2+ ions, which support the hydrogel network. Both processes contribute to change the hydrogel morphology as can be seen in Fig. 7.
 |
| | Fig. 7 SEM images of dry alginate–Ca2+/CS hydrogel and previously swelled in water and in buffer solutions (pH 2 and 8). | |
3.3 Mechanical properties
Fig. 8 shows the variation of the elastic moduli (E) calculated for the swollen hydrogel samples as a function of the immersion time at pH 2 and 8. The data show that the values of E are directly affected by increasing the immersion time and by the pH of the solution where the matrixes were swelled. At pH 2, the E values for the hydrogel samples decreased considerably after 1 h of immersion, which indicates that the hydrogel sample became more flexible. This is a consequence of the absorption of liquid by the hydrogel matrix. After 3 h of immersion, the E values tend to be constant and remain close to 0.6 kPa. At the equilibrium swelling the mechanical properties and morphology of the alginate–Ca2+/CS hydrogel did not present significant changes. From these results it is possible to infer that the hydrogel is able to resist acid conditions without compromise to its properties, which allows it to travel through the gastric system safely, for example. On the other hand, the samples swelled at pH 8 showed a gradual decrease of E values over the whole immersion time (up to 12 h of immersion). At the end of the experiments at pH 8 (Fig. 8) the E goes down to almost zero. After 12 h at pH 8 the hydrogel showed poor mechanical properties and became easily breakable and difficult to handle.
 |
| | Fig. 8 Time-dependent E values calculated for the alginate–Ca2+/CS hydrogel swollen at pH 2 and 8. | |
According to the discussion above, the process of formation, swelling and degradation of the alginate–Ca2+/CS hydrogel is illustrated in Fig. 9. The addition of the Ca2+ solution on the alginate–CS solutions causes quick physical crosslinking of alginate chains and the CS chains remain entrapped in the alginate–Ca2+ network. The Reptation Model developed by de Gennes33 helps to describe the process of CS releasing during the erosion of the hydrogel matrix. The model presented by de Gennes describes how a single chain, entrapped within a network, diffuses. The chain movements are similar to the snake movements through a hypothetical tube, where the contours are defined by the position of the entanglement points of the network. Therefore, the diffusional process described by the Reptation Model34 is similar to the behavior discussed in this work for the diffusion of CS chains. In this case, the CS chains are initially entrapped into the “egg-box”-type structure. The alginate–Ca2+ network is thought of as being fixed obstacles that limit the hypothetical tubes in which the CS chains move.
 |
| | Fig. 9 Illustrative scheme of alginate–Ca2+/CS hydrogel formation and its behavior when swelled in different pH conditions. | |
3.4
In vitro IND release studies
The data referring to the efficiency for the IND loading into the alginate–Ca2+ hydrogels are presented in Table 1.
Table 1 Encapsulation efficiency of IND in each hydrogel sample
| Sample |
IND content |
Encapsulation efficiency (%) |
| Added (mg) |
Supernatant (mg) |
Encapsulated (mg) |
| SIF1 |
1.20 |
0.14 |
1.06 |
88.4 |
| SIF2 |
2.40 |
0.17 |
2.23 |
92.9 |
| SIF3 |
4.80 |
0.21 |
4.59 |
95.7 |
| SIF4 |
7.20 |
0.18 |
7.02 |
97.5 |
| SGF1 |
1.20 |
0.16 |
1.04 |
87.0 |
| SGF2 |
2.40 |
0.18 |
2.22 |
92.8 |
| SGF3 |
4.80 |
0.20 |
4.60 |
95.9 |
| SGF4 |
7.20 |
0.25 |
6.95 |
98.5 |
Fig. 10 shows the fraction of IND released (in %) from the alginate–Ca2+/CS hydrogel when it is immersed in SIF (Fig. 10a) or SGF (Fig. 10b) as a function of time. The data demonstrate that the profile of IND released from the alginate–Ca2+/CS hydrogel is clearly pH-dependent. Comparing the two tested media (SGF – pH 1.2 and SIF – pH 6.8), it is possible to verify a great difference in relation to the fraction of IND released. In SIF (pH 6.8) all the hydrogel samples released more than 60% of the loaded IND. For all the samples, the release profiles showed that the fraction of IND released increased up to 12 h after immersion in SIF and then the equilibrium is achieved. On the other hand, in SGF (pH 1.2) the fractions of IND released were lower than 3% and no variation in the release curves was observed for all of the tested hydrogel samples. In SGF, the hydrogel matrix remains compacted and no disruption was observed, according to the photos of Fig. 