Bimetallic ZnSe–SnSe2 heterostructure functionalized separator for high-rate Li–S batteries†
Jiayi
Xue‡
a,
Daotong
Yang†
a,
Jianhua
Lin†
a,
Quan
Zhuang
 *a,
Mingxun
Jia
a,
Tong
Wu
a,
Lei
Ji
c,
Yingying
Zhang
*a,
Zhiqing
Niu
*a,
Mingxun
Jia
a,
Tong
Wu
a,
Lei
Ji
c,
Yingying
Zhang
*a,
Zhiqing
Niu
 b and
Jinghai
Liu
b and
Jinghai
Liu
 *a
*a
aInner Mongolia Engineering Research Center of Lithium-Sulfur Battery Energy Storage, Inner Mongolia Key Laboratory of Solid State Chemistry for Battery, College of Chemistry and Materials Science, Inner Mongolia Minzu University, Tongliao, China. E-mail: jhliu2008@sinano.ac.cn; zhuangquan21@outlook.com; zyy285127@163.com
bKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Haihe Laboratory of Sustainable Chemical Transformations, College of Chemistry, Nankai University, Tianjin, China
cState Key Laboratory of Low Dimensional Quantum Physics and Department of Physics, Tsinghua University, Beijing, China
First published on 28th November 2024
Abstract
Lithium polysulfide (LiPS) shuttling is still the core issue in advancing Li–S battery technologies towards high-power and fast-charging commercialized application. In this work, we demonstrate a confined catalysis of LiPSs by a functionalized separator to suppress shuttling and to improve the high rate capability and cycling stability. An oxygenated carbon nitride (OCN)-supported ZnSe–SnSe2 heterostructure (ZnSe–SnSe2@OCN) was designed for the functionalized separator. The ZnSe–SnSe2@OCN functionalized separator gives a high specific capacity of 609 mA h g−1 at 5 C, favorable cycling stability of 350 cycles at 1 C with a decay rate of 0.11% and coulombic efficiency of 98.6%. It also produces low voltage hysteresis (∼17 mV) after 600 h of cycling without significant voltage fluctuations in a Li|Li symmetric cell. The experimental evidence and density functional theory calculations reveal that the bimetallic ZnSe–SnSe2 sites regulate the density of states at the Fermi level and provide Se–Li, Zn–S and Sn–S chemical bonding interface for LiPS adsorption confinement. This work provides a viable functionalized separator solution for future high-rate Li–S batteries.
Lithium–sulfur (Li–S) batteries, offering a higher theoretical capacity at a lower cost than lithium-ion batteries, are expected to be strong candidates for next-generation commercialized batteries.1,2 However, various challenges hinder their large-scale application, including slow electrode reaction kinetics, severe self-discharge, rapid capacity decay, and significant corrosion of the lithium metal anode.3–5 These issues primarily arise from the shuttle effect of soluble lithium polysulfides (LiPSs) and the uneven deposition on the lithium (Li) metal surface during charging and discharging.6,7 Therefore, efficiently suppressing LiPS shuttling and inhibiting Li dendrite growth have become the primary concerns for achieving high rate and high energy density in Li–S batteries. The separator, responsible for separating the positive and negative electrodes to prevent short circuits, is a core component of such batteries.8 Unfortunately, commercially available separators with large pores allow LiPSs to easily penetrate through the separator under the driving force of external electric fields and concentration gradients, leading to Li electrode corrosion and LiPS shuttling. Coating the separator with an active material to form a functionalized separator with the ability to restrict the LiPSs at the cathode side provides a facile pathway to suppress shuttle effects while protecting the Li anode and achieving uniform Li deposition.9 Therefore, the use of functionalized separators in Li–S batteries has become an effective strategy for enhancing battery performance.10–12
Numerous studies have demonstrated that carbon nanomaterials can effectively inhibit LiPS diffusion due to their excellent conductivity and large specific surface area, making them a popular choice for functionalized separators.13–16 However, these functionalized separators fall short in further significant utilization for capturing LiPSs due to only physical LiPS adsorption.17,18 To improve the chemical affinity and catalytic conversion of LiPSs, integration of polar transition metal sites onto carbon has become an effective approach to reinforce the bonding interaction with LiPSs,19 where transition metal oxides,20–23 sulfides24–27 and selenides28,29 have been reported. The advantages of selenides include excellent electrical conductivity, large polarity and high catalytic activity compared with oxides and sulfides.17,30 Zinc selenide (ZnSe) semiconductor with its sulfophilic and lithiophilic sites can effectively inhibit LiPS diffusion.31–36 Tin(IV) selenide (SnSe2), a two-dimensional layered structure with a narrow band gap and large interlayer spacing, has been theoretically proved to have catalytic effects on LiPS conversion.37,38 Heterostructure interfaces provide interfacial charge modulation, LiPS entrapment and catalysis of bidirectional sulfur conversion.39–42 Considering the confined catalysis of LiPSs together with the ion-diffusion and charge-transfer kinetics, the design of a heterostructure consisting of ZnSe and SnSe2 with catalytically active sites, built-in electric field and density of states at the Fermi level can fundamentally meet the requirements for the suppression of LiPS shuttling and Li dendrite growth.
