Investigation of the leaching mechanism of NMC 811 (LiNi0.8Mn0.1Co0.1O2) by hydrochloric acid for recycling lithium ion battery cathodes
Abstract
This paper investigates the reactions involved when LiNi0.8Mn0.1Co0.1O2 (NMC 811), which is one of the most promising positive electrodes for the next generation of lithium-ion batteries, is leached by hydrochloric acid. This study shows that the leaching behaviour of lithium is quite different than those observed for nickel, cobalt and manganese contained in NMC 811 since lithium dissolution is faster than those observed for nickel, cobalt and manganese. Analysis of leaching kinetic data evidenced that NMC 811 dissolution occurs in two steps. In the first step, NMC is transformed into a new phase which contains less lithium (2.8 < n < 3.6): 1st step: 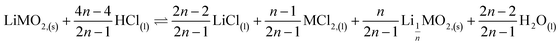 where M = Ni, Mn, Co. In the second step, the new phase is dissolved (limiting step): 2nd step:
where M = Ni, Mn, Co. In the second step, the new phase is dissolved (limiting step): 2nd step: 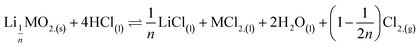 . Finally, the overall reaction of NMC 811 leaching by hydrochloric acid can be written as: 2LiMO2,(s) + 8HCl(l) ⇌ 2LiCl(l) + 2MCl2,(l) + 4H2O(l) + Cl2,(g).
. Finally, the overall reaction of NMC 811 leaching by hydrochloric acid can be written as: 2LiMO2,(s) + 8HCl(l) ⇌ 2LiCl(l) + 2MCl2,(l) + 4H2O(l) + Cl2,(g).

- This article is part of the themed collection: Editors' Collection: Lithium-ion batteries and beyond - materials, processes and recycling


 Please wait while we load your content...
Please wait while we load your content...