6 (at pH 8). This behavior restricts the amount of liquid absorbed and avoids the disintegration of the hydrogel matrix. So, the drug release by a diffusional process or by disintegration of the hydrogel matrix is very limited. The low fraction of IND released in SGF is related to the drug being loaded close the hydrogel surface. In SIF, as observed by the swelling test, the hydrogel matrix absorbs a high amount of liquid allowing the drug to be released through a diffusional process. In addition, when immersed at pH 6.8 the hydrogel matrix is destabilized due to removal of Ca2+ by OH− ions forming insoluble Ca(OH)2. This destabilization process decreases the mechanical properties of the hydrogel, which disintegrates and releases the drug. The data presented here are interesting because it demonstrates that the alginate–Ca2+/CS hydrogel is robust to prevent the drug release in acid conditions (e.g. gastric medium) and acts as an efficient device for controlled drug delivery in the colon region. The disintegration of the hydrogel matrix is not a problem because, as described in the introduction section, the alginate is a highly biocompatible polysaccharide, causing no damage or side effects to the body. In addition, CS has been utilized in the treatment of inflammatory processes of the ligaments. CS stimulates the synthesis of compounds related to the inhibition of enzymes that promote the degradation of cartilage and the CS, on the other side, also stimulates the synthesis of proteoglycans and collagen. The administration of CS helps the reestablishment of the balance of articular cartilage with improvement or disappearance of joint pain. Furthermore, CS can block the action of lytic enzymes and improve cartilage repair by stimulating the synthesis of proteoglycans and raising levels of hyaluronic acid.35,36
 |
| | Fig. 10 Time-dependent release curves of IND from the alginate–Ca2+/CS hydrogel samples loaded with different amounts of IND in (a) SIF and (b) SGF. | |
Furthermore, it was verified that in SIF media (Fig. 10a) the hydrogel sample with the lowest amount of loaded IND (sample SIF1) released the highest fraction of the drug (ca. 87%), whereas the sample with the highest amount of loaded IND (sample SIF4) released the lowest fraction (ca. 64%). This same behavior was observed for the hydrogel samples immersed in SGF (Fig. 10 b). The sample SGF1 released the highest fraction of the drug while the sample SGF4 released the lowest one.
Indomethacin (Fig. 11) is a non-steroid anti-inflammatory with low solubility in water. When high amounts of IND are encapsulated into the hydrogel matrix they can form strong hydrophobic interactions between the IND molecules and/or between IND and the different groups of the hydrogel matrix. When low amounts of IND are encapsulated into the hydrogel matrix the formation of these interactions reduces the IND release.
4. Conclusions
This work reports on the preparation and characterization of a novel alginate–Ca2+/CS hydrogel. The alginate chains were crosslinked due to the ionic interaction of carboxylates of alginate and Ca2+ ions and the CS was entrapped into the hydrogel matrix as observed by FTIR data and thermal analysis. Swelling data showed that the formed alginate–Ca2+/CS hydrogels possess pH-dependent water uptake capacity. Some changes in the hydrogel morphology occurred, as observed by SEM images after the hydrogels had been swelled in buffer solutions with different pHs. The E values calculated for the hydrogel samples, previously swelled at pH 2 and pH 8, decreased when they were immersed for long immersion times. This decrease is more pronounced at pH 8, which confirms that for this pH and long immersion times the hydrogel matrix disrupts due to the removal of Ca2+ ions by hydroxide groups. In vitro IND release studies showed that in SGF (pH 1.2) low fractions of the drug are released whereas in SIF (pH 6.8) all the tested samples released more than 60% of the encapsulated IND. The results presented and discussed in this work allow the inference that the alginate–Ca2+/CS hydrogel has great potential as a device for a colon-specific drug delivery system.
Acknowledgements
The authors thank CNPq/CAPES, Brazil, for the doctorate and post-doctorate fellowships (ARJ and JFP) and for the financial support (Proc. 400702/2012-5, 481424/2010-5 and 309005/2009-4).
References
- J. Berger, M. Reist, J. M. Mayer, O. Felt, N.A. Peppas and R. Gurny, Eur. J. Pharm. Biopharm., 2004, 57, 19–34 CrossRef CAS.
