Membrane fluidization & eryptotic properties of hesperidin–copper complex
Stalin
Selvaraj
,
Sridharan
Krishnaswamy
,
Venkappayya
Devashya
,
Swaminathan
Sethuraman
and
Uma Maheswari
Krishnan
*
Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology, SASTRA University, Thanjavur-613 401, India. E-mail: umakrishnan@sastra.edu; Fax: +91 4362 264120; Tel: +91 4362 264101 Ext: 677
First published on 18th September 2012
Abstract
Metal–flavonoid complexes have elicited much attention in recent years due to their enhanced pharmacological activities when compared with their parent flavonoid and hence have the potential to be used as therapeutic agents. The membrane interactions and localization of these molecules will determine their role as a clinically relevant therapeutic molecule. Hesperidin is a flavone glycoside present in citrus plants. In the present work, a room temperature synthesis of hesperidin–copper complex was carried out and its interactions with membranes were investigated on a self-assembled nano-dimensional lipid bilayer membrane using electrochemical techniques. The results reveal that the copper complex interacts strongly at the membrane–electrolyte interface and localizes in the outer bilayer leaflet in a dose-dependent manner causing extensive membrane fluidization. When incubated with erythrocytes, hesperidin–copper complex initiated eryptosis and triggered formation of echinocytes by disrupting the actin cytoskeletal network because of its surface interaction. The parent flavonoid did not show such extensive membrane perturbation effects which may be attributed to their deeper penetration. The hesperidin–copper complex exhibits lower anti-oxidant activity when compared with hesperidin. These alterations in the mode of interaction of hesperidin–copper complex compared with their parent flavonoid may influence their pharmacological activities.
1. Introduction
Flavonoids are a group of polyphenols ubiquitously distributed in vegetables, fruits, seeds, roots and stem either in the aglycone form or as glycone derivatives. Flavonoids are also taken as food supplements. All flavonoids exhibit a typical structure comprising three cyclic carbon rings denoted as A, B and C. The substitutions in the ‘C’ ring distinguish different flavonoids.1 Flavonoids possess numerous pharmacological activities, such as anti-cancer, anti-diabetic,2 anti-atherosclerotic, anti-oxidant, anti-inflammatory properties, etc.1 These activities of flavonoids have been mainly attributed to their ability to alter the membrane-mediated signaling pathways by modifying cell membrane permeability and binding with different proteins.1,2Hesperidin, a flavonone glycoside predominantly present in citrus fruits such as orange and lemon are reported to possess numerous pharmacological activities such as anti-inflammatory,3 anti-oxidant4 and anti-nociceptive properties.5 It also acts as a very good sedative probably due to its interactions with opioid and adenosine receptors.6,7 Reports on its cytotoxicity against cancer cells are available suggesting its involvement in the membrane signaling pathways.4 Earlier studies have reported that the bioavailability of flavonoid glycosides and their pharmacological activities are influenced by their structure.8 Isoflavones and flavonoid aglycones have been reported to be easily absorbed through the intestine, unlike their glucoside derivatives.8 Contrasting reports on the enhanced bioavailability of rhamnoside derivatives of flavonoids such as rutin and hesperidin due to permeation through the intestinal membrane layer are also available.8 The membrane permeation of a molecule is chiefly associated with its mode and extent of interaction with cellular membranes. A recent report has suggested that hesperidin may interact with the polar head groups of the cell membrane through its hydroxyl groups as well as with the fatty acyl chains through the rhamnoside residue.8 However, more detailed studies on its membrane interactions, localization and its implications on its bioavailability and pharmacological action are yet to be thoroughly explored.
Another key controversy in flavonoid research is conflicting reports on its antioxidant and pro-oxidant behaviour. While some reports have suggested that flavonoids have excellent anti-oxidant properties, a few studies have demonstrated that the flavonoids transform to pro-oxidants resulting in cell injury.9 The transition to pro-oxidant nature has been attributed to both increased concentration of the flavonoid as well as to the presence of transition metal ions which can be due to either the formation of hydroxyl radicals by flavonoids or generation of free radicals in the presence of metal ions.9 Flavonoids are strong metal ion chelators and, hence, it will be of interest to understand whether such chelation can result in exhibition of antioxidant character or pro-oxidant nature. Metal–flavonoid complexes have been reported to exhibit anti-oxidant,10 anti-microbial,11 DNA lytic12 and anti-cancer activities.13 It has also been shown that certain metal–flavonoid complexes can cause lipid peroxidation.14 Hence, this study attempts to address the questions as to whether formation of a hesperidin–copper complex could lead to membrane protective effects or contribute to membrane perturbation, using electrochemical and biochemical assays.
