Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface decorated metal carbonyl clusters: bridging organometallic molecular clusters and atomically precise ligated nanoclusters
Cristiana
Cesari
 ,
Cristina
Femoni
,
Cristina
Femoni
 ,
Francesca
Forti
,
Francesca
Forti
 ,
Maria Carmela
Iapalucci
,
Maria Carmela
Iapalucci
 ,
Giorgia
Scorzoni
,
Giorgia
Scorzoni
 and
Stefano
Zacchini
and
Stefano
Zacchini
 *
*
Dipartimento di Chimica Industriale “Toso Montanari”, Università di Bologna, Via P. Gobetti 85, 40129 Bologna, Italy. E-mail: stefano.zacchini@unibo.it
First published on 13th January 2025
Abstract
In this Frontier Article, the work carried out within our research group in Bologna in the field of surface decorated metal carbonyl clusters will be outlined and put in a more general context. After a short Introduction, clusters composed of a metal carbonyl core decorated on the surface by metal–ligand fragments will be analyzed. Both metal–ligand fragments behaving as Lewis acids and Lewis bases will be considered. Then, the focus will be moved to clusters composed of a naked metal core decorated and stabilized on the surface by metal-carbonyl fragments. The structure and bonding (where theoretical studies are available) of such surface decorated metal carbonyl clusters will be presented, and compared to atomically precise ligated nanoclusters.
1. Introduction
According to F. A. Cotton, metal atom clusters are “…compounds containing a finite group of metal atoms which are held together entirely, mainly, or at least to a significant extent, by bonds directly between the metal atoms even though some non-metal atoms may be associated intimately with the cluster…”.1 There are nowadays several categories of compounds that fall within Cotton's definition, including clusters composed of many types of transition and main group metals, in low to high oxidation states, and stabilized by a large variety of inorganic and organic ligands.2–8 Metal carbonyl clusters (MCC) represent one of the most important category of organometallic and low-valent molecular metal clusters.9–24 The chemistry of MCCs is well developed, and they played a fundamental role for Cotton's definition of metal clusters.A similar consideration applies to gold-phosphine clusters, another category of low valent metal clusters, that was developed almost contemporarily to MCCs.25 Indeed, following the pioneering work of Malatesta and Naldini in the 1960s,26–29 several gold-phosphine clusters were characterized between 1970 and 2000. Since the turn of the century the range of gold cluster compounds has been greatly extended by the study of organothiolato-gold clusters. Indeed, molecular gold clusters and nanoclusters protected by thiolate ligands represent a very important field of research in modern inorganic chemistry and nanochemsitry.30–35 This field has been further expanded, including other organic ligands, as well as other metals, and alloy nanoclusters.36–38 Molecular clusters find nowadays several applications, including catalysis, electrocatalysis, material chemistry, sensing, medicine, biology.39–43
As in the early days of metal cluster chemistry, single crystal X-ray diffraction (SC-XRD) represents the most important technique for the complete characterization of the structures of metal clusters. The SC-XRD analysis of [Au102(p-MBA)44] (p-MBA = p-mercaptobenzoic acid) represented somehow a milestone and showed some interesting features.44 First of all, its metal core does not possess a ccp structure, as found in larger Au nanoparticles and bulk Au, but is based on an icosahedral structure. Moreover, the thiolato ligands are not innocent, but strongly interact with surface Au atoms, forming [Aun(SR)n+1]− staple motives which decorate the surface of the cluster. Similar structures have been found for several other thiolate protected Au nanoclusters, and linear RS–Au–SR staple motives are nowadays a common features in cluster chemistry.45
Different models have been developed for the interpretation of bonding in molecular metal clusters. In the case of low valent metal clusters, including MCCs, the most noticeable models for their electron counting are the effective atomic number (EAN) rule, Wade's rules, the polyhedral skeletal electron pair theory (PSEPT), and topological electron counting (TEC).25,46 Regarding thiolate protected gold nanoclusters, and related molecular nanoclusters, their bonding has been usually rationalized based on the superatom model.47–49 Recently, the superatom model has been successfully applied also to some MCCs, in particular Pt MCCs decorated on the surface by Cd-halide or Au-phosphine fragments, as well as Au, Ag and Cu clusters stabilized on the surface by Fe–CO, Ni–CO, Nb–CO or Ta–CO fragments.50–53 These are representative examples of the two main categories of surface decorated MCCs, that is: (1) clusters composed of a metal carbonyl core decorated on the surface by ML fragments; (2) clusters composed of a naked metal core decorated and stabilized on the surface by metal-carbonyl fragments. These theoretical studies suggest some analogies between surface decorated MCCs and atomically precise ligated nanoclusters. Further analogies become evident when their structures are analyzed. Indeed, the metal–ligand and metal-CO fragments decorating the surfaces of such MCCs are somehow reminiscent of the staple motives found in atomically precise ligated nanoclusters. One of the aim of this Frontier Article is to stimulate further theoretical studies in order to investigate the possibility of extending the applicability of the superatom model to other MCCs and, at the same time, to compare it with other theoretical models. This might lead to a general unified approach for the interpretation of the different categories of molecular ligated metal clusters.
Our group has worked in the field of surface decorated MCCs over the last 15 years, and the most noticeable results are summarized in this Frontier Article. After this short Introduction, section 2 will be dedicated to clusters composed of a metal carbonyl core decorated on the surface by ML fragments. This will be further divided based on the fact that the surface ML fragments may act as Lewis acids (section 2.1) or Lewis bases (section 2.2). Then, section 3 will be focused on clusters composed of a naked metal core decorated and stabilized on the surface by metal-carbonyl fragments. For each category of surface decorated MCCs, the most representative examples taken from our work will be presented together with the most relevant compounds published in the literature. The structure and bonding (where theoretical studies are available) of such surface decorated MCCs will be presented, and compared to atomically precise ligated nanoclusters. The applications of MCCs in catalysis and electrocatalysis have been recently reviewed.9,13 A short perspective on the potential and prospects of MCCs in practical applications will be reported in the Conclusions. All the structures reported in the following figures have been determined by SC-XRD.
2. Metal carbonyl core decorated on the surface by metal–ligand fragments
2.1. Metal–ligand fragments as Lewis acids
There are several examples of MCCs decorated on the surface by metal–ligand fragments of general formula {MxLy}z (x, y = 1, 2, 3…; z = 0, positive or negative; M = transition or p-block metal; L = neutral or anionic ligand). These fragments may act as Lewis acids or Lewis bases. The former behaviour will be discussed in this section, the latter in section 2.2.Cationic [Au(PR3)]+ groups are the archetype of metal–ligand fragments that can coordinate as Lewis acids on the surface of MCCs. Indeed, several examples of MCCs decorated by [Au(PR3)]+ fragments are known, and this topic has been recently reviewed.15 [Au(PR3)]+ fragments possess 12-electrons, and the d10 Au(I) centre may accept electron density from the MCC surface on its empty orbitals (s, p or hybridized orbitals). It must be remarked that coordination of a 12 electron fragment does not alter the electron count of a MCC. This concept can be extended to other 12 electron ML fragments, including group 12 metals [ML]2+ and [MX]+ fragments (M = Zn, Cd, Hg; L = neutral ligand; X = anionic ligand), group 11 metals [ML]+ and [MX] fragments (M = Cu, Ag, Au), group 10 metals [ML] fragments (M = Ni, Pd, Pt), and group 9 metals [ML2]+ fragments (M = Co, Rh, Ir).
Earlier interest was due to the isolobal analogy between [Au(PR3)]+ and H+.54–57 As soon as structures of MCCs containing two or more [Au(PR3)]+ fragments were discovered, it was realized that Au(I) centres could reciprocally attract due to aurophilic interactions.58–63 Indeed, peraurated MCCs revealed to be suitable platforms to study aurophilicity. This generates [{Au(PR3)}n]n+ fragments, which are strictly related to the Au-thiolate staple motives found in molecular Au nanoclusters.
Since aurophilic interactions are rather weak, MCCs decorated by [Au(PR3)]+ fragments may show fluxionality and/or isomerism (Fig. 1 and 2).64–67
 | ||
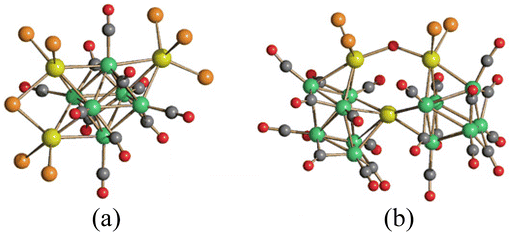
| Fig. 1 Three isomers of [Co6C(CO)12(AuPPh3)4] are formed upon crystallization, as found in the crystals of [Co6C(CO)12(AuPPh3)4], [Co6C(CO)12(AuPPh3)4]·THF and [Co6C(CO)12(AuPPh3)4]·4THF (blue, Co; yellow, Au; green, P; red, O; grey, C; white, H). Adapted with permission from ref. 64 Copyright 2014 American Chemical Society. | ||
Moreover, coordination of [Au(PR3)]+ fragments may favour the stabilisation and isolation of MCCs, that otherwise cannot be observed as free species.68 For instance, perauration allows the isolation of Ni carbide MCCs such as [Ni6(C)(CO)9(AuPPh3)4]2− and [Ni6(C)(CO)8(AuPPh3)8]2+, containing a carbide atom within an octahedral Ni6 cages (Fig. 3).69,70
 | ||
| Fig. 3 Perauration of Ni-carbide MCCs results in the encapsulation of the carbide within an octahedral Ni6 cage. Usually carbides are present within larger Ni cages, such as capped trigonal prismatic or square-antiprismatic. Molecular structure of (a) [Ni6(C)(CO)8(AuPPh3)8]2+, (b) its Ni6(C)Au8 cage, and (c) its [Ni6(C)(CO)8]6− core (green, Ni; yellow, Au; purple, P; red, O; grey, C; H-atoms have been omitted for clarity). Surface decoration with [Au(PR3)]+ fragments results in the stabilization in the otherwise unknown [Ni6(C)(CO)8]6− cluster. Adapted from ref. 70 with permission from The Royal Society of Chemistry. | ||
The reaction of [Pt19(CO)22]4− with CO affords a purported [Pt19(CO)22]4− cluster, which has never been isolated, since the reaction is reversed upon crystallization. Nonetheless, it has been possible to trap this elusive [Pt19(CO)24]4− species by addition of 3–4 [Au(PPh3)]+ fragments, affording the surface decorated clusters [Pt19(CO)24(AuPPh3)3]− and [Pt19(CO)24(AuPPh3)4] (Fig. 4).71 Interestingly, [Pt19(CO)24(AuPPh3)3]− is decorated by three isolated [Au(PPh3)]+ fragments, whereas [Pt19(CO)24(AuPPh3)4] contains two [{Au(PPh3)}2]2+ fragments showing one aurophilic interaction each.
The cluster [Os10C(CO)24Au(AuPCy3)3] is rather peculiar, since it may be viewed as a [Os10C(CO)24] tetrahedron of frequency two decorated on one edge by a [Au4(PCy3)3] tetrahedral unit (Fig. 5).72 This is a rare case of a MCC decorated by an Au-phosphine cluster.
The concepts developed for [Au(PR3)]+ fragments can be applied to related fragments obtained using other monodenate or polydentate ligands, or replacing Au(I) with other coinage metal ions, that is, Cu(I) and Ag(I). Several examples of MCCs decorated by [ML]+ (M = Cu, Ag, Au; L = neutral ligand) or MX (M = Cu, Ag, Au; X = anionic ligand) fragments are, indeed, known.