- S. Wittaya-Areekul and C. Prahsarn, Int. J. Pharm., 2006, 313, 123–128 CrossRef CAS.
- F. Song, L. M. Zhang, C. Yang and L. Yan, Int. J. Pharm., 2009, 373, 41–47 CrossRef CAS.
- M. R. Moura, A. F. Rubira and E. C. Muniz, Polimeros, 2008, 18, 132–137 CrossRef.
- Y. C. Ho, F. L. Mi, H. W. Sung and P. L. Kuo, Int. J. Pharm., 2009, 376, 69–75 CrossRef CAS.
- A. V. Reis, M. R. Guilherme, A. F. Rubira and E. C. Muniz, Polymer, 2006, 47, 2023–2029 CrossRef CAS.
- A. R. Fajardo, J. F. Piai, A. F. Rubira and E. C. Muniz, Carbohydr. Polym., 2010, 80, 934–943 CrossRef CAS.
- J. Pavez, J. F. Silva and F. Melo, J. Cryst. Growth, 2005, 282, 438–447 CrossRef CAS.
- K. I. Draget, G. Skjak-Braek and O. Smidsrod, Int. J. Biol. Macromol., 1997, 21, 47–55 CrossRef CAS.
- G. Pasparakis and N. Bouropoulos, Int. J. Pharm., 2006, 323, 34–42 CrossRef CAS.
- N. E. Simpson, N. Khokhlova, J. A. Oca-Cossio, S. S. McFarlane, C. P. Simpson and I. Coenstantinidis, Biomaterials, 2005, 26, 4633–4641 CrossRef CAS.
- J. S. Boateng, K. H. Matthews, H. N. E. Stevens and G. M. Eccleston, J. Pharm. Sci., 2008, 97, 2892–2923 CrossRef CAS.
- J. F. W. Keuren, S. J. H. Wielders, G. M. Willems, M. Morra, L. Cahalan, P. Cahalan and T. Lindhout, Biomaterials, 2003, 24, 1917–1924 CrossRef CAS.
- M. R. Guilherme, E. A. Toledo, A. F. Rubira and E. C. Muniz, J. Membr. Sci., 2002, 210, 129–136 CrossRef CAS.
- M. G. Cascone, N. Barbani, C. Cristallini, P. Giusti, G. Ciardelli and G. Lazzeri, J. Biomater. Sci., Polym. Ed., 2001, 12, 267–281 CrossRef CAS.
- D. S. Jones, D. W. McLaughlin, C. P. McCoy and S. P. Gorman, Biomaterials, 2005, 26(14), 1761–1770 CrossRef CAS.
- L. L. Hyland, M. B. Taraban, B. Hammouda and Y.B. Yu, Biopolymers, 2011, 95, 840–850 CrossRef CAS.
- P. Matricardi, M. Pontoriero, T. Coviello, M. A. Casadei and F. Alhaique, Biomacromolecules, 2008, 9, 2014–2020 CrossRef CAS.
- H. H. Zheng, H. Zucheng, Q. Zhang, M. Konno, S. Yang and J. Wang, Carbohydr. Polym., 1998, 35, 215–221 CrossRef CAS.
- A. Wasteson, Biochem J, 1971, 122, 477–485 CAS.
- USP30-NF25, United States Pharmacopeia-National Formulary, The United Pharmacopeia Convention, Rockville, MD, USA, 2007 Search PubMed.
- A. R. Fajardo, L. C. Lopes, A. J. M. Valente, A. F. Rubira and E. C. Muniz, Colloid Polym. Sci., 2011, 289, 1739–1748 CAS.
- S. Hua, H. Ma, L. Xun, H. Yang and A. Wang, Int. J. Biol. Macromol., 2010, 46, 517–523 CrossRef CAS.
- K. Wang and Z. He, Int. J. Pharm., 2002, 244, 117–126 CrossRef CAS.
- A. R. Fajardo, L. C. Lopes, A. G. B. Pereira, A. F. Rubira and E. C. Muniz, Carbohydr. Polym., 2012, 87, 1950–1955 CrossRef CAS.
- H. Tian, Y. Chen, C. Ding and G. Li, Carbohydr. Polym., 2012, 89, 542–550 CrossRef CAS.