Arora et al. have compared the membrane interactions of nine different flavonoids using unilamellar lipid vesicles and an attempt was made to correlate the membrane interactions with their antioxidant potency.15 The mode of membrane interaction of flavonoids is mainly attributed to their lipid composition and structure. Therefore, a suitable model of the cell membrane is necessary to understand the single molecule interactions at the membrane interface. Planar lipid bilayers offer a simple and versatile tool to understand the single molecule interactions at the membrane–electrolyte interface.16 Any slight alteration in the membrane electrical properties such as capacitance and resistance can be used to understand the mode of interaction. Hence, it is proposed to study the interactions of metal–flavonoid complexes with lipid membranes using self-assembled planar lipid bilayers. However, the biological membrane presents a complex milieu of proteins and lipids, which contribute to its selective permeability. Therefore, further validation using electrochemical studies can be obtained using cell membranes. Erythrocytes possess an extremely sensitive membrane that has extensive communication with its cytoskeletal network. Any alteration in its native environment or introduction of stress that disturbs its cell membrane is immediately reflected by a change in the erythrocyte morphology.17 Hence, the erythrocyte membrane can serve as an excellent cell model to understand dose-dependent interactions of molecules with cell membranes. Thus far, no investigations have been reported to study the influence of metal–flavonoid complexes with erythrocyte membranes. Therefore, the present work aims to explore the interactions of hesperidin–copper complex with lipid bilayers and study its implications on its anti-oxidant and biological activities.
2. Materials and methods
2.1 Reagents
Hesperidin (Sigma Aldrich, USA), copper(II) acetate and methanol (Merck, India) were used for the synthesis of hesperidin–copper complex. Potassium chloride (Merck, India) was used for electrochemical investigations. Phosphate buffered saline (PBS), glutaraldehyde, sodium chloride, magnesium chloride, Tween 80, Triton X100, sodium azide (Merck, India), phalloidin–rhodamine and fetal bovine serum (FBS) (Invitrogen, India) were used for erythrocyte destabilization studies. Dimethyl sulphoxide (Merck, India) was used as solvent.2.2 Synthesis and characterization of hesperidin–copper complex
0.610 g of hesperidin was dissolved in 50 mL of methanol, mixed with 0.199 g of copper(II) acetate in 25 mL of double distilled water and stirred for 6 h at room temperature. The pale green insoluble precipitate obtained was vacuum dried, washed repeatedly with water and methanol to remove the excess copper acetate and hesperidin. The product was then air dried. The carbon and hydrogen in the complex were analyzed using CHNS analyzer (Elementar Vario EL III, Germany). The amount of copper in the complex was estimated by atomic absorption spectrometry (AAnalyst 400/HGA 900/AS 800, Perkin Elmer, USA). The complexation of copper to hesperidin was confirmed using EPR spectroscopy (EMXPlus, Bruker, Germany). The UV–visible spectrum of the complex was recorded in dimethyl sulphoxide (DMSO) as solvent at room temperature (Lambda 25, Perkin Elmer, USA).2.3 UV-absorption studies
A Job plot was plotted using different ratios of equimolar concentrations of hesperidin and copper(II) acetate (0.1 mM) and the corresponding absorbance values. The various ratios of flavonoid to copper acetate used were 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2.5
0, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 2
0.5, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.5
2, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 0
2.5 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The absorption maximum was measured at 608 nm using UV–visible spectrophotometer (Lambda 25, Perkin Elmer, USA).