Here, we designed and synthesized an oxygenated carbon nitride (OCN)-supported ZnSe–SnSe2 heterostructure (ZnSe–SnSe2@OCN) by selenization of ZnSn(OH)6@OCN for a functionalized separator in Li–S batteries. The morphology, microstructure and surface chemical composition were examined to confirm the formation of ZnSe–SnSe2@OCN. The confined catalysis of LiPSs by the ZnSe–SnSe2@OCN functionalized separator to suppress shuttling to improve the high rate capability and cycling stability relating to electrochemical polarization, electron transfer and ionic diffusion was further explored in a Li–S cell. The effects of ZnSe–SnSe2@OCN on Li+ uniform deposition on the Li anode were analyzed in a Li|Li symmetric cell. Binding energy, differential charge density and projected density of states (PDOS) were calculated for elaborating the mechanism of bimetallic ZnSe–SnSe2 sites towards LiPS confinement.
The design of the ZnSe–SnSe2@OCN functionalized separator is schematically shown in Fig. 1, where the co-precipitation method is used to realize the self-assembly of ZnSn(OH)6 on the OCN surface. ZnSe–SnSe2@OCN was prepared by in situ selenization under hydrogen/argon (H2/Ar) atmosphere. ZnSe–SnSe2@OCN was then coated on a commercial Celgard 2400 (PP) separator for Li–S batteries. The crystalline ZnSn(OH)6 cubes with a size of 100 nm on the surface of the graphene-like OCN were confirmed (Fig. S1,†Fig. 2 and Fig. S2†). XRD patterns of ZnSe–SnSe2@OCN directly indicate the successful conversion of ZnSn(OH)6 after selenization. These patterns indicate the formation of cubic ZnSe (PDF 37-1463) with diffraction peaks at 27.2°, 45.2° and 53.6° corresponding to the (111), (220) and (311) crystal planes, and hexagonal SnSe2 (PDF 89-2939) with diffraction peaks at 14.4°, 30.7°, 40.1° and 47.7° matching the (001), (011), (012) and (110) crystal planes. The original cubic morphology collapses and aggregates into nanocrystals attaching to the graphene-like OCN after high-temperature selenization at 500 °C (Fig. 2c and d).
 | ||
| Fig. 1 Schematic illustration of the design of the ZnSe–SnSe2@OCN functionalized separator for Li–S batteries. | ||
The ZnSe–SnSe2 heterointerface was observed by high-resolution TEM (HRTEM) images (Fig. 2e and f), with the lattice spacing of 0.33 nm referring to the (111) crystal plane of ZnSe, together with that of 0.19 nm relating to the (110) crystal plane of SnSe2. The interface obviously exhibits a lattice distortion, indicating the formation of a heterostructure. ZnSe–SnSe2 nanocrystal was further verified by selected area electron diffraction (SAED) patterns, indicating the (111) and (220) crystal planes for ZnSe, and the (011) and (023) crystal planes for SnSe2 (Fig. 2g). Elemental mapping analysis (Fig. 2h) gives ZnSe–SnSe2@OCN consisting of six evenly distributed elements of carbon (C), nitrogen (N), oxygen (O), selenium (Se), tin (Sn) and zinc (Zn), demonstrating the uniform loading of ZnSe–SnSe2 nanocrystals on the OCN.