- W. Garnjanagoonchorn, L. Wongekalak and A. Engkagul, Chem. Eng. Process., 2007, 46, 465–471 CrossRef CAS.
- L. Li, Y. Fang, R. Vreeker, I. Appelqvist and E. Mendes, Biomacromolecules, 2007, 8, 464–468 CrossRef CAS.
- L. Han, D. Dean, D. A. Daher, A. J. Grodzinsky and C. Ortiz, Biophys. J., 2008, 95, 4862–4870 CrossRef CAS.
- B. Larsson, M. Nilsson and H. Tjalve, Biochem. Pharmacol., 1981, 30, 2963–2970 CrossRef CAS.
- Y. Xu, C. Zhan, L. Fan, L. Wang and H. Zheng, Int. J. Pharm., 2007, 336, 329–337 CrossRef CAS.
- T. C. Martinsen, K. Bergh and H. L. Waldum, Basic Clin. Pharmacol. Toxicol., 2005, 95, 94–102 CrossRef.
- P. G. de Gennes., J. Chem. Phys., 1971, 55, 572–579 CrossRef.
- J. Martel-Pelletier, S. K. Tat and J. P. Pelletier, Osteoarthritis Cartilage, 2010, 18, S7–S11 CrossRef.
- S. Verrier, R. Bareille, M. Dard and J. Amedee, J. Mater. Sci.: Mater. Med., 1996, 7, 46–51 CrossRef CAS.
- L. Yang, J. Kuang, Z. Li and L.-M. Zhang, Macromol. Biosci., 2008, 8, 279–286 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent. The linear correlation coefficient (R2) value was higher than 0.999. The loading efficiency was determined from the following equation:
1) as the solvent. The linear correlation coefficient (R2) value was higher than 0.999. The loading efficiency was determined from the following equation: 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) asymmetric and symmetric stretching at 1618 and 1425 cm−1, and the C–O–C stretching at 1037 cm−1.23,24 The spectrum of CS (1c) showed a broad band assigned to the –OH and to the N–H stretching, in which the –OH stretching overlaps with the N–H one, with a maximum at 3436 cm−1. In addition, the absorption bands assigned to the bending vibrations of the N–H (N-acetylated residues, amide band), C–O–C and C–O stretching, and O–H angular coupling, indicating the existence of free carboxyl groups, were observed at 1645, 1419, and 1037 cm−1, respectively. The absorption band assigned to the S
O) asymmetric and symmetric stretching at 1618 and 1425 cm−1, and the C–O–C stretching at 1037 cm−1.23,24 The spectrum of CS (1c) showed a broad band assigned to the –OH and to the N–H stretching, in which the –OH stretching overlaps with the N–H one, with a maximum at 3436 cm−1. In addition, the absorption bands assigned to the bending vibrations of the N–H (N-acetylated residues, amide band), C–O–C and C–O stretching, and O–H angular coupling, indicating the existence of free carboxyl groups, were observed at 1645, 1419, and 1037 cm−1, respectively. The absorption band assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching related to the sulfate groups on CS, appeared at 1238 cm−1 and the absorption band assigned to the α-(1,4) glycoside bond is observed at 925 cm−1.25–27 The spectrum of the alginate–Ca2+/CS hydrogel (1b) showed the characteristic absorption bands of the alginate–Ca2+ hydrogel. After the addition of CS it is possible to realize that some characteristic absorption bands proceeding from CS are present in the hydrogel spectrum. The spectrum of the alginate–Ca2+/CS hydrogel absorption shows the band assigned to the S
O stretching related to the sulfate groups on CS, appeared at 1238 cm−1 and the absorption band assigned to the α-(1,4) glycoside bond is observed at 925 cm−1.25–27 The spectrum of the alginate–Ca2+/CS hydrogel (1b) showed the characteristic absorption bands of the alginate–Ca2+ hydrogel. After the addition of CS it is possible to realize that some characteristic absorption bands proceeding from CS are present in the hydrogel spectrum. The spectrum of the alginate–Ca2+/CS hydrogel absorption shows the band assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching related to the sulfate groups on CS, at 1238 cm−1, which confirms that the CS was successfully entrapped into the alginate–Ca2+ matrix.
O stretching related to the sulfate groups on CS, at 1238 cm−1, which confirms that the CS was successfully entrapped into the alginate–Ca2+ matrix.