3. The absorption maximum was measured at 608 nm using UV–visible spectrophotometer (Lambda 25, Perkin Elmer, USA).
2.4 Formation of planar lipid bilayer membrane (BLM)
The planar lipid bilayer was prepared using the Mueller–Rudin method using egg phospholipids.18 A 2% (w/v) dispersion of phospholipids in n-decane was painted on an aperture of area 0.00793 cm2 bifurcating two chambers of volume of 3.5 mL each. All experiments were carried out in 0.1 M KCl bath medium and measurements were recorded at room temperature (25 °C). The bilayer set-up was kept on a vibration-isolated platform and shielded from electrical noise using a Faraday cage. The bilayer formation and attainment of stability for the lipid bilayer was confirmed from the constancy in the electrical parameters (capacitance and conductance). On addition of hesperidin/hesperidin–copper complex to the system, the bath solution was stirred for five minutes to ensure uniform concentration and stirring was stopped during electrical measurements.2.5 Electrochemical studies
A standard circuit for cyclic voltammogram analysis is shown in Fig. 1. A three-electrode system consisting of a platinum electrode as the working electrode, Ag/AgCl (3 M KCl) reference electrode and a platinum wire counter electrode was used for measurements. Electrical continuity was maintained using agar salt bridges. An external resistor was connected in series to the lipid bilayer. Finally, all the electrodes were connected to an electrochemical analyzer (Model 604C, CH Instruments, USA). All the measurements were carried out for 30 min after addition of hesperidin or its copper complex.19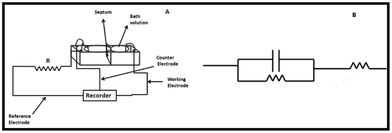 | ||
| Fig. 1 (A) Schematic representation of the measurement system; and (B) equivalent electric circuit used for electrochemical measurements. | ||
2.6 1,1′-Diphenyl-2-picrylhydrazyl (DPPH) assay
3.5 mL of 1,1′-diphenyl-2-picrylhydrazyl (DPPH) radical and different concentrations of hesperidin or hesperidin–copper complex in DMSO were added to obtain a final concentration of 50, 100, 150, 200 and 250 μM and then incubated at room temperature for 45 min. The absorbance of the test solutions was measured at 515 nm against a blank sample containing DPPH (negative control) using a UV–visible spectrophotometer.2.7 Erythrocyte destabilization study
0.5 mL of blood was collected from a healthy individual in 0.5 mL 0.1 M EDTA solution. Then, RBC cells were separated at 1000 rpm in the refrigerated centrifuge and washed with PBS thrice to remove proteins and other cells. The cells were then serially diluted with 500 μL PBS and incubated with 100 μM of hesperidin and hesperidin–copper complex separately. The cells were incubated at 37 °C for an hour, then fixed with 2% (v/v) glutaraldehyde in 400 μL of distilled water and stored at 4 °C for 20 h. The glutaraldehyde was removed by centrifugation at 1000 rpm. Finally, the cells were transferred to aluminum stub and coated with platinum for imaging using a cold field emission scanning electron microscope (JSM 6701F, JEOL, Japan).202.8 Cytoskeletal staining
Cytoskeletal staining of erythrocytes was performed to understand the changes in the F-actin network, which was viewed using laser scanning confocal microscopy (FV 1000, Olympus, Japan). The erythrocyte cell suspensions were prepared as explained in the previous section. The cells were incubated with 100 μM of hesperidin/hesperidin–copper complex for 1 h and the PBS was removed. Then, the erythrocytes were fixed with 2.5% (v/v) glutaraldehyde for half an hour and the glutaraldehyde was removed by centrifugation at 1000 rpm for 10 min. The cells were incubated with cytoskeleton buffer (5 mM NaCl, 150 mM MgCl2, 0.5 mM Tris base, and 0.5% Triton X100 in PBS solution) for 10 min and incubated with blocking buffer (5% FBS, 0.1% Tween 20, and 0.02% sodium azide in PBS) for 30 min at 37 °C. The blocking buffer was then removed. Finally, the erythrocytes were incubated with rhodamine–phalloidin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) for 1 h at 37 °C and visualized using a confocal microscope.21
200) for 1 h at 37 °C and visualized using a confocal microscope.21
2.9 Statistical analysis
DPPH assay and specific capacitance were expressed as the mean ± the standard deviation of three values. Comparison between mean values were made using one-way ANOVA followed by Tukey’s test at 95% confidence interval (p < 0.05)3. Results
3.1 Structure of hesperidin–copper complex
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
| Compound | Color | Elemental analysis (%) | |||||
|---|---|---|---|---|---|---|---|
| Experimental | Calculated | ||||||
| C | H | Cu2+ | C | H | Cu2+ | ||
| a Assuming ML2 complex. | |||||||
| Hesperidin | Yellow | 54.81 | 4.98 | — | 55.21 | 5.25 | — |
| Hesperidin–copper complex | Pale green | 51.03 | 5.18 | 4.82 | 52.40 | 4.99 | 5.09 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447 cm−1 indicates a d–d* transition implying a square planar geometry of the complex. Similar inferences have been reported for copper complexes in the literature.14,19 The stoichiometry of the complex was confirmed by UV-absorption titrimetry and using the well known Job plot shown in Fig. 2B. The presence of absorbance at 608 nm is the indication of the formation of hesperidin–copper complex. The peak value of absorbance is observed at a mole fraction of copper acetate of 0.3333. The Job plot also confirms that the mole ratio between hesperidin and copper is 2. This indicates the formation of a 1
