Catalytic conversion of polyethylene into aromatics with Pt/ZSM-5: insights into reaction pathways and rate-controlling step regulation†
Abstract
Catalytic pyrolysis of polyethylene (PE) can produce benzene, toluene, and xylene (BTX) as important building-block chemicals, and selectivity control is key to its economic and ecological efficiency. Here, we report a synergistic effect between Pt and ZSM-5 for selective production of BTX from catalytic pyrolysis of PE by providing mechanistic insights into reaction pathways of key intermediates and regulation of the rate-controlling step. A high yield (52%) of BTX was obtained at 450 °C under atmospheric pressure using a Pt/ZSM-5 catalyst, where Pt mainly exists in the metallic state with a cuboctahedral crystal structure. In contrast, thermal pyrolysis of PE produced linear alkenes/alkanes of wide carbon number distribution, and catalytic pyrolysis of PE over ZSM-5 led to a low BTX yield of 21% with C1–C4 alkenes/alkanes being the major products even at a higher temperature (500 °C). It was found that the introduction of Pt into ZSM-5 significantly decreased the aromatization onset temperature, suggesting a reduction of apparent activation energy for the aromatization of alkene intermediates during catalytic pyrolysis of PE. Density functional theory calculations reveal that the aromatization of intermediate ethylene proceeds via oligomerization, cyclization, and dehydrogenation, and the energy barrier of the rate-controlling step, i.e., dehydrogenation of 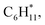 on Pt/ZSM-5 is much lower than that on ZSM-5. As a result, Pt enhanced the aromatization rates of light alkenes formed by PE cracking over the acid sites in ZSM-5, the synergistic effects of which contributed to the high BTX yield on Pt/ZSM-5.
on Pt/ZSM-5 is much lower than that on ZSM-5. As a result, Pt enhanced the aromatization rates of light alkenes formed by PE cracking over the acid sites in ZSM-5, the synergistic effects of which contributed to the high BTX yield on Pt/ZSM-5.

- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...