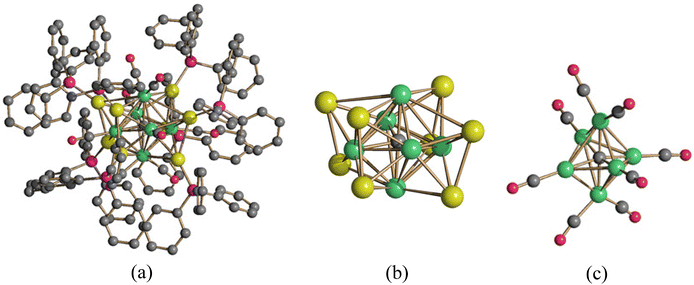
NHC ligands have recently attracted a large interest in catalysis, coordination and organometallic chemistry. Examples of MCCs decorated by [M(NHC)]+ (M = Cu, Ag, Au) fragments have been, also, reported, even though their nuclearity is usually rather limited.73–82 The largest species reported so far are [Pt6(CO)12(AgIPr)2] and [Pt9(CO)18(AgIPr)2] [IPr = C3N2H2(C6H3iPr2)2] obtained from the reactions of Chini clusters [Pt6(CO)12]2− and [Pt9(CO)18]2−, respectively, with Ag(IPr)Cl (Fig. 6).83 In these cases, the two [AgIPr]+ fragments cap the external triangular faces of the Chini clusters with retention of their prismatic structures.
 | ||
| Fig. 6 MCCs decorated by [M(NHC)]+ (M = Cu, Ag, Au) fragments. The molecular structure of (a) [Pt6(CO)12(AgIPr)2] and (b) [Pt9(CO)18(AgIPr)2] (purple, Pt; yellow, Ag; blue, N; red, O; grey, C; white, H). Adapted with permission from ref. 83 Copyright 2017 American Chemical Society. | ||
Clusters with higher nuclearities have been obtained upon capping Ni carbide MCCs with CuCl and [Cu(MeCN)]+ fragments (Fig. 7), as in the case of [HNi42C8(CO)44(CuCl)]7− and [H2Ni29+x(CO)33+x{Cu(MeCN)}2]4− (x = 0, 1).84–86 The two [Cu(MeCN)]+ fragments in the latter cluster may be formally replaced with isoelectronic [CdX]+ (X = Cl, Br, I) or Ni(CO) fragments, affording [H6−nNi30C4(CO)34(CdX)2]n− (n = 3–6), [H7−nNi32C4(CO)36(CdX)]n− (n = 5–7), and [H6−nNi34+xC4(CO)38+x]n− (n = 5, 6; x = 0, 1).87,88 All these clusters are based on the same tetracarbide Ni30C4 core. Similarly, replacing the CuCl fragment of [HNi42C8(CO)44(CuCl)]7− with [CdX]+ (X = Cl, Br) or Ni(CO) fragments results in related [Ni42+xC8(CO)44+x(CdCl)]7− (x = 0, 1), [HNi42+xC8(CO)44+x(CdBr)]6− (x = 0, 1), [HNi43C8(CO)45]7−, [HNi44C8(CO)46]7−, [H2Ni43C8(CO)45]6−, and [H2Ni44C8(CO)46]6−.84,85,89 All these clusters possess a common Ni42C8 core differently capped by miscellaneous [CuCl], [CdX]+, and [Ni(CO)] fragments and may be viewed as borderline compounds between molecular and quasi-molecular clusters. It must be remarked that from a synthetic point of view, clusters containing Cu and Cd are usually obtained from the reactions of preformed Ni carbide MCCs such as [Ni9C(CO)17]2−, [Ni10(C2)(CO)16]2−, [Ni16(C2)2(CO)23]4−, and [Ni38C6(CO)42]6−, with CuCl, [Cu(MeCN)4][BF4] and CdX2. Homometallic Ni species are obtained from the chemical oxidation of the same carbide species. Thus, since Cu(I) and Cd(II) salts may act also as oxidants, sometimes mixtures of species are obtained, that contain a variable amount of [CdX]+/Ni(CO), CuCl/Ni(CO) or [Cu(MeCN)]+/Ni(CO) fragments.
 | ||
| Fig. 7 Ni-carbide MCCs decorated by Cu and Cd-based fragments: (a) [H2Ni30C4(CO)34{Cu(MeCN)}2]4− (green, Ni; orange, Cu; grey, C; red, O; blue, N; white, H); (b) [Ni36C8(CO)36(Cd2Cl3)]5−, (c) [Ni42+xC8(CO)44+x(CdCl)]7− (x = 0.19) (green, Ni; yellow, Cd; orange, Cl; grey, C; red, O); (d) [HNi42C8(CO)44(CuCl)]7− and (e) [HNi43+xC8(CO)45+x]7− (two CO ligands with partial occupancy factors have not been located) (green, Ni; purple, Ni with partial occupancy factor; orange Cu; yellow, Cl; grey, C; red, O). Hydride ligands have not been located by SC-XRD. Adapted from ref. 84 and 85 with permission from Springer and Elsevier. | ||
Capping a cluster with Ni(CO) fragments is very common and, indeed, several Ni MCCs displaying the same metal core decorated by a variable amount of Ni(CO) fragments are known (Fig. 8).84,85,89–91 Even though apparently different, it must be remarked that both in the cases of Ni MCCs and Au thiolated nanoclusters, capping fragments and metal core are based on the same metals, that is, Ni in the case of Ni MCCs decorated by Ni(CO) fragments, Au in the case of Au nanoclusters with Au–SR staple motives.
 | ||
| Fig. 8 Different Ni(CO) capping modes found in the [HNi42C8(CO)44(CuCl)]7−, [HNi43+xC8(CO)45+x]7−, [H2Ni43+xC8(CO)45+x]6−, [Ni42+xC8(CO)44+x(CdCl)]7− and [HNi42+xC8(CO)44+x(CdBr)]6− clusters. (a) Uncapped Ni42C8(CuCl) core; (b) mono-capped Ni44C8 core; (c) mono-capped Ni43C8(CdX) core (green, Ni; grey, C; blue, Ni not bonded to carbide; purple, Ni with partial occupancy factor; orange, Cu; cyan, Cd; yellow, Cl or Br). Hydride ligands have not been located by SC-XRD. Adapted from ref. 84 with permission from Springer. | ||
As an alternative to Ni(CO), MCCs may also be decorated by M(PR3) or M(NHC) (M = Ni, Pd, Pt) fragments (Fig. 9).92–96
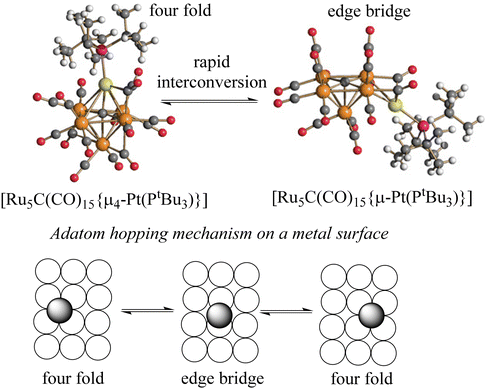 | ||
| Fig. 9 (Top) Molecular structures of the two isomers [Ru5C(CO)15{μ4-Pt(PtBu3)}] and [Ru5C(CO)15{μ-Pt(PtBu3)}] (orange, Ru; yellow, Pt; green, P; red, O; grey, C; white, H). The two isomers rapidly interconvert in solution, mimicking the shift of a metal atom from a 4-fold to a 2-fold bonding site and back on a metal surface during the hopping process. (Bottom) A schematic representation of the adatom hopping mechanism on a metal surface. Empty circles represent the metal surface; the shaded circle represent the atom that moves. Adapted with permission from ref. 92 Copyright 2003 American Chemical Society. | ||
Some impressive examples of MCCs decorated by Ni(PR3) and Pd(PR3) fragments are the homo and heterometallic Pd CO/PR3-ligated clusters described by Dahl and Mednikov (Fig. 10).12,97,98
 | ||
| Fig. 10 MCCs decorated by Ni(PR3) fragments: (a) [Pd16Ni4(CO)22(PPh3)4]2− and (b) [Pd33Ni9(CO)41(PPh3)6]4− (orange, Pd; green, Ni; purple, P; grey, C; red, O). H-atoms have been omitted for clarity. | ||
Group 9 metal fragments of the type [ML2]+ (M = Rh, Ir; L = neutral ligand) are isoelectronic to those described above and, thus, may be used in order to decorate MCCs. Nonetheless, very few examples are known.99,100
As shown above, some MCCs decorated by [CdX]+ fragments have been reported, and the literature may be extended to related [ZnX]+ and [HgX]+ fragments. Cd(II) is rather versatile for this purpose, and other Cd-based fragments may be found, that is, CdX2, [Cd2Cl3]+ and [Cd5(μ-Br)5Br5−x(solvent)x]x+.101–105
Thermal decomposition in dfm at 120 °C of Chini clusters [Pt3n(CO)6n]2− (n = 2–6) in the presence of CdBr2·H2O under different experimental conditions selectively afford the surface decorated Pt–Cd nanoclusters [Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2−, [Pt19(CO)17{Cd5(μ-Br)5Br3(Me2CO)2}{Cd5(μ-Br)5Br(Me2CO)4}]2−, and [H2Pt26(CO)20(CdBr)12]8−.106,107
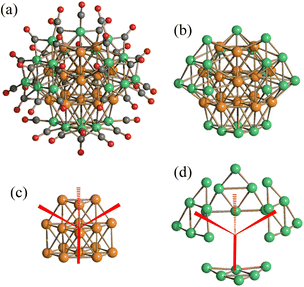
[Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2− is composed of a Pt13(CO)12 core decorated by two {Cd5(μ-Br)5Br2(dmf)3} rings (Fig. 11). Its electron count may be justified adopting either an ionic or covalent (radical) model. In the ionic model, the oxidation states of Cd and Br are assumed to be +2 and −1, respectively, and the cluster may be partitioned into two cationic [{Cd5(μ-Br)5Br2(dmf)3}]3+ rings and one [Pt13(CO)12]8− anionic core. The two rings act as Lewis acids via the Cd(II) centers, and overall [Pt13(CO)12]8− displays 162 cluster valence electrons (CVE) [13(Pt) × 10 + 12(CO) × 2 + 8(negative charge)]. In the covalent model, each {Cd5(μ-Br)5Br2(dmf)3} ring is assumed as neutral, and binds the inner [Pt13(CO)12]2− cluster as a pseudo π-allyl ligand, contributing three electrons (one per each Cd bonded to dmf). The overall electron count is still 162 CVE [13(Pt) × 10 + 12(CO) × 2 + 2(negative charge) + 2(Cd rings) × 3]. Based on the cluster-borane analogy, an icosahedral transition metal cluster should display 170 CVE rather than 162 CVE.108 Nonetheless, most gold icosahedral clusters display 162 CVE, as in the case of [Au13Cl2(PMe2Ph)10]3+ and [Au9M4Cl4(PMe2Ph)10]+ (M = Cu, Ag, Au).109 Mingos rationalized the low electron count of such clusters based on the predominance of radial over tangential bonding.110 Pyykko further supported the explanation of Mingos, by performing DFT calculations of the hypothetical 162 CVE [WAu12(CO)12] cluster.111 The same electron count has been found also in [Pt13{Au2(PPh3)2}2(CO)10(PPh3)4] (Fig. 12), composed of a [Pt13(CO)10(PPh3)4]4− core [13(Pt) × 10 + 10(CO) × 2 + 4(PPh3) × 2 + 4(negative charge) = 162 CVE] decorated by two [Au2(PPh3)2]2+, acting as Lewis acids.112 Using a similar approach based on Lewis Dot Formulas, staple motives such as [Au(SR)2]− and [Au2(SR)3]−, found in thiolated gold clusters, may be viewed essentially as four-electron donors. Within this framework, the [Au25(SCH2CH2Ph)18]− nanocluster may be viewed as composed of a [Au13]5+ core [13(Au) × 11 − 5(positive charge) = 138 CVE] and six [Au2(SR)3]− staple motives [contributing 6 × 4 = 24 CVE], with an overall electron count of 162 CVE, as in the above mentioned organometallic carbonyl and phosphine clusters.
 | ||
| Fig. 11 [Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2− (a) may be described as composed of a [Pt13(CO)12]8− anionic core (b) decorated by two cationic [{Cd5(μ-Br)5Br2(dmf)3}]3+ rings (c, tow views). The resulting Pt13 and Pt13Cd10 cores are represented in (d) and (e), respectively (purple, Pt; yellow, Cd; orange, Br; blue, N; red, O; grey, C; white, H). Adapted with permission from ref. 106 Copyright 2011 American Chemical Society. | ||
Saillard et al. have analyzed the same clusters within the framework of the superatom model.51 [Au13Cl2(PMe2Ph)10]3+ is a superatom with 8 free electrons (fe = free electrons; nfe = number of free electrons) [nfe = 13(Au13) − 2 (Cl2) − 3(positive charge) = 8] and 1S2 1P6 closed-shell configuration. In the case of [Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2−, the 8− charge of the [Pt13(CO)12]8− core provides the 8 free electrons for the same superatomic 1S2 1P6 configuration. This point has been further corroborated by the Kohn–Sham orbital diagram of [Pt13(CO)12]8−, where the 1S and 1P orbitals lie at the bottom of the fully occupied 5d block, whereas the empty 1D level is located above the π*(CO) orbitals (Fig. 13). Similar conclusions apply to [Pt13{Au2(PPh3)2}2(CO)10(PPh3)4], which is again a superatom with nfe = 8. These bonding analyses may be extended to other Pt–Cd carbonyl clusters, and the reader may find details in the cited literature.51
 | ||
| Fig. 13 Kohn–Sham MO diagram of [Pt13(CO)12]8−, as found in the structure of [Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2−. Adapted from ref. 51 with permission from The Royal Society of Chemistry. | ||
Surface decoration of MCCs with Lewis acids may be extended also to main group metal-based fragments. For instance, reactions of [Ni6(CO)12]2− with InBr3 under different experimental conditions lead to the selective formation of [Ni6(μ3-InBr3)(η2-μ6-In2Br5)(CO)11]3−, [Ni6(η2-μ6-In2Br5)2(CO)10]4−, and [Ni12(μ6-In)(η2-μ6-In2Br4OH)(CO)22]4− (Fig. 14).113 These clusters are composed of [Ni6(CO)11]4− or [Ni6(CO)10]6− carbonyl cores decorated by Lewis-acid fragments such as InBr3, [In2Br5]+, [In2Br4OH]+ and [In]3+.