To investigate the surface chemical bonds of ZnSe–SnSe2@OCN, XPS tests were performed. The high-resolution XPS spectral analysis of O 1s (Fig. 3a) indicates four bond forms including quinone/pyridone oxygen (530.8 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Se–O (531.7 eV), C–O–C (532.9 eV), and carboxylic oxygen (535.8 eV). The C 1s spectrum in Fig. 3b shows the main graphite-like carbon (248.8 eV), along with C–N/C–O (285.6 eV), C–O–C/Se–C (286.6 eV), C
O/Se–O (531.7 eV), C–O–C (532.9 eV), and carboxylic oxygen (535.8 eV). The C 1s spectrum in Fig. 3b shows the main graphite-like carbon (248.8 eV), along with C–N/C–O (285.6 eV), C–O–C/Se–C (286.6 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV), and C(O)OH (289.6 eV). The N 1s spectrum in Fig. 3c displays four major forms of N, namely pyridinic N (398.3 eV), pyrrolic/pyridone N (399.2 eV), quaternary N (401.1 eV), and pyridine oxide N (403.2 eV). The high-resolution Sn 3d XPS spectrum in Fig. 3d exhibits peaks at 487.0 eV and 495.6 eV of Sn 3d5/2 and Sn 3d3/2, suggesting the presence of Sn–Se bonds and Sn4+ oxidation state. The Zn 2p3/2 and Zn 2p1/2 peaks at 1021.9 eV and 1044.9 eV in Fig. 3e confirm the existence of Zn–Se bonds, verifying the presence of Zn2+ oxidation state. The high-resolution Se 3d XPS spectrum in Fig. 3f could be divided into six peaks, where the peaks at 54.1 eV and 55.0 eV correspond to Se 3d5/2 and Se 3d3/2 in Zn–Se/Sn–Se, and the two main peaks at 56.0 eV and 56.9 eV correspond to Se 3d5/2 and Se3d3/2 of the Se–C bond. The peaks at 58.7 eV and 59.5 eV represent the Se–O bond, which could result from the slight oxidation in air condition. The XPS survey scan of ZnSe–SnSe2@OCN shows a chemical composition of six elements of O, C, N, Zn, Sn and Se (Fig. S3a†).
O (288.1 eV), and C(O)OH (289.6 eV). The N 1s spectrum in Fig. 3c displays four major forms of N, namely pyridinic N (398.3 eV), pyrrolic/pyridone N (399.2 eV), quaternary N (401.1 eV), and pyridine oxide N (403.2 eV). The high-resolution Sn 3d XPS spectrum in Fig. 3d exhibits peaks at 487.0 eV and 495.6 eV of Sn 3d5/2 and Sn 3d3/2, suggesting the presence of Sn–Se bonds and Sn4+ oxidation state. The Zn 2p3/2 and Zn 2p1/2 peaks at 1021.9 eV and 1044.9 eV in Fig. 3e confirm the existence of Zn–Se bonds, verifying the presence of Zn2+ oxidation state. The high-resolution Se 3d XPS spectrum in Fig. 3f could be divided into six peaks, where the peaks at 54.1 eV and 55.0 eV correspond to Se 3d5/2 and Se 3d3/2 in Zn–Se/Sn–Se, and the two main peaks at 56.0 eV and 56.9 eV correspond to Se 3d5/2 and Se3d3/2 of the Se–C bond. The peaks at 58.7 eV and 59.5 eV represent the Se–O bond, which could result from the slight oxidation in air condition. The XPS survey scan of ZnSe–SnSe2@OCN shows a chemical composition of six elements of O, C, N, Zn, Sn and Se (Fig. S3a†).
 | ||
| Fig. 3 Surface chemical analysis of ZnSe–SnSe2@OCN. High-resolution XPS spectra of (a) O 1s, (b) C 1s, (c) N 1s, (d) Sn 3d, (e) Zn 2p and (f) Se 3d. | ||
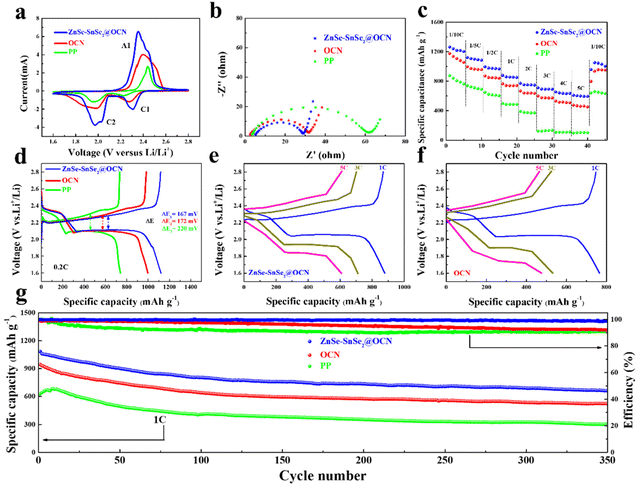
The electrochemical performances of the ZnSe–SnSe2@OCN functionalized separator in tailoring the confined catalysis of LiPSs to suppress shuttling and to improve the high rate capability and cycling stability were evaluated in a Li–S cell. A CR2032 coin cell was used with Li metal anode and sulfur cathode comprising Super P/sulfur (SP/S) composite (70.2 wt% in Fig. S4†). The confined catalysis of LiPSs can be reflected by cyclic voltammetry (CV), where the ZnSe–SnSe2@OCN functionalized separator exhibits low overpotential, high current density and small polarization. The oxidation potential at the A1 peak shows 42.6 mV shift to the left direction in comparison with the OCN functionalized separator and 83.6 mV shift compared with the PP separator. Meanwhile, the reduction potential at the C1 peak gives 32.6 mV shift to the right direction in comparison with OCN and 82.9 mV compared with PP, and at the C2 peak are 21.0 mV and 68.3 mV respectively (Fig. 4a). The influence of ZnSe–SnSe2@OCN on charge transfer and ion diffusion was analyzed by electrochemical impedance spectroscopy (EIS). The Nyquist plot in Fig. 4b depicts a charge transfer resistance (Rct) of 24.65 Ω, compared with 30.53 Ω for OCN and 56.35 Ω for PP.