447 cm−1 indicates a d–d* transition implying a square planar geometry of the complex. Similar inferences have been reported for copper complexes in the literature.14,19 The stoichiometry of the complex was confirmed by UV-absorption titrimetry and using the well known Job plot shown in Fig. 2B. The presence of absorbance at 608 nm is the indication of the formation of hesperidin–copper complex. The peak value of absorbance is observed at a mole fraction of copper acetate of 0.3333. The Job plot also confirms that the mole ratio between hesperidin and copper is 2. This indicates the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex of metal
2 complex of metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) flavonoid.23
flavonoid.23
 | ||
Fig. 2 (A) UV-visible spectra for hesperidin and its copper complex. Inset: Visible spectra of hesperidin and its copper complex; (B) Job plot for hesperidin–copper complex for understanding the M–L2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) complex. 2) complex. | ||
The EPR spectrum is additional proof for the formation of the copper complex. The paramagnetic signal obtained in the EPR spectrum of the complex measured in the solid state at room temperature also indicates the formation of a complex (Fig. 3). The EPR spectrum for copper acetate has been reported in the literature to exhibit a multiplet pattern with a g∥ (g-parallel) value of 2.42 and g⊥ (g-perpendicular) value of 2.08.24 In the hesperidin–copper complex, we observed a g∥ value of 2.36 and g⊥ value of 2.046. in which g∥ > g⊥ > 2. This suggests the coordination of copper with the oxygen atom from the flavonoid and also indicates the presence of unpaired spinning electron in the dx2−y2 orbital of Cu2+ (Fig. 3).19,24
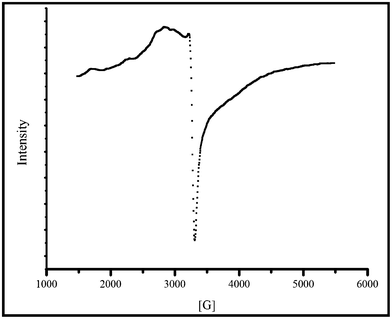 | ||
| Fig. 3 EPR spectrum for hesperidin–copper complex. | ||
The FTIR vibration bands observed for free hesperidin and its copper complex are summarized in Table 2. The ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration band for the free ligand at 1655 cm−1 is shifted to 1647 cm−1 in the complex indicating coordination of the carbonyl oxygen with the copper ion.14,19 The vibration band for ν(C–O–C) at 1296 cm−1 in the ligand is shifted to 1277 cm−1 in the hesperidin–copper complex implying the coordination of 5 –OH in hesperidin with copper. The presence of a metal–oxygen band at 615 cm−1 further confirms formation of the copper complex.
O) vibration band for the free ligand at 1655 cm−1 is shifted to 1647 cm−1 in the complex indicating coordination of the carbonyl oxygen with the copper ion.14,19 The vibration band for ν(C–O–C) at 1296 cm−1 in the ligand is shifted to 1277 cm−1 in the hesperidin–copper complex implying the coordination of 5 –OH in hesperidin with copper. The presence of a metal–oxygen band at 615 cm−1 further confirms formation of the copper complex.
| Molecule | Vibration frequency (cm−1) | |||
|---|---|---|---|---|
| ν(C–O) | ν(C–O–C) | ν(C–C) | ν(M–O) | |
| Hesperidin | 1655 | 1296 | 1505 | — |
| Hesperidin–copper complex | 1647 | 1277 | 1519 | 615 |
From the elemental analysis and spectral data, the structure of hesperidin–copper complex is proposed to contain two hesperidin molecules coordinated to copper via the 5 –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O as shown in Fig. 4.
O as shown in Fig. 4.
 | ||
| Fig. 4 Proposed structure of cationic hesperidin–copper complex. | ||
 | ||
| Fig. 5 Percentage reductions in the absorbance of DPPH in the presence of different concentrations of hesperidin and hesperidin–copper complex. (Values are expressed as mean ± SD n = 3). | ||
3.2 Membrane interactions using planar lipid bilayers
 | ||
| Fig. 6 Comparison of cyclic voltammograms and admittance Cole–Cole plot for bilayer in the presence of hesperidin/hesperidin–copper complex. (A) Current–voltage curve for unmodified bilayer in the presence of different concentrations of hesperidin; (B) admittance Cole–Cole plot for bilayer in the presence of different concentrations of hesperidin; (C) cyclic voltammogram for bilayer in the presence of different concentrations of hesperidin–copper complex; and (D) admittance Cole–Cole plot for bilayer in the presence of different concentrations of hesperidin–copper complex. | ||
The cyclic voltammograms for the lipid bilayer in the presence of different concentrations of hesperidin–copper complex is presented in Fig. 6C. The cyclic voltammograms reveal that the addition of different concentrations (20–100 μM) of hesperidin–copper complex gradually increases the current flow without disturbing the capacitive nature of lipid bilayer. The complex admittance plot of the bilayer in the presence of different concentrations (20–100 μM) of hesperidin–copper complex given in Fig. 6D displays the capacitive semicircle pattern for the lipid bilayer. However, the addition of hesperidin–copper complex (20–100 μM) causes a progressive reduction in the diameter and height of the semi-circle implying increasing current flow.