2.2. Metal–ligand fragments as Lewis bases
Metal–ligand fragments with a Lewis base character may coordinate to the surface of a MCC as monodentate or polydentate ligands. Such fragments may replace one or more CO ligands in existing MCCs, or stabilize non-existing MCC cores. Therefore, these metal–ligand Lewis base fragments may be viewed as organometallic analogues of organic ligands, such as monodentate or polydentate phosphines.Among these, Sn(II) based fragments are very effective and versatile. Simple fragments such as [SnCl3]−, SnCl2 and [SnCl]+ may be viewed as two-electron donor ligands, related to alkyl, carbene and carbyne ligands, respectively (Scheme 1). The [Cl2Sn(μ-OR)SnCl2]− and [Br2Sn(μ-Br)SnBr2]− fragments may be described as bidentate (four electron) ligands, whereas [Cl2SnOCOSnCl2]2− is a tridentate ligand, donating four electrons via the two Sn(II)-centers and two electrons via the C-atom. The species [Pt8(CO)10(SnCl2)4]2−, [Pt5(CO)5{Cl2Sn(OR)SnCl2}3]3− (R = H, Me, Et, iPr), [Pt6(CO)6(SnCl2)2(SnCl3)4]4−, [Pt9(CO)8(SnCl2)3(SnCl3)2(Cl2SnOCOSnCl2)]4− and [Pt10(CO)14{Cl2Sn(OH)SnCl2}2]2− can be obtained from the reactions of [Pt3n(CO)6n]2− (n = 2–5) Chini clusters with SnCl2 under different experimental conditions (Fig. 15).114–116 These may be further transformed into [Pt6(CO)8(SnCl2)(SnCl3)4]4− and [Pt6(CO)8(SnCl2)(SnCl3)2(PPh3)2]2− upon reactions with CO and PPh3. All these species may be viewed as Pt–CO clusters decorated by the above mentioned Sn-based fragments.
 | ||
| Fig. 15 Pt carbonyl clusters decorated by Sn-fragments acting as Lewis bases. Molecular structures of (a) [Pt6(CO)6(SnCl2)2(SnCl3)4]4−, (b) [Pt8(CO)10(SnCl2)4]2−, (c) [Pt9(CO)8(SnCl2)3(SnCl3)2(Cl2SnOCOSnCl2)]4− and (d) [Pt10(CO)14{Cl2Sn(OH)SnCl2}2]2− (purple, Pt; orange, Sn; green, Cl; red, O; grey, C; white, H). Adapted from ref. 115 with permission from The Royal Society of Chemistry. | ||
Reaction of [Pt15(CO)30]2− with GeCl4 affords [Pt8(CO)10(GeCl2)4]2−, isostructural to [Pt8(CO)10(SnCl2)4]2−, that contains four carbene-like GeCl2 ligands.117 Several examples of Ru, Os and Ir carbonyl clusters decorated by [GeR]+ and GeR2 (R = H, alkyl, aryl) have been reported by Adams and Captain.118–120 For instance, thermal reaction of Ru3(CO)12 and tBuGeH3 in heptane affords a mixture of six clusters, that is, Ru3(CO)9(μ3-GetBu)2, Ru2(CO)6(μ-GetBuH)3, Ru4(CO)10(μ4-Ge2tBu2)(μ-GetBuH)2, Ru4(CO)8(μ4-Ge2tBu2)(μ-GetBuH)2(μ3-GetBu)(H), Ru5(CO)12(μ3-GetBu)2(μ4-GetBu)(H), and Ru6(CO)12(μ3-GetBu)4(H)2, which can be separated by TLC (Scheme 2). All these Ru–Ge carbonyl clusters are composed of Ru2, Ru3, Ru4, Ru5 and Ru6 carbonyl cores decorated by [GetBu]+ and GetBuH ligands.
 | ||
| Scheme 2 The products of the thermal reaction of Ru3(CO)12 and tBuGeH3. The products have been separated by thin layer chromatography (TLC). CO ligands have been omitted for clarity. Adapted from ref. 9 with permission from The Royal Society of Chemistry. | ||
The clusters [Rh6(CO)15(GaCp*)], [Rh6(CO)14(GaCp*)2], [Rh6(CO)13(GaCp*)3], and [Rh6(CO)12(GaCp*)4] may be viewed as octahedral Rh6 clusters decorated by GaCp* fragments (Fig. 16).121,122 This is a two-electron donor fragment and, thus, these species are formally obtained upon replacement of 1–4 CO ligands in [Rh6(CO)16] by 1–4 GaCp*. A related InCp* species has been also reported, that is, [Rh6(CO)15(InCp*)]. The cluster [Ni4(CO)6(GaCp*)4] is a tetrahedral 60 CVE species, analogue to the homoleptic [Ni4(CO)10] cluster, which has never been observed (Fig. 17).123
 | ||
| Fig. 16 Octahedral Rh carbonyl clusters decorated by GaCp* and InCp* fragments. Molecular structures of (a) [Rh6(CO)12(μ3-GaCp*)4] and (b) [Rh6(CO)15(μ3-InCp*]) (blue, Rh; yellow, Ga (a) or In (b); red, O; grey, C; white, H). These compounds have been obtained upon reactions of [Rh6(CO)16] with GaCp* and [Rh6(CO)15(MeCN)] with InCp*, respectively (Cp* = pentamethylcyclopentadienyl). Adapted from ref. 9 with permission from The Royal Society of Chemistry. | ||
3. Metal core decorated on the surface by metal-carbonyl fragments
Metal-carbonyl fragments may behave as ligands and coordinate to single metal ions, dimers or metal clusters. This applies to monometallic M–CO fragments, that is, [M(CO)n]z−, or polynuclear MCC fragments, that is, [Mm(CO)n]z−. Such M–CO fragments may exist or not exist as free species.There are several complexes where a single d10 metal ion (Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+) is linearly bonded to two MCC fragments. Some noticeable examples are represented in Fig. 18.124–126 Such complexes are closely related to MCCs decorated on the surface by ML fragments. In the case of compounds described in section 2.1, the central ion is bonded to one MCC fragment and one L (or X−) ligand, whereas in the cases reported in Fig. 18, M is bonded to two MCC fragments.
In most of the cases so far reported, the single metal ion is coordinated to anionic MCC fragments. An interesting exception is represented by [MFe2(CO)10]+ (M = Cu, Ag, Au), where the M+ ions are linearly bonded to two neutral Fe(CO)5 molecules.127 There are also a few examples where a d10 ion is coordinated to extended cyclic or acyclic MCC fragments, such as [MRu6(CO)22]− (M = Cu, Ag), [AuRu5(CO)19]−,128 and [HgOs6(μ-PPh2)2(CO)20].129
In all cases, the MCC fragments act as Lewis bases, and they can also bind to larger Mn fragments. This point is well exemplified by the Hg–Ru clusters [HgRu6(CO)22]2−, [Hg2Ru7(CO)26]2−, [Hg3Ru8(CO)30]2−, and [Hg4Ru10(CO)32]4− (Fig. 19).130 These contain [Hg]2+, [Hg2]4+, linear [Hg3]6+, and rectangular [Hg4]8+ cores, respectively, stabilized by [Ru(CO)4]2−, [Ru3(CO)11]2−, and [Ru4(CO)12]2− MCC anions. Triangular [Hg3]6+ cores have been found in [Hg3Os9(CO)33] and [Hg3Os18(C)2(CO)42]2− (Fig. 20).131,132 The former cluster possesses a slightly twisted 2-D structure, since the three [Os3(CO)11]2− units are side-by-side bonded to the three edges of the [Hg3]6+ triangle. Conversely, in the case of [Hg3Os18(C)2(CO)42]2−, the two [Os9C(CO)21]4− fragments are bonded to the two triangular faces of [Hg3]6+, resulting in a sandwich compound. Mercurophilic interactions are present in all these compounds.
 | ||
| Fig. 19 [Hgn]2n+ (n = 1–4) cores stabilised upon coordination to metal carbonyl fragments. Molecular structures of (a) [HgRu6(CO)22]2−, (b) [Hg2Ru7(CO)26]2−, (c) [Hg3Ru8(CO)30]2−, and (d) [Hg4Ru10(CO)32]4− (blue, Hg; orange, Ru; red, O; grey, C). Adapted from ref. 130 with permission from Elsevier. | ||
Square or rectangular [M4]n+ cores (n = 4, M = Cu, Ag, Au; n = 8, M = Cd, Hg) stabilized by MCC fragments are rather common. Representative examples are [M4Fe4(CO)16]4− (M = Ag, Au),133 [M4Co4(CO)16],63 (M = Cu, Ag), [M4Mo4(Cp)4(CO)12] (M = Ag, Au), [M4Mo4(C5H4NMe2)4(CO)12] (M = Ag, Au),134 [Cd4Fe4(CO)16], [Hg4Ru4(CO)16],135 and [Hg4Mn4(MeCp)4(CO)8] (Fig. 21).136 These 2-D clusters contain [M4]4+ (M = Cu, Ag, Au) or [M4]8+ (M = Cd, Hg) cores stabilized by [Fe(CO)4]2−, [Ru(CO)4]2−, [Co(CO)4]−, [CpMo(CO)3]−, and [(MeCp)Mn(CO)2]2− fragments. Anionic [M4Fe4(CO)16]4− (M = Ag, Au) may be further decorated by [Au2(dppm)]2+ and [Au2(dppe)]2+ fragments affording the larger 2-D clusters [Ag8Fe4(CO)16(dppm)2], [Ag4Au4Fe4(CO)16(dppe)2], [Au8Fe4(CO)16(dppe)2], and, [Au6Cu2Fe4(CO)16(dppe)2].137,138 The species [Au8Mo4(CO)20(PPh3)4] is closely related.139
Polymerization isomerism has been observed in the case of [M4Fe4(CO)16]4− (M = Ag, Au). Indeed, it has been possible to synthesize and structurally characterize their triangular isomers [M3Fe3(CO)12]3− (M = Ag, Au) (Scheme 3).133 Conversely, only [Cu3Fe3(CO)12]3− is known in the case of copper.
 | ||
| Scheme 3 Syntheses of the clusters [M3Fe3(CO)12]3− (M = Cu, Ag, Au) and [M4Fe4(CO)16]4− (M = Ag, Au). In the case of Ag and Au, the trimeric and tetrameric clusters may be viewed as polymerization isomers. Adapted with permission from ref. 133 Copyright 2019 American Chemical Society. | ||
The species [M5Fe4(CO)16]3− (M = Cu, Ag, Au) contain a centered rectangular [M5]5+ core decorated on the surface by four [Fe(CO)4]2− fragments, and formally originate upon addition of a M+ ion at the center of [M4Fe4(CO)16]4−. Interestingly, complex mixtures of 2-D molecular alloy clusters of the type [MxM′5−xFe4(CO)16]3− (M, M′ = Cu, Ag, Au; M ≠ M′) can be prepared in an almost continuum of compositions.140
In the case of copper, further addition of a Cu+ ion affords the 3-D cluster [Cu6Fe4(CO)16]2−, composed of an octahedral [Cu6]6+ core and four [Fe(CO)4]2− fragments. Alternatively, this cluster may be viewed as a sp3 four-electron [Cu6]2+ superatom bonded to four [Fe(CO)4]˙− MCC radical fragments (Fig. 22). The same model has been applied to anionic [Au6Ni12(CO)24]2− and cationic [Ag6M4(CO)24]2+ (M = Nb, Ta), which contain a [M6]2+ (M = Ag, Au) superatom tetrahedrally bonded to four [Ni3(CO)6]˙− or four [M(CO)6]˙ (M = Nb, Ta) radical metal carbonyl fragments (Fig. 23 and 24).52,53 The closely related [Ag6Nb5(CO)30]+ has been interpreted as composed of a trigonal prismatic [Ag6]+ five electrons core bonded to five [Nb(CO)6]˙ radicals, which adopt a trigonal bypiramidal arrangement. Octahedral cores have been found also in [Au6Rh16(CO)36]6− and [HPd6Fe6(CO)24]3−,141,142 but theoretical studies for their possible rationalization within the superatom model have not been yet carried out.