The rate capability for the ZnSe–SnSe2@OCN functionalized separator gives a specific capacity of 609 mA h g−1 at 5 C, and values of 1265, 1121, 997, 879, 771, 711 and 654 mA h g−1 at current densities of 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 3 C and 4 C (Fig. 4c). Then, it maintains a specific capacity of 957.6 mA h g−1 after returning to initial 0.1 C. The GCD curves in Fig. 4d consist of two typical discharge plateaus representing the conversion of S8 into LiPSs (Li2Sn, 4 ≤ n ≤ 8) and the reduction of LiPSs into final Li2S2/Li2S solid. The initial discharge capacity is 1121 mA h g−1 at 0.2 C, 124 mA h g−1 higher than that of OCN and 380 mA h g−1 higher than that of PP. The polarization (ΔE) was 167 mV for the ZnSe–SnSe2@OCN functionalized separator at 0.2 C, 5 mV lower than that for OCN and 53 mV lower than that for PP, together with holding the typical discharge plateau even at high current densities of 1 C, 3 C and 5 C (Fig. 4e and f).
In order to further evaluate the effects on cycling life and capacity decay, we checked the cycling performance at 1 C for the ZnSe–SnSe2@OCN functionalized separator. As shown in Fig. 4g, the discharge specific capacity still remains at 661 mA h g−1, with an average decay rate of 0.11% per cycle and a coulombic efficiency (CE) of 98.6% after 350 cycles. Similarly, a value of 791 mA h g−1 can be obtained after 200 cycles at 0.5 C. We also provide a comparison of the electrochemical cycling stability for previously reported functionalized separators in Table S1† to show the competitive advantage of ZnSe–SnSe2@OCN.
We further investigated the influence of the ZnSe–SnSe2@OCN functionalized separator on the Li+ diffusion kinetics and Li deposition/stripping. The Li+ ion diffusion and polarization kinetics were investigated by CV under variable scan rates (Fig. 5a and Fig. S7a, S7b†) and evaluated using the classic Randles–Sevcik equation, where Ip = (2.69 × 105)n1.5ADLi+0.5CLi+ν0.5. The slope, Ip/v0.5, should be correlated with the diffusion coefficient of lithium ions (DLi+), and it shows a larger slope for the ZnSe–SnSe2@OCN functionalized separator than for OCN and PP. To further investigate the role of the ZnSe–SnSe2@OCN functionalized separator in uniform Li deposition/stripping, the long-term cycling of a Li|Li symmetric cell was carried out at 0.5 mA cm−2 with a stripping/plating capacity of 0.5 mA h cm−2 (Fig. 5d). It shows a low voltage hysteresis (∼17 mV) after 600 h of cycling without significant voltage fluctuations, illustrating a great potential to ensure uniform Li deposition. In comparison, a Li|Li symmetric cell with PP showed an unstable voltage hysteresis as well as obvious current fluctuations and large overpotentials (∼60 mV) after 350 h of cycling, suggesting uncontrolled SEI and inhomogeneous Li deposition/stripping. In addition, ZnSe–SnSe2@OCN endows the Li|Li symmetric cell with good rate performances with overpotentials of 16, 23, 34, and 59 mV at current densities of 0.5, 1, 2, and 4 mA cm−2, respectively (Fig. 5c). It can return to 14 mV after current density switching to 0.5 mA cm−2, indicating the excellent regulation of uniform redistribution of Li+ ion fluxes and Li deposition/stripping.