Fig. 7A shows the average specific capacitance (n = 3) of the lipid bilayer calculated from the cyclic voltammograms using eqn (1) before and after addition of hesperidin or its copper complex.
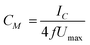 | (1) |
 | ||
| Fig. 7 (A) Influence of different concentrations of hesperidin and hesperidin–copper complex on bilayer specific capacitance. Values are expressed as average. (B) The effect of different concentrations of hesperidin and hesperidin–copper complex on bilayer resistance for charge transfer. Values are obtained from admittance Cole–Cole plot. | ||
Fig. 7B shows the relative decrease in the charge transfer resistance (RCT) of the lipid bilayer on addition of increasing concentrations of hesperidin or its copper complex. It is observed that while both hesperidin and its copper complex exhibit progressive reduction in the RCT values, the decrease is more pronounced for hesperidin–copper complex at concentrations below 80 μM. Hesperidin initially causes a lower magnitude of decrease in the RCT values which becomes higher than the change produced by its copper complex at concentrations above 100 μM.
 | ||
| Fig. 8 Charge–discharge profiles for bilayer in the presence of different concentrations of (A) hesperidin and (B) hesperidin–copper complex. (C) Relative change in charging peak current in the presence of hesperidin and hesperidin–copper complex | ||
 | ||
| Fig. 9 Scanning electron micrographs of RBC cell morphology in the presence of 100 μM of hesperidin and hesperidin–copper complex. Panels A, C and E are low magnification images and panels B, D and F are high magnification images. (A) and (B) Normal RBC; (C) and (D) 100 μM of hesperidin; (E) and (F) 100 μM of hesperidin–copper complex. | ||
 | ||
| Fig. 10 Confocal micrographs for cytoskeleton of RBC incubated with 100 μM of hesperidin/hesperidin–copper complex. (A) Normal RBC; (B) treated with 100 μM hesperidin; and (C) treated with 100 μM hesperidin–copper complex. | ||
4. Discussion
The various pharmacological activities of flavonoids, such as anti-cancer, anti-inflammatory, anti-atherosclerotic, anti-diabetic properties, etc., are profoundly influenced by the nature of their interactions with cell membranes as well as by their localization at the membrane–electrolyte interface.1 Modification of membrane fluidity and permeability due to reorganization of the membrane lipids can also influence the conformation and function of membrane-associated proteins.14,15 Though the biological activities for most flavonoids have been identified, the mechanisms contributing to these effects are yet to be elucidated. Structure–activity studies on flavonoids have shown that the presence of –OH groups at 3′, 4′ and 5′ positions in the B ring, 5th carbon in the A ring and a 2,3 double bond in the C ring confer enhanced bioactivity to flavonoids.27 However, reports on pro-oxidant characteristics of these flavonoids in the presence of metal ions reported in the literature indicate that there exists a possibility of destruction of these sites due to chelation with metal ions.14 The propensity of flavonoids to form chelates with transition metal ions at ambient conditions have been reported in the literature. The phenolic group in the A rings and the adjacent keto group in the C ring of hesperidin enable it to chelate with copper(II) ions easily. This is indicated in the bathochromic shifts in the electronic spectrum and the shifts in the vibration frequency in the FTIR spectrum. The metal/ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is also on expected lines for copper(II) complexes which have a coordination number of 4 (Table 1 and 2; Fig. 2, 3 and 4). Similar 1
2 is also on expected lines for copper(II) complexes which have a coordination number of 4 (Table 1 and 2; Fig. 2, 3 and 4). Similar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes have been reported for chrysin–copper, morin–copper, hesperitin–vanadyl and quercetin–copper complexes.11,13,14,19
2 complexes have been reported for chrysin–copper, morin–copper, hesperitin–vanadyl and quercetin–copper complexes.11,13,14,19
Flavonoids are well known antioxidants that can scavenge free radicals either by electron transfer and hydrogen atom removal (chain breaking antioxidants) or by chelating with transition metal ions, key contributors to generation of free radicals (preventive antioxidants).1,19 The structure of the flavonoid and its complex can exert a pronounced effect on its antioxidant property. Three important structural characteristics that contribute to the antioxidant property of a flavonoid are: (i) an ortho dihydroxy catechol ring for better metal chelation; (ii) a 2,3 double bond in conjugation with a 4-keto group aiding electron delocalization; and (iii) hydroxyl groups at the 3rd and 5th carbon enabling formation of a stable quinonic structure during oxidation.14 In the case of hesperidin, both the 2,3 double bond as well as the 5-OH are available that contributes to its antioxidant properties. However, absence of the dihydroxy catechol ring and 3-OH reduces its antioxidant potential when compared with quercetin. When hesperidin forms a complex with copper(II), the 5-OH group forms a coordinate bond with copper(II), thereby further reducing its antioxidant property when compared with its parent flavonoid (Fig. 5).