 | ||
| Fig. 22 (a) The molecular cluster [Cu6Fe4(CO)16]2− may be viewed as (b) a sp3 four-electron [Cu6]2+ superatom bonded to four [Fe(CO)4]˙− MCC radical fragments (blue, Fe; orange, Cu; red, O; grey, C). | ||
 | ||
| Fig. 23 Schematic structural formulae and structures of the metallic cores of the cationic cluster cores of [Ag6Nb5(CO)30]+ (3+) and [Ag6M4(CO)24]2+ (M = Nb, Ta) (42+). The counterions [Al(ORF)4]− and the CO ligands are omitted for clarity in all structures. Ellipsoids were drawn at the 50 % probability level. Sum of the formally available valence electrons (VE) of the metal cores (Ag+: 10 VE and M−: 6 VE). Adapted from ref. 52 with permission from Wiley. | ||
 | ||
| Fig. 24 The molecular cluster [Au6Ni12(CO)24]2− (a) may be viewed as a sp3 four-electron [Au6]2+ superatom bonded to four [Ni3(CO)6]˙− MCC radical fragments (green, Ni; yellow, Au; red, O; grey, C). The interaction of one of these radical fragments with the octahedral core is schematically represented (b). Adapted from ref. 52 with permission from Wiley. | ||
A further intriguing example where M–CO fragments act as ligands toward a gold cluster is [Au6Co2(PPh3)4(CO)8].143 Its [Au6]2+ core consists of two tetrahedra with a common edge, bonded to four PPh3 and two [Co(CO)4]− units all acting as terminal ligands. Alternatively, it could be interpreted as composed of a neutral [Au6] core, four PPh3 ligands and two [Co(CO)4]˙ radicals.
A few examples of larger nanoclusters containing metal cores decorated by MCC fragments have been reported, that is, [Ag9Os13(CO)48]−, [Ag9Os9(μ3-O)2(CO)30]−,144 [Ag13Fe8(CO)32]n− (n = 3, 4, 5),140 [Ag16Ni24(CO)40]4−,145 [H3−nPd9Co15C3(CO)38]n− (n = 0–3), [H6−nPd16Co20C4(CO)48]n− (n = 3–6),146–148 [Au21Fe10(CO)40]5−, [Au22Fe12(CO)48]6−, [Au28Fe14(CO)52]8−, and [Au34Fe14(CO)50]10−.149
The two Ag–Os clusters are actually composed of three [Ag3]3+ units stabilized upon coordination to four [Os3(CO)11]2− and one [Os(CO)4]2− fragments in the case of [Ag9Os13(CO)48]−, and two [Os3O(CO)9]2− and three [Os(CO)4]2− units in the case of [Ag9Os9(μ3-O)2(CO)30]−.144
The very impressive [Ag16Ni24(CO)40]4− cluster reported by Dahl et al.,145 is composed of a ccp Ag16 kernel whose surface is connected to four tetrahedrally disposed triangular Ni6(CO)10 fragments. Taking into consideration the negative charge, it may be viewed as a 16 electrons Ag16 core bonded to four [Ni6(CO)10]˙− radicals (Fig. 25).
[H3−nPd9Co15C3(CO)38]n− (n = 0–3) and [H6−nPd16Co20C4(CO)48]n− (n = 3–6) display some interesting features (Fig. 26).146–148 First of all, they are composed of compact Pd9 and Pd16 cores decorated by three or four [Co5C(CO)12] MCC fragments arranged in a trigonal planar and tetrahedral geometry, respectively (Fig. 27). Moreover, they are poly-hydrides and each single hydride species display some reversible redox processes, as assessed by electrochemical studies. SQUID measurements indicate that [HPd9Co15C3(CO)38]2− is paramagnetic with two unpaired electrons. The structure of the Pd9 core of [H3−nPd9Co15C3(CO)38]n− (n = 0–3) reversibly changes from trigonal prismatic to octahedral upon protonation/deprotonation reactions. Core isomerism has been observed in the case of [HPd9Co15C3(CO)38]2− that may adopt both trigonal prismatic and octahedral structures of the Pd9 core, depending on packing forces.
 | ||
| Fig. 26 Synthesis of [H6−nCo20Pd16C4(CO)48]n− (n = 3–6) and its transformation into [HPd9Co15C3(CO)38]2− upon reaction with HBF4 in CH2Cl2. Protonation/deprotonation reactions of [H3−nCo15Pd9C3(CO)38]n− (n = 0–3) are reported. [H3Co15Pd9C3(CO)38] and [H2Co15Pd9C3(CO)38]− display an Oh-Pd9 core, whereas [Co15Pd9C3(CO)38]3− adopt a TP-Pd9 structure (Oh = octahedron; TP = trigonal prism). Both isomers have been found in the case of [HCo15Pd9C3(CO)38]2− (orange, Pd; blue, Co; red, O; grey, C). Hydride ligands have not been located by SC-XRD. Adapted from ref. 147 with permission from The Royal Society of Chemistry. | ||
The [Au21Fe10(CO)40]5−, [Au22Fe12(CO)48]6−, [Au28Fe14(CO)52]8−, and [Au34Fe14(CO)50]10− nanoclusters may be viewed as organometallic counterparts of thiolate-protected gold nanoclusters.149 Indeed, these Au–Fe–CO molecular nanoclusters are stabilized on the surface by linear Fe–Au–Fe fragments, reminiscent of the S–Au–S staple motives present in ligand protected Au nanoclusters. Because of this, the real nuclearity of the metal cores of such Au–Fe–CO and Au–SR nanoclusters are reduced compared to the nominal ones. The linear fragments on the surface may generate larger motives, such as Au(SR)2 and Au2(SR)3 in the case of Au–SR nanoclusters, and Au{Fe(CO)4}2 and Au2{Fe(CO)4}3 for Au–Fe–CO MCCs. Both an ionic and neutral model may be used in order to interpret their bonding. Using the ionic model, [Au21Fe10(CO)40]5− may be partitioned into a [Au11]5+ core and two [Au5{μ-Fe(CO)4}5]5− rings containing linear Fe–Au–Fe motives (Fig. 28).106,149 Similarly, [Au25(SCH2CH2Ph)18]− may be viewed as composed by a [Au13]5+ core and six [Au2(SR)3]− staple motives.
Häkkinen and Femoni have further analyzed these iron-carbonyl-protected gold clusters by near-infrared (NIR) and Raman spectroscopy in conjunction with linear-response time-dependent density functional theory (LR-TDDFT).50 In particular, the analyses of [Au21Fe10(CO)40]5− and [Au22Fe12(CO)48]6− (Fig. 29) indicate that their bonding and electronic structures display some analogies to thiolate-monolayer-protected Au clusters, and the frontier orbitals responsible for their NIR absorption may be rationalized in the framework of the gold superatom model.
 | ||
| Fig. 29 NIR absorption spectrum recorded in CH3CN, molecular structure and Au22Fe12 frame of [Au22Fe12(CO)48]6− (yellow, Au; green, Fe; grey, C; red, O). Adapted with permission from ref. 50 Copyright 2009 American Chemical Society. | ||
The species [Au16S{Fe(CO)4}4(IPr)4]2+ (IPr = C3N2H2(C6H3iPr2)2) was obtained upon thermal decomposition of [Fe(CO)4(AuIPr)2] in DMSO.150 It consists of a μ12-S centered Au12-cubeoctahedron, decorated on the surface by four μ3-Fe(CO)4 and four μ3-AuIPr fragments, with pseudo-Td symmetry (Fig. 30 and 31). Icosahedral Au13 and Au12M cages have been found in several ligand-protected molecular gold nanoclusters,30,31,35,44,151,152 whereas the cubeoctahedral core is less common.153 The electron count of [Au16S{Fe(CO)4}4(IPr)4]2+ can be derived assuming that the μ3-AuIPr fragments, isolobal to μ3-H, contribute one electron each, the μ3-Fe(CO)4 groups are four electron donors, and the interstitial μ6-S atom donates six electrons.150 Overall, [Au16S{Fe(CO)4}4(IPr)4]2+ possesses 156 CVE [11 × 12 (Au) + 6 × 1 (μ6-S) + 4 × 1 (μ3-AuIPr) + 4 × 4 (μ3-Fe(CO)4) −2 (charge +2)]. According to the EAN (Effective Atomic Number) rule, a cubeoctahedron should have 168 CVE. PSEPT (Polyhedral Skeletal Electron Pair Theory) predicted 170 CVE by interpreting a cubeoctahedron as a four-connected polyhedron. Conversely, assuming that radial bonding predominates, on the basis of Mingos Rules a cubeoctahedron should have 162 CVE. In all cases, [Au16S{Fe(CO)4}4(IPr)4]2+ results electron poor, as often found for gold clusters.
 | ||
| Fig. 30 Two different views of the molecular structure of [Au16S{Fe(CO)4}4(IPr)4]2+ (IPr = C3N2H2(C6H3iPr2)2). Au–C(O) contacts [2.636(4)–2.723(4) Å] were represented as fragmented lines. Hydrogen atoms have been omitted (green Fe; yellow Au; orange S; blue N; red O; grey C). Adapted with permission from ref. 150 Copyright 2020 American Chemical Society. | ||
4. Conclusions
Two general types of molecular clusters may be classified as surface decorated MCCs. These include clusters composed of a metal carbonyl core decorated on the surface by ML fragments, as well as clusters composed of a naked metal core decorated and stabilized on the surface by metal-carbonyl fragments. All of these possess a metal core of various geometries stabilized upon coordination to its surfaces of different metal-ligands or metal-CO fragments. Most of the work appeared to date concerns the syntheses of surface decorated MCCs and their structural characterization by SC-XRD. Some of the most noticeable examples appeared in the literature have been reported in this Frontier Article, mainly outlining the contribution of our research group in Bologna to this field.The examples herein reported show the rich structural and chemical diversity of surface decorated MCCs, as well as the possibility of obtained higher nuclearity clusters. Beside the aesthetical pleasure generated by these species, they allow to access unusual structural motives and geometries. At this regard, some structural analogies can be drawn among some of these surface decorated MCCs and other categories of ligated clusters, such as ligand protected coinage metal nanoclusters. In particular, the M-ligand and M–CO fragments decorating some of these surface decorated MCCs display some resemblances to the staple motives reported for ligand protected Au nanoclusters and related species. Moreover, the superatom model, usually applied to coinage metal nanoclusters, has been recently applied also to some representative examples of surface decorated MCCs.50–53 Further structural and theoretical work will be required in order to fully explore the potentialities of these analogies, hopefully leading to a general unified approach for the interpretation of the different categories of molecular ligated metal clusters.
At this regard, it is noteworthy that, adopting the electron counting rules of low valent metal clusters (whose classically employed for MCCs), the surface decorated MCCs [Pt13(CO)12{Cd5(μ-Br)5Br2(dmf)3}2]2− and [Pt13{Au2(PPh3)2}2(CO)10(PPh3)4], the Au-phosphine cluster [Au13Cl2(PMe2Ph)10]3+, and the Au-thiolate cluster [Au25(SCH2CH2Ph)18]−, all possess 162 CVE (see section 2.1).106–109 As predicted by Mingos, this electron counting is consistent with an icosahedral M13 core where radial bonds predominate over tangential ones.110,111 Applying the superatom model to the same four icosahedral clusters, they result to be superatom with 8 free electrons.51 This apparent “abnormal” difference in the electron counting (162 vs. 8) is due to how and which electrons are counted in the two schemes. CVE rules add all the valence electrons of the cluster, without differentiating between core electrons and those used for binding the ligands. Moreover, also the d electrons of the transition metals are included, even if the question might be debated in the case of Au. Of course, the metal framework (M–M bonding) is due to a reduced number of electrons (as a function of geometry). Most electrons are used to bind ligands (σ and π interactions) or are inert low lying core electrons. Conversely, in the superatom model only the electrons responsible for M–M bonds are counted, and d electrons are excluded. After considering these two points, the two models perfectly agree for the above mentioned four icosahedral clusters. Somehow, they say the same thing, but in a different manner. It would be important to extend this theoretical comparison among different electron counting models to other molecular metal clusters, in order to better appreciate such analogies.