The mechanism of bimetallic ZnSe–SnSe2 sites towards LiPS confinement was further experimentally and theoretically probed. Observation of the LiPS shuttling block for the ZnSe–SnSe2@OCN functionalized separator was carried out using an H-type module with Li2S6 electrolyte in the left-hand chamber. After 6 hours of LiPS permeation, colorless and transparent electrolyte in the right-hand chamber proves the significant adsorption confinement of ZnSe–SnSe2@OCN for LiPSs, while a light yellow color was observed for OCN and dark yellow for PP (Fig. 6a). UV-visible spectra further give a quantitative measure of Li2S6 for the adsorption of electrolyte in the right-hand chamber, where there was no broad absorption peak in the wavelength range from 400 nm to 550 nm for ZnSe–SnSe2@OCN (Fig. 6b). The mechanical stability of a ZnSe–SnSe2@OCN film coating on a PP separator was also checked by folding the functionalized separator twice, and there was no detachment upon recovery (Fig. S8†).
The confinement interaction of bimetallic ZnSe–SnSe2 sites towards LiPSs was studied by density functional theory (DFT) calculations. The structural optimization, binding energy, and electronic density of states (DOS) were simulated by DS-PAW software,43 and Bader charge analysis and differential charge density plotting were performed by the VASP package.44 The adsorption geometry of Li2Sn and S8 on ZnSe–SnSe2, SnSe2, ZnSe and OCN was optimized (Fig. S11 to S14†). Regarding SnSe2, ZnSe and OCN, ZnSe–SnSe2 has a higher binding energy with Li2Sn (−1.12 eV to −3.81 eV), indicating strong adsorption confinement of ZnSe–SnSe2 for Li2Sn (Fig. 6c and Fig. S10b, Table S2†). The sites on ZnSe–SnSe2 were further elucidated through Bader charge analysis and differential charge density. With the Li2S4 molecule as an example, strong bonding between Sn–S, Zn–S, and Li–Se atoms was detected, accompanied by a significant charge transfer of 0.473e− from Li2S4 to the ZnSe–SnSe2 interface. This can also be reflected by the differential charge density map (Fig. 6d), where the yellow isosurface near ZnSe–SnSe2 indicates electron gain, and the cyan isosurface around Li2S4 represents electron loss. The high-resolution XPS spectra of Sn 3d and Zn 2p experimentally show the bimetallic sites with binding energy towards a lower shift after 200 cycles of charging or discharging (Fig. 6e and Fig. S9†), indicating an increase of electron density at Sn4+ and Zn2+ sites, consistent with the theoretical prediction.
Furthermore, the PDOS was used to investigate in detail the chemical bonding between bimetallic ZnSe–SnSe2 sites and Li2S4 molecules. The electronic structures of ZnSe–SnSe2, SnSe2 and ZnSe were firstly investigated, where the DOS arising at the Fermi level for ZnSe–SnSe2 and the typical semiconductor bandgap of SnSe2 and ZnSe can be detected (Fig. S10a†). From the PDOS analysis, the Se2− site with p orbital in bimetallic ZnSe–SnSe2 brings about chemical bonding with Li s orbital in Li2S4 (Fig. 6f). The Zn2+ and Sn4+ sites with p orbital and d orbital overlap with the S p orbital in Li2S4 (Fig. 6g). The orbital hybridization degrees between Li–Se, S–Zn and S–Sn for ZnSe–SnSe2 are stronger than the ones for SnSe2 and ZnSe, providing the strong adsorption confinement of bimetallic ZnSe–SnSe2 sites.
In summary, a ZnSe–SnSe2@OCN functionalized separator has been developed to realize the confined catalysis of LiPSs and uniform Li deposition/stripping for high-rate Li–S batteries. The bimetallic ZnSe–SnSe2 sites contribute to the suppression of LiPS shuttling by providing Zn2+, Sn4+ and Se2− sites with strong chemical bonding interactions. The confinement effects of the bimetallic heterostructure interface combining LiPS catalysis, electrochemical polarization, electron transfer and ionic diffusion, and Li deposition could represent a new functionalized separator strategy for future fast-charging Li–S batteries.
Data availability
Data for this article are available within the article and its ESI.†Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 22361035, 12264038), Inner Mongolia Grassland Talents-Young Leading Talent Program (KYCYYC23001, KYCYYC24001), Natural Science Foundation of Inner Mongolia of China (2024QN02013), Program for Innovative Research Team in Universities of Inner Mongolia Autonomous Region (NMGIRT2417), Basic Research Funds for University at Inner Mongolia (no. GXKY22063, GXKY22087), Excellent Students in School to Improve the Basic Scientific Research Ability Project (GXKY22234), Fund for Supporting the Reform and Development of Local Universities (Discipline Construction), Doctoral Scientific Research Foundation of Inner Mongolia University for Nationalities (no. KYQD23005, KYQD18047, KYQD21002), Inner Mongolia Grassland Talents-Young Innovative Talent Program (2023QNCXRC02), and Open Projects Funded by Inner Mongolia Engineering Research Center of Lithium–Sulfur Battery Energy Storage (MDK2023088). We gratefully acknowledge HZWTECH for providing computation facilities.References
- W. Hua, H. Li, C. Pei, J. Xia, Y. Sun, C. Zhang, W. Lv, Y. Tao, Y. Jiao, B. Zhang, S. Z. Qiao, Y. Wan and Q. H. Yang, Selective catalysis remedies polysulfide shuttling in lithium–sulfur batteries, Adv. Mater., 2021, 33, 2101006 CAS
.