Lipid bilayers composed of an exterior hydrophilic layer on either side sandwiching an inner hydrophobic layer behave analogously to a parallel plate capacitor with the hydrophobic core as the dielectric and the hydrophilic head groups forming the conducting plates.26 The tight packing of the lipids forming the bilayer is reflected in the low current flowing through the system. The membrane capacitance is directly proportional to the dielectric constant of the acyl chains forming the hydrophobic core and the surface area of the membrane and inversely proportional to the membrane thickness (Fig. 6). The increase in capacitance of the lipid bilayer on addition of hesperidin–copper complex implies an increase in the membrane area and/or a decrease in its thickness due to the dose dependent interaction of the compound.24–26 The bulky nature of the hesperidin–copper complex suggests that the former increase in membrane area is most likely to contribute to the increase in capacitance (Fig. 7A). The glycoside rings in the complex will tend to interact extensively at the membrane–electrolyte interface and these surface interactions are expected to create defects in the compact bilayer architecture. This is reflected in the increased membrane permeability implied by the progressive decrease in the RCT values calculated from the admittance Cole–Cole plot and the dose-dependent reduction in the width of the transportation lag spike in the chronoamperometric studies (Fig. 6B, 6D, 8A, 8B & 8C). The relative increase in the charging current for the lipid bilayer in the presence of the hesperidin–copper complex (Fig. 8B) further confirms the surface localization of the bulky hesperidin–copper complex. Similar localization has been reported for quercetin by Movileanu et al.16 As the concentration of the copper complex increases, the molecular stresses at the membrane–electrolyte interface increases, which may promote the penetration of these molecules into the lipid bilayer. However, the bulky molecular size, presence of glycoside residues and the cationic nature of the complex restrict its penetration into the outer leaflet of the bilayer. This can cause pronounced fluidization of the lipid bilayer reflected in the increased current flow in the system (Fig. 8C). Procyanidin dimers have also been observed to exhibit similar membrane fluidization effects due to its surface accumulation.28 In the case of the parent flavonoid hesperidin, a similar interaction is expected to occur at the membrane–electrolyte interface. However, the lack of charge and relatively smaller size of hesperidin when compared to its copper complex enables it to localize more deeply into the lipid bilayer at higher concentrations. This is evident from the greater reduction in the RCT values of the lipid bilayer at higher concentrations of hesperidin (≥80 μM) (Fig. 7B). The initial increase in the charging current for the lipid bilayer in the presence of lower concentrations of hesperidin (≤40 μM) followed by a decrease indicates that the hesperidin, which initially accumulated at the surface, starts penetrating into the lipid bilayer (Fig. 8A). A similar kind of localization has been reported for another flavonoid glycoside, namely rutin, as well as for anandamide.29 The permeation of hesperidin into cell membranes has been reported to impart the ability to cross the blood–brain barrier by hesperidin.30 The electrochemical data presented in this study also correlates with this observation.