From a practical point of view, MCCs already found several applications in catalysis and electrocatalysis.9,13,128,154–158 Enhanced molecular electrocatalysts might be obtained based on the electron sponge behavior of larger MCCs.19,159 Paramagnetic MCCs could be exploited as single molecular magnets.160 MCCs were employed as models in surface science, particularly for Fischer–Tropsch chemistry, and gave a fundamental contribution to the development of the cluster-surface analogy.161,162 More recently, iron carbide MCCs garnered attention in bioinorganic chemistry, as spectroscopic, structural and functional models of the nitrogenase active site cluster.163 Such studies require the partial replacement of carbonyls with other ligands, and the introduction of organic and inorganic sulfur based ligands is rather challenging.164 CO-substitution may lead also to water-soluble MCCs, that might have potential biological and pharmaceutical applications.165 Isomerism and chirality are two other fields of increasing interest in molecular cluster chemistry,166,167 whose investigation in the case of MCCs is at its beginning.168,169 New molecular materials integrated with MCCs can be obtained by self-assembly phenomena exploiting the formation of homometallic or heterometallic bonds, or employing suitable bidentate ligands.11,18,101 Indeed, self-assembly can be exploited for the construction of cluster-based polymers for optoelectronics, magnetism, catalysis and nanotechnology.11,170,171 A further strategy for the preparation of MCC based materials is represented by their encapsulation within metal organic framework (MOF),172 affording nanostructures materials for potential applications in catalysis and electrocatalysis.173 Finally, MCCs may be used as molecular precursors in controlled thermal processes for the synthesis of supported metal catalysts, metal nanocrystals embedded in porous matrices, magnetic nanoalloys, and conductive sub-micrometric metal wires.18,174–178
Data availability
No primary research results, software, or code have been included and no new data were generated or analyzed as part of this Frontier Article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financed by the European Union – NextGenerationEU through the Italian Ministry of University and Research under PNRR – Mission 4 Component 1, Investment 4.1 (DM 118/2023) – CUP J33C23002200002. We thank the referees for useful suggestions in revising the manuscript.References
- F. A. Cotton, Transition-metal compounds containing clusters of metal atoms, Q. Rev., Chem. Soc., 1966, 20, 389–401 RSC.
- A. V. Virovets, E. Peresypkina and M. Scheer, Structural Chemistry of Giant Metal Based Supramolecules, Chem. Rev., 2021, 121, 14885–14554 CrossRef PubMed.
- J. E. McGrady, F. Weigend and S. Dehnen, Electronic structure and bonding in endohedral Zintl clusters, Chem. Soc. Rev., 2002, 51, 628–649 RSC.
- J. Heine, B. Peerless, S. Dehnen and C. Lichtenberg, Charge Makes a Difference: Molecular Ionic Bismuth Compounds, Angew. Chem., Int. Ed., 2023, 62, e202218771 CrossRef CAS.
- M. Schütz, C. Gemel, W. Klein, R. A. Fischer and T. F. Fässler, Intermetallic phases meet intermetalloid clusters, Chem. Soc. Rev., 2021, 50, 8496–8510 RSC.
- J. Qian, Z. Yang, J. Lyu, Q. Yao and J. Xie, Molecular Interactions in Atomically Precise Metal Nanoclusters, Precis. Chem., 2024, 2, 495–517 CrossRef CAS PubMed.
- K. Wang, T. Iwano and S. Uchida, Keplerate polyoxometalate compounds: a multifunctional nano-platform for advanced materials, Dalton Trans., 2024, 53, 16797–16806 RSC.
- E. L. Albright, T. I. Levchenko, V. K. Kulkarni, A. I. Sullivan, J. F. DeJesus, S. Malola, S. Takano, M. Nambo, K. Stamplecoskie, H. Häkkinen, T. Tsukuda and C. M. Crudden, N-Heterocyclic Carbene-Stabilized Atomically Precise Metal Nanoclusters, J. Am. Chem. Soc., 2024, 146, 5759–5780 CrossRef CAS PubMed.
- C. Cesari, J.-H. Shon, S. Zacchini and L. A. Berben, Metal carbonyl clusters of groups of 8–10: synthesis and catalysis, Chem. Soc. Rev., 2021, 50, 9503–9539 RSC.
- P. R. Raithby, The growth of higher nuclearity carbonyl clusters of ruthenium and osmium, J. Organomet. Chem., 2024, 1005, 122979 CrossRef.
- M. Shieh, Y.-H. Liu, Y.-H. Li and R. Y. Lin, Metal carbonyl cluster-based coordination polymers: diverse syntheses, versatile network structures, and special properties, CrystEngComm, 2019, 21, 7341–7364 RSC.
- E. G. Mednikov and L. F. Dahl, Syntheses, structures and properties of primarily nanosized homo/heterometallic palladium CO/PR3-ligated clusters, Philos. Trans. R. Soc., A, 2010, 368, 1301–1332 CrossRef PubMed.
- C. Cesari, C. Femoni, F. Forti, M. C. Iapalucci, G. Scorzoni and S. Zacchini, Molecular hydride carbonyl clusters and nanoclusters, Inorg. Chim. Acta, 2025, 574, 122394 CrossRef.
- C. Cesari, C. Femoni, M. C. Iapalucci and S. Zacchini, Molecular Fe, Co and Ni carbide carbonyl clusters and nanoclusters, Inorg. Chim. Acta, 2023, 544, 121235 CrossRef.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, S. Ruggieri and S. Zacchini, The role of gold in transition metal carbonyl clusters, Coord. Chem. Rev., 2018, 355, 27–38 CrossRef.
- B. Berti, C. Femoni, M. C. Iapalucci, S. Ruggieri and S. Zacchini, Functionalization, Modification, and Transformation of Platinum Chini Clusters, Eur. J. Inorg. Chem., 2018, 3285–3296 CrossRef.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Platinum Carbonyl Clusters Chemistry: Four Decades of Challenging Nanoscience, J. Cluster Sci., 2014, 25, 115–146 CrossRef.
- S. Zacchini, Using Metal Carbonyl Clusters To Develop a Molecular Approach towards Metal Nanoparticles, Eur. J. Inorg. Chem., 2011, 4125–4145 CrossRef.
- C. Femoni, M. C. Iapalucci, F. Kaswalder, G. Longoni and S. Zacchini, The possible role of metal carbonyl clusters in nanoscience and nanotechnologies, Coord. Chem. Rev., 2006, 250, 1580–1604 CrossRef.
- B. F. G. Johnson and S. McIndoe, Spectroscopic and mass spectrometric methods for the characterisation of metal clusters, Coord. Chem. Rev., 2000, 200–202, 901–932 CrossRef.
- K. H. Whitmire, Transition metal complexes of the naked pnictide elements, Coord. Chem. Rev., 2018, 376, 114–195 CrossRef.
- J. A. Cabeza and P. G. Álvarez, The N-heterocyclic carbene chemistry of transition-metal carbonyl clusters, Chem. Soc. Rev., 2011, 40, 5389–5405 RSC.
- G. Hogarth, S. E. Kabir and E. Nordlander, Cluster chemistry in the Noughties: new developments and their relationship to nanoparticles, Dalton Trans., 2010, 39, 6153–6174 RSC.
- M. Sellin and I. Krossing, Homoleptic Transition Metal Carbonyl Cations: Synthetic Approaches, Characterization and Follow-Up Chemistry, Acc. Chem. Res., 2023, 56, 2776–2787 CrossRef CAS PubMed.
- D. M. P. Mingos, Structural and bonding patterns in gold clusters, Dalton Trans., 2015, 44, 6680–6695 RSC.
- M. L. Ganadu, F. Demartin, A. Panzanelli, E. Zangrando, M. Peana, S. Medici and M. A. Zoroddu, Gold Clusters: From the Dispute on a Gold Chair to the Golden Future of Nanostructures, Molecules, 2021, 26, 5014 CrossRef CAS PubMed.
- L. Malatesta, Cluster compounds of gold, Gold Bull., 1975, 8, 48–52 CrossRef CAS.
- L. Naldini, F. Cariati, G. Simonetta and L. Malatesta, Gold-tertiary phosphine derivatives with intermetallic bonds, Chem. Commun., 1966, 647–648 RSC.
- L. Malatesta, L. Naldini, G. Simonetta and F. Cariati, Triphenylphosphine-gold(0)-gold(I) compounds, Coord. Chem. Rev., 1966, 1, 255–262 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS.
- S. Li, N.-N. Li, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chemical Flexibility of Atomically Precise Metal Clusters, Chem. Rev., 2024, 124, 7262–7378 CrossRef CAS PubMed.
- X. Zou, X. Kang and M. Zhu, Recent developments in the investigation of driving forces for transforming coinage metal nanoclusters, Chem. Soc. Rev., 2023, 52, 5892–5967 RSC.
- M. F. Matus and H. Häkkinen, Understanding ligand-protected noble metal nanoclusters at work, Nat. Rev. Mater., 2023, 8, 372–389 CrossRef CAS.
- W. W. Xu, X. C. Zeng and Y. Gao, The structural isomerism in gold nanoclusters, Nanoscale, 2018, 10, 9476–9483 RSC.
- C. Sun, B. K. Teo, C. Deng, J. Lin, G.-G. Luo, C.-H. Tung and D. Sun, Hydrido-coinage-metal-clusters: Rational design, synthetic protocols and structural characteristics, Coord. Chem. Rev., 2021, 427, 213576 CrossRef.
- T.-H. Chiu, J.-H. Liao, R. P. B. Silalahi, M. N. Pillay and C. W. Liu, Hydride-doped coinage metal superatom and their catalytic applications, Nanoscale Horiz., 2024, 9, 675–692 RSC.
- X. Kang, Y. Li, M. Zhu and R. Jin, Atomically precise alloy nanoclusters: syntheses, structures, and properties, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- T. Kawawaki, T. Okada, D. Hirayama and Y. Negishi, Atomically precise metal nanoclusters as catalysts for electrocatalytic CO2 reduction, Green Chem., 2024, 26, 122–163 RSC.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures, Chem. Rev., 2021, 121, 567–648 CrossRef PubMed.
- N. D. Loewen, T. V. Neelakantan and L. A. Berben, Renewable Formate from C-H Bond Formation with CO2: Using Iron Carbonyl Clusters as Electrocatalysts, Acc. Chem. Res., 2017, 50, 2362–2370 CrossRef PubMed.
- S. Mai, J. Sun, Z. Fang, G.-B. Xiao and J. Cao, Metal Clusters Based Multifunctional Materials for Solar Cells, Chem. – Eur. J., 2024, 30, e202303973 CrossRef PubMed.
- F. Zaera, Designing Sites in Heterogeneous Catalysis: Are We Reaching Selectivities Competitive With Those of Homogeneous Catalysis?, Chem. Rev., 2022, 122, 8594–8757 CrossRef PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution, Science, 2007, 318, 430–433 CrossRef PubMed.
- H. Häkkinen, The gold-sulfur interface at the nanoscale, Nat. Chem., 2012, 4, 443–455 CrossRef PubMed.
- S. M. Owen, Electron counting in clusters: a view of the concepts, Polyhedron, 1988, 7, 253–283 CrossRef.
- W. W. Xu, X. C. Zeng and Y. Gao, Application of Electronic Counting Rules for Ligand-Protected Gold Nanoclusters, Acc. Chem. Res., 2018, 51, 2739–2747 CrossRef CAS.
- H. Häkkinen, Atomic and electronic structure of gold clusters: understanding flakes, cages and superatoms from simple concepts, Chem. Soc. Rev., 2008, 37, 1847–1859 RSC.
- C. M. Aikens, R. Jin, X. Roy and T. Tsukuda, From atom-precise nanoclusters to superatom materials, J. Chem. Phys., 2022, 156, 170401 CrossRef CAS PubMed.
- O. Lopez-Acevedo, J. Rintala, S. Virtanen, C. Femoni, C. Tiozzo, H. Grönbeck, M. Pettersson and H. Häkkinen, Characterization of Iron-Carbonyl Protected Gold Clusters, J. Am. Chem. Soc., 2009, 131, 12573–12575 CrossRef CAS.
- J. Wei, R. Marchal, D. Astruc, S. Kahlal, J.-F. Halet and J.-Y. Saillard, Looking at platinum carbonyl nanoclusters as superatoms, Nanoscale, 2022, 14, 3946–3957 RSC.