- J. Shi, X. Wu, Y. Chen, Y. Zhang, X. Hou, R. Lv, J. Liu, M. Jiang, K. Huang and S. Feng, Structure factors dictate the ionic conductivity and chemical stability for cubic garnet-based solid-state electrolyte, Chin. Chem. Lett., 2024, 29, 109938 CrossRef
.
- X. B. Cheng, J. Q. Huang and Q. Zhang, Review—Li metal anode in working lithium–sulfur batteries, J. Electrochem. Soc., 2017, 165, A6058–A6072 CrossRef
.
- X. Fang and H. Peng, A revolution in electrodes: Recent progress in rechargeable lithium–sulfur batteries, Small, 2015, 11, 1488–1511 CrossRef CAS
.
- M. Jia, T. Li, D. Yang, L. Lu, L. Duan, J. Liu and T. Wu, Polymer electrolytes for lithium–sulfur batteries: progress and challenges, Batteries, 2023, 9, 488 CrossRef CAS
.
- F. Li, Q. Liu, J. Hu, Y. Feng, P. He and J. Ma, Recent advances in cathode materials for rechargeable lithium–sulfur batteries, Nanoscale, 2019, 11, 15418–15439 RSC
.
- A. I. Kamisan, T. I. T. Kudin, A. S. Kamisan, A. F. C. Omar, M. F. M. Taib, O. H. Hassan, A. M. M. Ali and M. Z. A. Yahya, Recent advances on graphene-based materials as cathode materials in lithium–sulfur batteries, Int. J. Hydrogen Energy, 2022, 47, 8630–8657 CrossRef CAS
.
- J.-Q. Huang, Q. Zhang and F. Wei, Multi-functional separator/interlayer system for high-stable lithium–sulfur batteries: Progress and prospects, Energy Storage Mater., 2015, 1, 127–145 CrossRef
.
- A. Kim, S. H. Oh, A. Adhikari, B. R. Sathe, S. Kumar and R. Patel, Recent advances in modified commercial separators for lithium–sulfur batteries, J. Mater. Chem. A, 2023, 11, 7833–7866 RSC
.
- M. Chen, M. Shao, J. Jin, L. Cui, H. Tu and X. Fu, Configurational and structural design of separators toward shuttling-free and dendrite-free lithium–sulfur batteries: A review, Energy Storage Mater., 2022, 47, 629–648 CrossRef
.
- P. Zuo, J. Hua, M. He, H. Zhang, Z. Qian, Y. Ma, C. Du, X. Cheng, Y. Gao and G. Yin, Facilitating the redox reaction of polysulfides by an electrocatalytic layer-modified separator for lithium–sulfur batteries, J. Mater. Chem. A, 2017, 5, 10936–10945 RSC
.
- W. Kang, N. Deng, J. Ju, Q. Li, D. Wu, X. Ma, L. Li, M. Naebe and B. Cheng, A review of recent developments in rechargeable lithium–sulfur batteries, Nanoscale, 2016, 8, 16541–16588 RSC
.
- H. Yao, K. Yan, W. Li, G. Zheng, D. Kong, Z. W. Seh, V. K. Narasimhan, Z. Liang and Y. Cui, Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode-separator interface, Energy Environ. Sci., 2014, 7, 3381–3390 RSC
.
- G. Zhou, L. Li, D. W. Wang, X. Y. Shan, S. Pei, F. Li and H. M. Cheng, A flexible sulfur-graphene-polypropylene separator integrated electrode for advanced Li–S batteries, Adv. Mater., 2015, 27, 641–647 CrossRef CAS PubMed
.
- G. Zhou, S. Pei, L. Li, D. W. Wang, S. Wang, K. Huang, L. C. Yin, F. Li and H. M. Cheng, A graphene-pure-sulfur sandwich structure for ultrafast, long-life lithium–sulfur batteries, Adv. Mater., 2014, 26, 625–631 CrossRef CAS
.