Further confirmation of the extensive membrane fluidizing effects and localization of hesperidin–copper complex is obtained from its interactions with erythrocytes. Erythrocytes are biconcave, non-nucleated and discoid structures containing hemoglobin.17 The cell membranes of erythrocytes contain a heterogeneous distribution of phospholipids and proteins. The outer leaflet of the erythrocyte membrane is reported to contain high quantities of phosphatidyl choline and sphingomyelin while the inner leaflet of the membrane contains the charged phospholipids phosphatidyl ethanolamine and phosphatidyl serine.17 The cytoskeletal network beneath the cell membrane consisting of spectrin and actin maintain the unique discoid shape of the erythrocytes, which is responsible for its oxygen carrying capability.20 The shape of the erythrocytes can be altered by chemical, osmotic, magnetic and radiation stresses.20 Chemical stresses can cause shape deformation of erythrocytes from discoid to star-shaped echinocytes or cup-shaped spherocytes.31 This shape alteration may be attributed to many factors. These include membrane fluidization, calcium ion efflux, ceramide pathway activation, cytoskeletal protein abnormalities, flipping of phosphatidyl serine from the inner leaflet to the outer leaflet of the bilayer.20,30 The localization of a molecule in the membrane can trigger one or many of the above factors leading to shape deformation of the erythrocytes. The hesperidin molecules can localize deeper within the lipid bilayer causing only mild disturbance to the phospholipid packing as also indicated by the electrophysiological data. The reduced membrane fluidization causes only small changes to the shape of the erythrocytes (Fig. 9). The cytoskeletal staining also indicates that hesperidin does alter the distribution of F-actin network but does not completely destroy the cytoskeletal architecture (Fig. 10). Unlike hesperidin, its copper complex shows dramatic changes in the erythrocyte morphology and forms echinocytes. Echinocyte formation has been mainly attributed to an increase in membrane fluidization and, according to the bilayer-couple hypothesis, an increase in the curvature of the outer layer when compared with the inner layer of the bilayer.17,30 This confirms the findings from the electrophysiological experiments that the hesperidin–copper complex exhibits strong surface interactions due to its cationic nature causing greater membrane fluidization. The cytoskeletal staining confirms the disruption of the F-actin network implying that the membrane fluidization is a major driving factor for echinocyte formation. Fig. 11 shows the schematic indicating the interactions of hesperidin and its copper complex with the erythrocyte membrane.
 | ||
| Fig. 11 Schematic representations for the interaction of hesperidin and hesperidin–copper complex with erythrocyte membrane. (Blue: hesperidin, green: hesperidin–copper complex). | ||
The anti-oxidant quercetin has also been reported to induce the formation of echinocytes, which has been attributed to the surface interaction of quercetin with the erythrocyte membrane. However, no such phenomenon has been investigated earlier for any flavonoid–metal ion complex. Our results clearly indicated that the hesperidin–copper complex localized predominately in the membrane surface like quercetin while hesperidin localizes in the hydrophobic core like rutin. This in turn alters the membrane permeability and the arrangement of integral membrane proteins, which may have implications in membrane-mediated signaling pathways.
5. Conclusion
Room temperature synthesis of hesperidin–copper complex was achieved and the structural elucidation confirmed the formation of a cationic complex with a metal-to-ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The copper complex was found to exhibit strong surface interactions and due to its bulkiness was able to fluidize the membrane to a greater extent. Its antioxidant potential was lower than its parent flavonoid and it initiated formation of echinocyte spicules on the surface of erythrocytes probably due to an alteration in the membrane curvature at the outer leaflet. Thus, formation of a hesperidin–copper complex in vivo is likely to lead to detrimental effects. However, its cytotoxicity can be explored further for anti-cancer applications in the future.
2. The copper complex was found to exhibit strong surface interactions and due to its bulkiness was able to fluidize the membrane to a greater extent. Its antioxidant potential was lower than its parent flavonoid and it initiated formation of echinocyte spicules on the surface of erythrocytes probably due to an alteration in the membrane curvature at the outer leaflet. Thus, formation of a hesperidin–copper complex in vivo is likely to lead to detrimental effects. However, its cytotoxicity can be explored further for anti-cancer applications in the future.
Acknowledgements
The authors wish to acknowledge financial support from the Department of Science & Technology, Government of India, under the Grant SR/SO/BB–35/2004, and the infrastructural support from SASTRA University.References
- H. H. Bent, Pharmacol. Ther., 2002, 96, 67–202 CrossRef.
- S. D. Quine and P. S. Raghu, Pharmacol. Reports., 2005, 57, 610–615 CAS.
- J. A. Emim, A. B. Oliveira and A. J. Lapa, J. Pharm. Pharmacol., 1994, 46, 118–122 CrossRef CAS.
- A. Hirata, Y. Murakami, M. Shoji, Y. Kadoma and S. Fujisawa, Anticancer Res., 2005, 25, 3367–3374 CAS.
- A. L. Martínez, M. E. Gonzalez–Trujano, M. Chávez, F. Pellicer, J. Moreno and F. J. Lopez-Munoz, Pharmacol., Biochem. Behav., 2011, 97, 683–689 CrossRef.