- W. Unkrig, K. Kloiber, B. Butschke, D. Kratzert and I. Krossing, Altering Charges on Heterobimetallic Transition-Metal Carbonyl Clusters, Chem. – Eur. J., 2020, 26, 12373–12381 CrossRef CAS PubMed.
- A. Muñoz-Castro, sp3-hybridization in superatomic clusters. Analogues to simple molecules involving the Au6 core, Chem. Sci., 2014, 5, 4749–4754 RSC.
- J. W. Lauher and K. Wald, Synthesis and Structure of [FeCo3(CO)12AuPPh3]: A Trimetallic Trigonal-Bipyramidal Cluster. Gold Derivatives as Structural Analogues of Hydrides, J. Am. Chem. Soc., 1981, 103, 7648–7650 CrossRef CAS.
- P. Braunstein, J. Rosé, Y. Dusausoy and J.-P. Mangeot, Complexes à liaisons métal-métal. XXI. Synthèse et structure du “cluster” (Ph3P)AuRuCo3(CO)12: une bipyramide trigonale à trois métaux différents, C. R. Chim., 1982, 294, 967–970 CAS.
- P. Braunstein and J. Rosé, Gold in Bimetallic Molecular Clusters. Their Synthesis, Bonding and Catalytic Reactivities, Gold Bull., 1985, 18, 17–30 CrossRef CAS.
- P. Braunstein, H. Lehner, D. Matt, A. Tiripicchio and M. Tiripicchio-Camellini, Synthesis of the first Pt-Au cluster by an unexpected H+-substitution at trans-PtH(Cl)L2, Angew. Chem., Int. Ed. Engl., 1984, 23, 304–304 CrossRef.
- S. Sculfort and P. Braunstein, Intramolecular d10-d10 interactions in heterometallic clusters of the transition metals, Chem. Soc. Rev., 2011, 40, 2741–2760 RSC.
- H. Schmidbaur and A. Schier, Aurophilic interactions as a subject of current research: an up-date, Chem. Soc. Rev., 2012, 41, 370–412 RSC.
- P. Pyykkö, Strong closed-shell interactions in inorganic chemistry, Chem. Rev., 1997, 97, 597–636 CrossRef.
- A. Das, U. Das and A. K. Das, Relativistic effects on the chemical bonding properties of the heavier elements and their compounds, Coord. Chem. Rev., 2023, 479, 215000 CrossRef.
- N. Mirzadeh, H. Privér, A. J. Blake and H. Schmidbaur, Innovative Molecular Design Strategies in Materials Science Following the Aurophilicity Concept, Chem. Rev., 2020, 120, 7551–7591 CrossRef.
- C. Cesari, B. Berti, F. Calcagno, C. Femoni, M. Garavelli, M. C. Iapalucci, I. Rivalta and S. Zacchini, Polymerization isomerism in Co-M (M = Cu, Ag, Au) carbonyl clusters: synthesis, structures and computational investigation, Molecules, 2021, 26, 1529 CrossRef.
- I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci, A. Ienco, G. Longoni, G. Manca and S. Zacchini, Octahedral Co-Carbide Carbonyl Clusters Decorated by [AuPPh3]+ Fragments: Synthesis, Structural Isomerism, and Aurophilic Interactions of Co6C(CO)12(AuPPh3)4, Inorg. Chem., 2014, 53, 9761–9770 CrossRef.
- C. Cesari, M. Bortoluzzi, C. Femoni, F. Forti, M. C. Iapalucci and S. Zacchini, Peraurated ruthenium hydride carbonyl clusters: aurophilicity, isolobal analogy, structural isomerism, and fluxionality, Dalton Trans., 2024, 53, 3865–3879 RSC.
- M. Bortoluzzi, I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci, G. Longoni and S. Zacchini, Hydride Migration from a Triangular Face to a Tetrahedral Cavity in Tetranuclear Iron Carbonyl Clusters upon Coordination of [AuPPh3]+ Fragments, Angew. Chem., Int. Ed., 2014, 53, 7233–7237 CrossRef PubMed.
- M. Bortoluzzi, I. Ciabatti, C. Cesari, C. Femoni, M. C. Iapalucci and S. Zacchini, Synthesis of the highly reduced [Fe6C(CO)15]4− carbonyl carbide cluster and its reactions with H+ and [Au(PPh3)]+, Eur. J. Inorg. Chem., 2017, 3135–3143 CrossRef.
- I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci and S. Zacchini, Co5C and Co4C carbido carbonyl clusters stabilized by [AuPPh3]+ fragments, Inorg. Chim. Acta, 2015, 428, 203–211 CrossRef.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, A. Ienco, G. Longoni, G. Manca and S. Zacchini, Intramolecular d10-d10 Interactions in a Ni6C(CO)9(AuPPh3)4 Bimetallic Nickel-Gold carbide Carbonyl Cluster, Inorg. Chem., 2013, 52, 10559–10565 CrossRef.
- M. Bortoluzzi, I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci, G. Longoni and S. Zacchini, Peraurated nickel carbide carbonyl clusters: the cationic [Ni6(C)(CO)8(AuPPh3)8]2+ monocarbide and the [Ni12(CO)(C2)(CO)17(AuPPh3)3]− anion containing one carbide and one acetylide unit, Dalton Trans., 2014, 43, 13471–13475 RSC.
- A. Ceriotti, P. Macchi, A. Sironi, S. El Afefey, M. Daghetta, S. Fedi, F. Fabrizi de Biani and R. Della Pergola, Cooperative Effects of Electron Donors and Acceptors for the Stabilization of Elusive Metal Cluster Frameworks: Synthesis and Solid-State Structures of [Pt19(CO)24(μ4-AuPPh3)3]− and [Pt19(CO)24{μ4-Au2(PPh3)2}2], Inorg. Chem., 2013, 52, 1960–1964 CrossRef PubMed.
- V. Dearing, S. R. Drake, B. F. G. Johnson, J. Lewis, M. McPartlin and H. R. Powell, The synthesis of the first tetra- and penta-gold decaosmium clusters, X-ray structure analysis of [Os10Au4C(CO)24{P(C6H11)3}3], J. Chem. Soc., Chem. Commun., 1988, 1331–1333 RSC.
- M. Bortoluzzi, C. Cesari, I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci, R. Mazzoni and S. Zacchini, Bimetallic Fe-Au Carbonyl Clusters Derived from Collman's Reagent: Synthesis, Structure and DFT Analysis of Fe(CO)4(AuNHC))2 and [Au3Fe2(CO)8(NHC)2]−, J. Cluster Sci., 2017, 28, 703–723 CrossRef CAS.
- R. Della Pergola, A. Sironi, A. Rosehr, V. Colombo and A. Sironi, N-heterocyclic carbene copper complexes tethered to iron carbidocarbonyl clusters, Inorg. Chem. Commun., 2014, 49, 27–29 CrossRef CAS.
- R. D. Adams, J. Tedder and Y. O. Wong, Phenyl-gold complexes of Ru6 and Ru5 carbonyl clusters, J. Organomet. Chem., 2015, 795, 2–10 CrossRef CAS.
- C.-N. Lin, C.-Y. Huang, C.-C. Yu, Y.-M. Chen, W.-M.- Ke, G.-J. Wang, G.-A. Lee and M. Shieh, Iron carbonyl cluster-incorporated Cu(I) NHC complexes in homocoupling of arylboronic acids: an effective [TeFe3(CO)9]2− ligand, Dalton Trans., 2015, 44, 16675–16679 RSC.
- M. K. Karunananda, S. R. Parmelee, G. W. Waldhart and N. P. Mankad, Experimental and Computational Characterization of the Transition State for C-X Bimetallic Oxidative Addition at a Cu-Fe Reaction Center, Organometallics, 2015, 34, 3857–3864 CrossRef CAS.
- R. D. Adams and G. Elpitiya, The addition of Gold and Tin to Bismuth-Triiridium Carbonyl Complexes, Inorg. Chem., 2015, 54, 8042–8048 CrossRef CAS PubMed.
- R. D. Adams, P. Dhull, V. Rassolov and Y. O. Wong, Synthesis and Reactivity of Electronically Unsaturated Dirhenium Carbonyl Compounds Containing Bridging Gold-Carbene Groups, Inorg. Chem., 2016, 55, 10475–10483 CrossRef CAS.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni, F. Vacca and S. Zacchini, Synthesis and Characterization of Heterobimetallic Carbonyl Clusters with Direct Au-Fe and Au⋯Au Interactions Supported by N-Heterocyclic Carbene and Phosphine Ligands, Eur. J. Inorg. Chem., 2019, 3084–3093 CrossRef CAS.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni and S. Zacchini, A Comparative Experimental and Computational Study of Heterometallic Fe-M (M = Cu, Ag, Au) Carbonyl Clusters Containing N-Hetericyclic Carbene Ligands, Eur. J. Inorg. Chem., 2020, 2191–2202 CrossRef CAS.
- C. Cesari, B. Berti, F. Calcagno, C. Lucarelli, M. Garavelli, R. Mazzoni, I. Rivalta and S. Zacchini, Bimetallic Co-M (M = Cu, Ag, and Au) Carbonyl Complexes Supported by N-Heterocyclic Carbene Ligands: Synthesis, Structures, Computational Investigation, and Catalysis for Ammonia Borane Dehydrogenation, Organometallics, 2021, 40, 2724–2735 CrossRef CAS.
- M. Bortoluzzi, C. Cesari, I. Ciabatti, C. Femoni, M. C. Iapalucci and S. Zacchini, Reactions of Platinum Carbonyl Chini Clusters with Ag(NHC)Cl Complexes: Formation of Acid-Base Lewis Adducts and Heteroleptic Clusters, Inorg. Chem., 2017, 56, 6532–6544 CrossRef CAS.
- C. Cesari, I. Ciabatti, C. Femoni, M. C. Iapalucci and S. Zacchini, Capping [H8−nNi42C8(CO)44]n− (n = 6, 7, 8) Octa-carbide Carbonyl Nanoclusters with [Ni(CO)] and [CuCl] Fragments, J. Cluster Sci., 2017, 28, 1963–1979 CrossRef.
- A. Bernardi, I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Molecular nickel poly-carbide carbonyl nanoclusters: The octa-carbide [HNi42C8(CO)44(CuCl)]7− and the deca-carbide [Ni45C10(CO)46]6−, J. Organomet. Chem., 2016, 812, 229–239 CrossRef.
- A. Bernardi, I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Ni-Cu tetracarbide carbonyls with vacant Ni(CO) fragments as borderline compounds between molecular and quasi-molecular clusters, Dalton Trans., 2013, 42, 407–421 RSC.
- A. Bernardi, C. Femoni, M. C. Iapalucci, G. Longoni, F. Ranuzzi, S. Zacchini, P. Zanello and S. Fedi, Synthesis, Molecular Structure and Properties of the [H6−nNi30C4(CO)34(CdCl)2]n− (n = 3–6) Bimetallic Carbide Carbonyl Cluster: A Model for the Growth of Noncompact Interstitial Metal Carbides, Chem. – Eur. J., 2008, 14, 1924–1934 CrossRef.
- A. Bernardi, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Cadmium-substitution promoted by nucleophilic attack of [Ni30C4(CO)34(CdX)2]6− (X = Cl, Br, I) carbide carbonyl clusters: Synthesis and characterization of the new [H7−nNi32C4(CO)36(CdX)]n− (X = Cl, Br, I; n = 5, 6, 7), Inorg. Chim. Acta, 2009, 362, 1239–1246 CrossRef.
- A. Bernardi, C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini, S. Fedi and P. Zanello, Synthesis, Structures and Electrochemistry of New Carbonylnickel Octacarbide Clusters: The Distorting Action of Carbide Atoms in the Growth of Ni Cages and the First Example of the Inclusion of a Carbon Atom within a (Distorted) Ni Octahedral Cage, Eur. J. Inorg. Chem., 2010, 4831–4842 CrossRef.
- C. Capacci, C. Cesari, C. Femoni, M. C. Iapalucci, F. Mancini, S. Ruggieri and S. Zacchini, Structural Diversity in Molecular Nickel Phosphide Carbonyl Nanoclusters, Inorg. Chem., 2020, 59, 16016–16026 CrossRef PubMed.
- C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini, S. Fedi and F. Fabrizi de Biani, Nickel poly-acetylide carbonyl clusters: structural features, bonding and electrochemical behaviour, Dalton Trans., 2012, 41, 4649–4663 RSC.