- T. Zhang, L. Zhang and Y. Hou, MXenes: Synthesis strategies and lithium-sulfur battery applications, eScience, 2022, 2, 164–182 CrossRef
.
- H. Wang, N. Deng, S. Wang, X. Wang, Y. Li, Q. Zeng, S. Luo, X. Cui, B. Cheng and W. Kang, Advanced preparation and application of transition metal selenides in lithium–sulfur batteries: a review, J. Mater. Chem. A, 2022, 10, 23433–23466 RSC
.
- L. Ji, X. Wang, Y. Jia, X. Qin, Y. Sui, H. Yan, Z. Niu, J. Liu and Y. Zhang, Oxygen and nitrogen tailoring carbon fiber aerogel with platinum electrocatalysis interfaced lithium/sulfur (Li/S) batteries, Chin. Chem. Lett., 2022, 34, 107123 CrossRef
.
- M. K. Aslam, S. Jamil, S. Hussain and M. Xu, Effects of catalysis and separator functionalization on high-energy lithium–sulfur batteries: a complete review, Energy Environ. Mater., 2023, 6, e12420 CrossRef CAS
.
- S. Imtiaz, Z. Ali Zafar, R. Razaq, D. Sun, Y. Xin, Q. Li, Z. Zhang, L. Zheng, Y. Huang and J. A. Anderson, Electrocatalysis on separator modified by molybdenum trioxide nanobelts for lithium–sulfur batteries, Adv. Mater. Interfaces, 2018, 5, 1800243 CrossRef
.
- W. Li, J. Hicks-Garner, J. Wang, J. Liu, A. F. Gross, E. Sherman, J. Graetz, J. J. Vajo and P. Liu, V2O5 Polysulfide Anion Barrier for Long-Lived Li–S Batteries, Chem. Mater., 2014, 26, 3403–3410 CrossRef CAS
.
- R. Song, R. Fang, L. Wen, Y. Shi, S. Wang and F. Li, A trilayer separator with dual function for high performance lithium–sulfur batteries, J. Power Sources, 2016, 301, 179–186 CrossRef CAS
.
- D. Yang, T. Wu, H. Gao, M. Jia, L. Ji, J. Wang, Q. Zhuang, B. Yu, L. Lu, Y. Zhang and J. Liu, Rutile titanium dioxide and graphene-like OCN tailoring free-standing carbon fiber aerogel as polysulfide anchoring materials for lithium–sulfur batteries, Mater. Today Sustainability, 2023, 24, 100573 CrossRef
.
- J. L. Yang, S. X. Zhao, Y. M. Lu, X. T. Zeng, W. Lv and G. Z. Cao, ZnS spheres wrapped by an ultrathin wrinkled carbon film as a multifunctional interlayer for long-life Li–S batteries, J. Mater. Chem. A, 2020, 8, 231–241 RSC
.
- Q. C. Li, H. Liu, B. Jin, L. Li, Q. D. Sheng, M. Y. Cui, Y. Y. Li, X. Y. Lang, Y. F. Zhu, L. J. Zhao and Q. Jiang, Anchoring polysulfides via a CoS2/NC@1T MoS2 modified separator for high-performance lithium–sulfur batteries, Inorg. Chem. Front., 2023, 10, 959–971 RSC
.
- H. Li, L. Sun, Y. Zhao, T. Tan and Y. Zhang, A novel CuS/graphene-coated separator for suppressing the shuttle effect of lithium/sulfur batteries, Appl. Surf. Sci., 2019, 466, 309–319 CrossRef CAS
.
- C. Y. Zhang, C. Zhang, J. L. Pan, G. W. Sun, Z. Shi, C. Li, X. Chang, G. Z. Sun, J. Y. Zhou and A. Cabot, Surface strain-enhanced MoS2 as a high-performance cathode catalyst for lithium–sulfur batteries, eScience, 2022, 2, 405–415 CrossRef
.
- X. Zhou, Y. Cui, X. Huang, Q. Zhang, B. Wang and S. Tang, Interface engineering of Fe3Se4/FeSe heterostructures encapsulated in MXene for boosting LiPS conversion and inhibiting shuttle effect, Chem. Eng. J., 2023, 457, 141139 CAS
.
- M. Wang, Y. Zhu, Y. Sun, Y. Zhao, X. Yang, X. Wang, Y. Song, H. Ci and J. Sun, A universal graphene-selenide heterostructured reservoir with elevated polysulfide evolution efficiency for pragmatic lithium–sulfur battery, Adv. Funct. Mater., 2022, 33, 2211978 Search PubMed
.