- L. M. Loscalzo, C. Wasowski, A. C. Paladini and M. Marder, Eur. J. Pharmacol., 2008, 580, 306–313 CrossRef CAS.
- S. L. Guzman-Gutierrez and A. Navarrete, Planta Med., 2009, 75, 295–301 CrossRef CAS.
- J. Londono-Londono, V. R. Lima, C. Jaramillo and T. Creczynski-Pasa, Arch. Biochem. Biophys., 2010, 499, 6–16 CrossRef CAS.
- N. R. Perron, C. R. Garcia, J. R. Pinzon, M. N. Chaur and J. L. Brumaghim, J. Inorg. Biochem., 2011, 105, 745–753 CrossRef CAS.
- V. A. Kostyuk, A. I. Potapovich, E. N. Vladykovskaya, L. G. Korkina and I. B. Afanasev, Arch. Biochem. Biophys., 2001, 385, 129–137 CrossRef CAS.
- M. Kopacz, E. Woźnicka and J. Gruszecka, Acta Pol. Pharm., 2005, 62, 65–67 CAS.
- T. Jun, W. Bochu and Z. Liancai, Colloids Surf., B, 2007, 55, 149–152 CrossRef.
- S. B. Etcheverry, E. G. Ferrer, L. Naso, J. Rivadeneira, V. Salinas and P. A. Williams, JBIC, J. Biol. Inorg. Chem., 2008, 13, 435–447 CrossRef CAS.
- S. Dowling, F. Regan and H. Hughes, J. Inorg. Biochem., 2010, 104, 1091–1098 CrossRef CAS.
- A. Arora, T. M. Byrem, M. G. Nair and G. M. Strasburg, Arch. Biochem. Biophys., 2000, 373, 102–109 CrossRef CAS.
- L. Movileanu, I. Neagoe and M. Flonta, Int. J. Pharm., 2000, 205, 135–146 CrossRef CAS.
- M. P. Sheetz and S. J. Singer, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 4457–4461 CrossRef CAS.
- P. Mueller, D. O. Rudin, H. T. Tien and W. C. Wescott, J. Phys. Chem., 1963, 67, 534–535 CAS.
- S. Selvaraj, S. Krishnaswamy, V. Devashya, S. Sethuraman and U. M. Krishnan, Langmuir, 2011, 27, 13374–13382 CrossRef CAS.
- M. Suwalsky, P. Orellana, M. Avello and F. Villena, Food Chem. Toxicol., 2007, 45, 130–135 CrossRef CAS.
- P. Kuppan, K. S. Vasanthan, D. Sundaramurthi, U. M. Krishnan and S. Sethuraman, Biomacromolecules, 2011, 12, 3156–3165 CrossRef CAS.
- U. Valentina, F. B. Stefania, A. Victoria, A. Corina-Cristina, B. R. O. Mihaela and M. Dana, Molecules, 2010, 15, 1578–1589 CrossRef.
- P. Job, Annali di Chimica Applicata, 1928, 9, 113–203 CAS.
- B. Atanu, M. Beena, S. Liang, M. Hari, R. M. Kadamc, S. Duttad, Z. Hong-Yu and K. Indira Priyadarsini, Free Radical Biol. Med., 2005, 39, 811–822 CrossRef.
- F. Valerij, A. Antonov, A. Andrej, P. A. Vladimir, A. Norik, A. Y. Evgenija and Korepanova, Eur. Biophys. J., 2003, 32, 55–59 Search PubMed.
- P. I. Oteiza, A. G. Erlejman, S. V. Verstraeten, C. L. Keen and C. G. Fraga, Clin. Dev. Immunol., 2005, 12, 19–25 CrossRef CAS.
- K. U. Maheswari, T. Ramachandran and D. Rajaji, Biochim. Biophys. Acta, Biomembr., 2000, 1463, 230–240 CrossRef CAS.
- S. V. Verstraeten, P. I. Oteiza and C. G. Fraga, Bol. Res., 2004, 37, 293–300 Search PubMed.
- E. D. Pasquale, H. Chahinian, P. Sanchez and J. Fantini, PLoS One, 2009, 4, e4989 Search PubMed.
- K. A. K. A. Youdim, M. S. Dobbie, G. Kuhnle, A. R. Proteggente, N. J. Abbott and C. Rice-Evans, J. Neurochem., 2003, 85, 180–192 CrossRef CAS.
- M. Suwalsky, P. Orellana, M. Avello and F. Villena, Food Chem. Toxicol., 2007, 45, 130–135 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