- R. D. Adams, B. Captain, W. Fu, P. J. Pellechia and M. D. Smith, Remarkable Dynamical Opening and Closing of Platinum and Palladium Pentaruthenium Carbido Carbonyl Cluster Complexes, Inorg. Chem., 2003, 42, 2094–2101 CrossRef PubMed.
- R. D. Adams, B. Captain, W. Fu and M. D. Smith, Lewis Acid-Base Interactions between Metal Atoms and Their Applications for the Synthesis of Bimetallic Cluster Complexes, J. Am. Chem. Soc., 2002, 124, 5628–5629 CrossRef.
- S. Saha and B. Captain, Synthesis and Structural Characterization of Ruthenium Carbonyl Cluster Complexes Containing Platinum with a Bulky N-Heterocyclic Carbene Ligand, Inorg. Chem., 2014, 21, 1210–1216 CrossRef PubMed.
- R. D. Adams and B. Captain, Unusual Structures and Reactivity of Mixed Metal Cluster Complexes Containing the Palladium/Platinum Tri-t-butylphosphine Grouping, Acc. Chem. Res., 2009, 42, 409–418 CrossRef PubMed.
- A. Koppaka, V. Zollo Jr., S. Etezadi, D. C. Lever and B. Captain, Addition of Pt(IPr) Groupings to Ru5 Carbide Gives New Mixed-Metal Pt-Ru Cluster Complexes, J. Cluster Sci., 2016, 27, 1671–1681 CrossRef.
- M. Kawano, J. W. Bacon, C. F. Campana, B. E. Winger, J. D. Dudek, S. A. Sirchio, S. L. Scruggs, U. Geiser and L. F. Dahl, High-Nuclearity Close-Packed Palladium-Nickel Carbonyl Phosphine Clusters: Heteropalladium [Pd16Ni4(CO)22(PPh3)4]2− and [Pd33Ni9(CO)41(PPh3)6]4− Containing Pseudo-Td ccp Pd16Ni4 and Pseudo-D3h hcp Pd33Ni9 Cages, Inorg. Chem., 2001, 40, 2554–2569 CrossRef.
- J. D. Erickson, E. G. Mednikov, S. A. Ivanov and L. F. Dahl, Isolation and Structural Characterization of a Makay 55-Metal-Atom Two-Shell Icosahedron of Pseudo-Ih Symmetry, Pd55L12(μ3-CO)20 (L = PR3, R = isopropyl): Comparative Analysis with Interior Two-Shell Icosahedral Geometries in Capped Three-Shell Pd145, Pt-Centered Four-Shell Pd-Pt M165, and Four-Shell Au133 Nanoclusters, J. Am. Chem. Soc., 2016, 138, 1502–1505 CrossRef PubMed.
- M. Bortoluzzi, I. Ciabatti, C. Femoni, M. Hayatifar, M. C. Iapalucci and S. Zacchini, [H3−nFe4(CO)12(IrCOD)]n− (n = 1, 2) and [H2Fe3(CO)10(IrCOD)]− Bimetallic Fe-Ir Hydride Carbonyl Clusters, Organometallics, 2015, 34, 189–197 CrossRef.
- F. Forti, C. Cesari, M. Bortoluzzi, C. Femoni, T. Funaioli, M. C. Iapalucci and S. Zacchini, Heterometallic Ru-Ir hydride carbonyl clusters, J. Cluster Sci., 2025, 36, 6 CrossRef.
- C. Femoni, F. Kaswalder, M. C. Iapalucci, G. Longoni and S. Zacchini, Copolymerization of Pt-carbonyl clusters with Lewis acids: synthesis and crystal structure of the molecular {Cd2Cl4[Pt9(CO)18]2−}( 1-D polymer, Chem. Commun., 2006, 2135–2137 RSC.
- A. Bernardi, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, The problems of detecting hydrides in metal carbonyl clusters by 1H NMR: the case study of [H4−nNi22(C2)4(CO)28(CdBr)2]n− (n = 2–4), Dalton Trans., 2009, 4245–4251 RSC.
- C.-Y. Miu, H.-H. Chi, S.-W. Chen, J.-J. Cherng, M.-H. Hsu, Y.-X. Huang and M. Shieh, Reactions of the μ3-sulfido triiron cluster [SFe3(CO)9]2− with functionalized organic halides and mercury salts: selective reactivity, electrochemistry, and theoretical calculations, New J. Chem., 2011, 35, 2442–2455 RSC.
- U. Brand and J. R. Shapley, Carbidohexarhenate Cluster Cores Bicapped by Mercury with Acetate or Thiolate Ligands, Inorg. Chem., 2000, 39, 32–36 CrossRef PubMed.
- C. A. Wright and J. R. Shapley, Carbidoheptarhenate Cluster Complexes of Cadmium and Zinc Units: The Structure of [PPh4]2[Re7C(CO)21(μ3-ZnCl)], Inorg. Chem., 2001, 40, 6338–6340 CrossRef PubMed.
- C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini and S. Zarra, Icosahedral Pt-Centered Pt13 and Pt19 Carbonyl Clusters Decorated by [Cd5(μ-Br)5Br5−x(solvent)x]x+ Rings Reminiscent of the Decoration of Au-Fe-CO and Au-Thiolate Nanoclusters: A Unifying Approach to Their Electron Counts, J. Am. Chem. Soc., 2011, 133, 2406–2409 CrossRef.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini and S. Zarra, Surface decorated platinum carbonyl clusters, Nanoscale, 2012, 4, 4166–4177 RSC.
- J.-F. Halet, D. G. Evans and D. M. P. Mingos, Effect of Cavity Size on the Charge Distribution in Carbido-Metal Carbonyl Clusters and Its Possible Catalytic Implications, J. Am. Chem. Soc., 1988, 110, 87–90 CrossRef.
- C. E. Briant, B. R. C. Theobald, J. W. White, L. K. Bell, D. M. P. Mingos and A. J. Welch, Synthesis and X-ray structural characterization of the centred icosahedral gold cluster compound [Au13(PMe2Ph)10Cl2](PF6)3; the realization of a theoretical prediction, J. Chem. Soc., Chem. Commun., 1981, 201–202 RSC.
- D. M. P. Mingos, Theoretical analyses and electron counting rules for high nuclearity clusters, J. Chem. Soc., Chem. Commun., 1985, 1352–1354 RSC.
- M. P. Johansson and P. Pyykkö, WAu12(CO)12?, Chem. Commun., 2010, 46, 3762–3764 RSC.
- N. de Silva and L. F. Dahl, Synthesis and Structural Analysis of the First nanosized Platinum-Gold Carbonyl/Phosphine Cluster, Pt13[Au2(PPh3)2]2(CO)10(PPh3)4, Containing a Pt-Centered [Ph3Pau-AuPPh3]-Capped Icosahedral Pt12 Cage, Inorg. Chem., 2005, 44, 9604–9606 CrossRef PubMed.
- F. Demartin, M. C. Iapalucci and G. Longoni, Synthesis and Characterization of Novel Types of Adducts of Nickel Carbonyl Clusters with Indium Halides: X-ray Structures of [NEt4]3[Ni6(μ3-InBr3)(η2-μ6-In2Br5)(CO)11]·Me2CO, [NEt4]4[Ni6(η2-μ6-In2Br5)2(CO)10]·Me2CO, and [NEt4]4[Ni12(μ6-In)(η2-μ6-In2Br4OH)(CO)22], Inorg. Chem., 1993, 32, 5536–5543 CrossRef.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci and S. Zacchini, Reactions of [Pt6(CO)6(SnX2)2(SnX3)4]4− (X = Cl, Br) with Acids: Syntheses and molecular structures of [Pt12(CO)10(SnCl)2(SnCl2)4{Cl2Sn(μ-OH)SnCl2}2]2− And [Pt7(CO)6(SnBr2)4{Br2Sn(μ-OH)SnBr2}{Br2Sn(μ-Br)SnBr2}]2− Platinum carbonyl clusters decorated by Sn(II)-Fragments, Inorg. Chim. Acta, 2020, 503, 119432 CrossRef.
- M. Bortoluzzi, A. Ceriotti, I. Ciabatti, R. Della Pergola, C. Femoni, M. C. Iapalucci, A. Storione and S. Zacchini, Platinum carbonyl clusters stabilized by Sn(II)-based fragments: syntheses and structures of [Pt6(CO)6(SnCl2)2(SnCl3)4]4−, [Pt9(CO)8(SnCl2)3(SnCl3)2(Cl2SnOCOSnCl2)]4− and [Pt10(CO)14{Cl2Sn(OH)SnCl2}2]2−, Dalton Trans., 2016, 45, 5001–5013 RSC.
- M. Bortoluzzi, A. Ceriotti, C. Cesari, I. Ciabatti, R. Della Pergola, C. Femoni, M. C. Iapalucci, A. Storione and S. Zacchini, Syntheses of [Pt6(CO)8(SnCl2)(SnCl3)4]4− and [Pt6(CO)8(SnCl2)(SnCl3)2(PPh3)2]2− Platinum-Carbonyl Clusters Decorated by SnII Fragments, Eur. J. Inorg. Chem., 2016, 3929–3949 Search PubMed.
- E. Brivio, A. Ceriotti, L. Garlaschelli, M. Manassero and M. Sansoni, Coordination of SnCl2 and GeCl2 onto a one dimensional platinum network: synthesis and structural characterization of the [PPh4]2[Pt8(ECl2)4(CO)10] (E = Sn, Ge) complexes, J. Chem. Soc., Chem. Commun., 1995, 2055–2056 RSC.
- S. Saha, D. Isrow and B. Captain, Build-up of a Ru6 octahedral cluster core stabilized by tert-butyl germyl ligands, J. Organomet. Chem., 2014, 751, 815–820 CrossRef.
- A. B. Hungria, R. Raja, R. D. Adams, B. Captain, J. M. Thomas, P. A. Midgley, V. Golovko and B. F. G. Johnson, Single-Step Conversion of Dimethyl Terephthalate into Cyclohexanedimethanol with Ru5PtSn, a Trimetallic Nanoparticle Catalyst, Angew. Chem., Int. Ed., 2006, 45, 4782–4785 CrossRef.
- R. D. Adams, B. Captain and W. Fu, Facile introduction of bridging MPh2 groups (M = Ge, Sn, Pb) into platinum-pentaruthenium and hexaruthenium carbide carbonyl cluster complexes, J. Organomet. Chem., 2003, 671, 158–165 CrossRef.
- E. V. Grachova, P. Jutzi, B. Neumann, L. O. Schebaum, H.-G. Stammler and S. P. Tunik, Unusual selective substitution of triply bridging carbonyl ligands for GaCp* in Rh6(CO)16. Synthesis and structural characterization of the Rh6(μ3-CO)4−x(μ3-GaCp*)x(CO)12 clusters, x = 1–4, J. Chem. Soc., Dalton Trans., 2002, 302–304 RSC.
- E. V. Grachova and G. Linti, Reactivity of InCp* Towards Transition Metal Carbonyl Clusters: Synthesis and Structural Characterization of the Rh6(CO)16−x(InCp*)x Mixed-Metal Cluster Compounds, x = 1–2, Eur. J. Inorg. Chem., 2007, 3561–3564 CrossRef.
- P. Jutzi, B. Neumann, G. Reunmann and H.-G. Stammler, Organometallics, 1998, 17, 1305–1314 CrossRef.
- M. Bortoluzzi, I. Ciabatti, C. Femoni, T. Funaioli, M. Hayatifar, M. C. Iapalucci, G. Longoni and S. Zacchini, Homoleptic and heteroleptic Au(I) complexes containing the new [Co5C(CO)12]− cluster as ligand, Dalton Trans., 2014, 43, 9633–9646 RSC.
- I. Ciabatti, C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini, S. Fedi and F. Fabrizi de Biani, Syntehsis, Structure, and Electrochemsitry of the Ni-Au Carbonyl Cluster [Ni12Au(CO)24]3− and Its Relation to [Ni32Au6(CO)44]6−, Inorg. Chem., 2012, 51, 11753–11761 CrossRef PubMed.
- G. Manca, F. Fabrizi de Biani, M. Corsini, C. Cesari, C. Femoni, M. C. Iapalucci, S. Zacchini and A. Ienco, Inverted Ligand Field in a Pentanuclear Bow Tie Au/Fe Carbonyl Cluster, Inorg. Chem., 2022, 61, 3484–3492 CrossRef PubMed.