- X. Kang, Z. Jin, H. Peng, Z. Cheng, L. Liu, X. Li, L. Xie, J. Zhang and Y. Dong, The role of selenium vacancies functionalized mediator of bimetal (Co, Fe) selenide for high-energy-density lithium–sulfur batteries, J. Colloid Interface Sci., 2023, 637, 161–172 CAS
.
- D. Yang, C. Zhang, J. J. Biendicho, X. Han, Z. Liang, R. Du, M. Li, J. Li, J. Arbiol, J. Llorca, Y. Zhou, J. R. Morante and A. Cabot, ZnSe/N-doped carbon nanoreactor with multiple adsorption sites for stable lithium–sulfur batteries, ACS Nano, 2020, 14, 15492–15504 CAS
.
- Z. Ye, Y. Jiang, T. Yang, L. Li, F. Wu and R. Chen, Engineering catalytic CoSe-ZnSe heterojunctions anchored on graphene aerogels for bidirectional sulfur conversion reactions, Adv. Sci., 2021, 9, 2103456 CrossRef
.
- J. Liu, C. Lin, Q. Xie, D.-L. Peng and R.-J. Xie, Core-shell zeolite imidazole framework-derived ZnSe@CoSe2/C heterostructure enabling robust polysulfide adsorption and rapid Li+ diffusion in high-rate and high-loading lithium–sulfur batteries, Chem. Eng. J., 2022, 430, 133099 CrossRef CAS
.
- B. Wang, D. Sun, Y. Ren, X. Zhou, Y. Ma, S. Tang and X. Meng, MOFs derived ZnSe/N-doped carbon nanosheets as multifunctional interlayers for ultralong-Life lithium–sulfur batteries, J. Mater. Sci. Technol., 2022, 125, 97–104 CrossRef CAS
.
- J. Feng, C. Shi, H. Dong, C. Zhang, W. Liu, Y. Liu, T. Wang, X. Zhao, S. Chen and J. Song, Design of ZnSe-CoSe heterostructure decorated in hollow N-doped carbon nanocage with generous adsorption and catalysis sites for the reversibly fast kinetics of polysulfide conversion, J. Energy Chem., 2023, 86, 135–145 CrossRef CAS
.
- Q. Sun, Y. Zhang, H. Zhou, C. Ma, Y. Zhang, J. Wang, W. Qiao and L. Ling, Boosting polysulfide confinement and redox kinetics via ZnSe/NC@rGO as separator modifier for high-performance lithium–sulfur batteries, Electrochim. Acta, 2023, 445, 142026 CrossRef CAS
.
- W. Xiao, Q. He and Y. Zhao, Virtual screening of two-dimensional selenides and transition metal doped SnSe for lithium-sulfur batteries: A first-principles study, Appl. Surf. Sci., 2021, 570, 151213 CrossRef CAS
.
- X. Zhou, W. Mao, X. Cao, S. He, P. Wang, W. Wang, X. Wang and S. Tang, Bidirectional tandem catalysis coupled with interface engineering and Se vacancies for accelerating the polysulfide conversion, Nano Energy, 2024, 125, 109563 CrossRef CAS
.
- J. Lee, C. Choi, J. B. Park, S. Yu, J. Ha, H. Lee, G. Jang, Y. S. Park, J. Yun, H. Im, S. Moon, S. Lee, J.-I. Choi, D.-W. Kim and J. Moon, Optimally arranged TiO2@MoS2 heterostructures with effectively induced built-in electric field for high-performance lithium–sulfur batteries, J. Energy
Chem., 2023, 83, 496–508 CrossRef CAS
.
- Y. Liu, T. Y. Lei, Y. Y. Li, W. Chen, Y. Hu, J. W. Huang, J. W. Chu, C. Y. Yan, C. Y. Wu and C. T. Yang, Entrapment of polysulfides by a BiFeO3/TiO2 heterogeneous structure on separator for high-performance Li–S batteries, J. Power Sources, 2023, 556, 232501 CrossRef CAS
.
- P. Liu, J. Han, K. Zhu, Z. Dong and L. Jiao, Heterostructure SnSe2/ZnSe@PDA nanobox for stable and highly efficient sodium–ion storage, Adv. Energy Mater., 2020, 10, 2000741 CrossRef CAS
.
- W. Zhou, X. Wang, J. Shan, L. Yue, D. Lu, L. Chen, J. Zhang and Y. Li, Engineering hollow core-shell hetero-structure box to induce interfacial charge modulation for promoting bidirectional sulfur conversion in lithium–sulfur batteries, J. Energy Chem., 2023, 80, 128–139 CrossRef CAS
.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B:Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed
.
- G. Kresse and J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B:Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02476a |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2025 |