- S. Pan, S. M. N. V. T. Gorantla, D. Parasar, H. V. R. Dias and G. Frenking, Chemical Bonding in Homoleptic Carbonyl Cations [M{Fe(CO)5}2]+ (M = Cu, Ag, Au), Chem. – Eur. J., 2021, 27, 6936–6944 CrossRef PubMed.
- C. Cesari, M. Bortoluzzi, F. Forti, L. Gubbels, C. Femoni, M. C. Iapalucci and S. Zacchini, 2-D Molecular Alloy Ru-M (M = Cu, Ag, and Au) Carbonyl Clusters: Synthesis, Molecular Structure, Catalysis, and Computational Studies, Inorg. Chem., 2022, 61, 14726–14741 CrossRef PubMed.
- H. Egold, M. Schraa, U. Flörke and J. Partyka, Synthesis of Novel Mixed Metal Cluster Complexes of Osmium and Mercury: Formation of the Wheel-Shaped Cluster Complexes Os6(μ6-Hg)(μ-PR2)2(CO)20 (R = Ph, i-Bu), Organometallics, 2002, 21, 1925–1932 CrossRef.
- C. Cesari, M. Bortoluzzi, C. Femoni, M. C. Iapalucci and S. Zacchini, Mercurophilic interactions in heterometallic Ru-Hg carbonyl clusters, Inorg. Chim. Acta, 2023, 545, 121281 CrossRef.
- M. Fajardo, H. D. Holden, B. F. G. Johnson, J. Lewis and P. R. Raithby, Syntehsis and structural characterisation of the heteronuclear raft complex [Os3(CO)11Hg]3, J. Chem. Soc., Chem. Commun., 1984, 24–25 RSC.
- L. H. Gade, B. F. G. Johnson, J. Lewis, M. McPartlin, T. Kotch and A. J. Lee, Photochemical Core Manipulation in High-Nuclearity Os-Hg Clusters, J. Am. Chem. Soc., 1991, 113, 8698–8704 CrossRef.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni, F. Vacca and S. Zacchini, Polymerization Isomerism in [{MFe(CO)4}n]n− (M = Cu, Ag, Au; N = 3, 4) Molecular Clusters Supported by Metallophilic Interactions, Inorg. Chem., 2019, 58, 2911–2915 CrossRef.
- P. Croizat, S. Sculfort, R. Welter and P. Braunstein, Hexa- and Octanuclear Heterometallic Clusters with Copper-, Silver-, or Gold-Molybdenum Bonds and d10-d10 Interactions, Organometallics, 2016, 35, 3949–3958 CrossRef.
- N. Masciocchi, P. Cairati, F. Ragaini and A. Sironi, Ab initio XPRD structure determination of metal carbonyl clusters: the case of [HgRu(CO)4]4, Organometallics, 1993, 12, 4499–4502 CrossRef.
- W. Gäde and E. Weiss, [(η5-CH3C5H4)Mn(CO)2Hg]4, a Compound with an Mn4Hg4 Eight-Membered Ring and Additional Hg-Hg Bonds, Angew. Chem., Int. Ed. Engl., 1981, 20, 803–804 CrossRef.
- V. G. Albano, C. Castellari, C. Femoni, M. C. Iapalucci, G. Longoni, M. Monari and S. Zacchini, Synthesis, Chemical Characterization, and Molecular Structure of Au8{Fe(CO)4}4(dppe)2 and Au6Cu2{Fe(CO)4}4(dppe)2, J. Cluster Sci., 2001, 12, 75–87 CrossRef.
- V. G. Albano, M. C. Iapalucci, G. Longoni, M. Monari, A. Paselli and S. Zacchini, Synthesis, Chemical Characterization, and Molecular Structures of Ag8Fe4(CO)16(dppm)2 and Ag4Au4Fe4(CO)16(dppe)2, Organometallcis, 1998, 17, 4438–4443 CrossRef.
- R. Della Pergola, L. Garlaschelli, M. C. Malatesta, C. Manassero and M. Manassero, A Traditional Synthetic Method, and a New Structural Motif, for Molybdenum-Gold Clusters: Synthesis and Solid-State Structure of Au8{Mo(CO)5}4(PPh3)4, Inorg. Chem., 2006, 45, 8465–8467 CrossRef PubMed.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, L. Soleri and S. Zacchini, Synthesis, Structural Characterization, and DFT Investigations of [MxM’5−xFe4(CO)16]3− (M, M′ = Cu, Ag, Au; M ≠ M′) 2-D Molecular Alloy Clusters, Inorg. Chem., 2020, 59, 15936–15952 CrossRef.
- C. Femoni, M. C. Iapalucci, S. Ruggieri and S. Zacchini, From Mononuclear Complexes to Molecular Nanoparticles: The Buildup of Atomically Precise Heterometallic Rhodium Carbonyl Nanoclusters, Acc. Chem. Res., 2018, 51, 2748–2755 CrossRef PubMed.
- G. Longoni, M. Manassero and M. Sansoni, Synthesis and characterization of new iron-palladium and iron-platinum carbonyl anionic clusters, J. Am. Chem. Soc., 1980, 102, 3242–3244 CrossRef.
- J. W. A. van der Velden, J. J. Bour, W. P. Bosman and J. H. Noordik, Reactions of Cationic Gold Clusters with Lewis Bases. Preparation and X-ray Structure Investigation of [Au8(PPh3)7](NO3)2·2CH2Cl2 and Au6(PPh3)4[Co(CO)4]2, Inorg. Chem., 1983, 22, 1913–1918 CrossRef.
- Y.-B. Lee and W.-T. Wong, Synthesis and characterization of high-nuclearity osmium-silver mixed-metal clusters, Chem. Commun., 2007, 3924–3926 RSC.
- J. Zhang and L. F. Dahl, First-known high-nuclearity silver-nickel carbonyl cluster: nanosized [Ag16Ni24(CO)40]4− possessing a new 40-atom cubic Td closed-packed metal-core geometry, J. Chem. Soc., Dalton Trans., 2002, 1269–1274 RSC.
- B. Berti, I. Ciabatti, C. Femoni, M. C. Iapalucci and S. Zacchini, Cluster Core Isomerism Induced by Crystal Packing Effects in the [HCo15Pd9C3(CO)38]2− Molecular Nanocluster, ACS Omega, 2018, 3, 13239–13250 CrossRef PubMed.
- I. Ciabatti, C. Femoni, M. Gaboardi, M. C. Iapalucci, G. Longoni, D. Pontiroli, M. Riccò and S. Zacchini, Structural rearrangements induced by acid-base reactions in metal carbonyl clusters: the case of [H3−nCo15Pd9C3(CO)38]n– (n = 0-3), Dalton Trans., 2014, 43, 4388–4399 RSC.
- I. Ciabatti, F. Fabrizi de Biani, C. Femoni, M. C. Iapalucci, G. Longoni and S. Zacchini, Metal Segregation in Bimetallic Co-Pd Carbide Carbonyl Clusters: Synthesis, Structure, Reactivity and Electrochemistry of [H6−nCo20Pd16C4(CO)48]n– (n = 3-6), ChemPlusChem, 2013, 78, 1456–1465 CrossRef PubMed.
- C. Femoni, M. C. Iapalucci, G. Longoni, C. Tiozzo and S. Zacchini, An Organometallic Approach to Gold Nanoparticles: Synthesis and X-Ray Structure of CO-Protected Au21Fe10, Au22Fe12, Au28Fe14, and Au34Fe14 Clusters, Angew. Chem., Int. Ed., 2008, 47, 6666–6669 CrossRef PubMed.
- B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni, F. Vacca and S. Zacchini, Thermal Growth of Au-Fe Heterometallic Carbonyl Clusters Containing N-Heterocyclic Carbene and Phosphine Ligands, Inorg. Chem., 2020, 59, 2228–2240 CrossRef PubMed.
- K. Konishi, M. Iwasaki and Y. Shichibu, Phosphine-Ligated Gold Clusters with Core + exo Geometries: Unique Properties and Interactions at the Ligand-Cluster Interface, Acc. Chem. Res., 2018, 51, 3125–3133 CrossRef PubMed.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C.-T. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, N-heterocyclic carbine-functionalized magic-number gold nanoclusters, Nat. Commun., 2019, 11, 419–425 Search PubMed.
- S. Zhou, W. Pei, Q. Du and J. Zhao, Foreign atom encapsulated Au12 golden cages for catalysis of CO oxidation, Phys. Chem. Chem. Phys., 2019, 21, 10587–10593 RSC.
- M. R. Radzhabov and N. P. Mankad, Activation of robust bonds by carbonyl complexes of Mn, Fe and Co, Chem. Commun., 2023, 59, 11932–11946 RSC.
- T. Kawawaki, Y. Mitomi, N. Nishi, R. Kurosaki, K. Oiwa, T. Tanaka, H. Hirase, S. Miyajima, Y. Niihori, D. J. Osborn, T. Koitaya, G. F. Metha, T. Yokoyama, K. Iida and Y. Negishi, Pt17 nanocluster electrocatalysts: preparation and origin of high oxygen reduction reaction activity, Nanoscale, 2023, 15, 7272–7279 RSC.
- C. Schmitt, N. Da Roit, M. Neumaier, C. B. Maliakkal, D. Wang, T. Henrich, C. Kübel, M. Kappes and S. Behrens, Continuous flow synthesis of atom-precise platinum clusters, Nanoscale Adv., 2024, 6, 2459–2468 RSC.
- Y. Negishi, Metal-nanocluster science and technology: my personal history and outlook, Phys. Chem. Chem. Phys., 2022, 24, 7569–7594 RSC.
- F. Forti, C. Cesari, M. Bortoluzzi, C. Femoni, M. C. Iapalucci and S. Zacchini, Heterometallic Ru-Ir clusters as catalyst precursors for hydrogenation and hydrogen transfer reactions, New J. Chem., 2023, 47, 19289–19303 RSC.
- G. Bussoli, A. Boccalini, M. Bortoluzzi, C. Cesari, M. C. Iapalucci, T. Funaioli, G. Scorzoni, S. Zacchini, S. Ruggieri and C. Femoni, Atomically precise rhodium-indium carbonyl nanoclusters: synthesis, characterization, crystal structure and electron-sponge features, Nanoscale, 2024, 16, 17852–17867 RSC.
- C. Femoni, M. C. Iapalucci, G. Longoni, J. Wolowska, S. Zacchini, P. Zanello, S. Fedi, M. Riccò, D. Pontiroli and M. Mazzani, Magnetic Behavior of Odd- and Even-Electron Metal Carbonyl Clusters: The Case Study of [Co8Pt4C2(CO)24]n− (n = 1, 2) Carbide Cluster, J. Am. Chem. Soc., 2010, 132, 2919–2927 CrossRef PubMed.
- E. I. Muetterties, T. N. Rhodin, E. Band, C. F. Brucker and W. R. Pretzer, Clusters and Surfaces, Chem. Rev., 1979, 79, 91–137 CrossRef.
- E. I. Muetterties and J. Stein, Mechanistic Features of Catalytic Carbon Monoxide Hydrogenation Reactions, Chem. Rev., 1979, 79, 479–490 CrossRef.
- C. R. Cobb, R. K. Ngo, E. J. Dick, V. M. Lynch and M. J. Rose, Multi-phosphine-chelated iron-carbide clusters via redox-promoted ligand exchange on an inert hexa-iron-carbide carbonyl cluster, [Fe6(μ6C)(μ2-CO)4(CO)12]2−, Chem. Sci., 2024, 15, 11455–11471 RSC.
- C. Joseph, C. R. Cobb and M. J. Rose, Single-Step Sulfur Insertion into Iron Carbide Carbonyl Clusters: Unlocking the Synthetic Door to FeMoco Analogues, Angew. Chem., Int. Ed., 2021, 60, 3433–3437 CrossRef.
- L. K. Batchelor, B. Berti, C. Cesari, I. Ciabatti, P. J. Dyson, C. Femoni, M. C. Iapalucci, M. Mor, S. Ruggieri and S. Zacchini, Water soluble derivatives of platinum carbonyl Chini clusters: synthesis, molecular structures and cytotoxicity of [Pt12(CO)20(PTA)4]2− and [Pt15(CO)25(PTA)5]2−, Dalton Trans., 2018, 47, 4467–4477 RSC.
- X. Wang and M. Zhu, Structural Isomerism in Atomically Precise Nanoclusters, Chem. Mater., 2021, 33, 39–62 CrossRef.
- J.-H. Huang, X.-Y. Dong, Y.-J. Wang and S.-Q. Zang, Generation and manipulation of chiroptical activities in coinage-metal clusters, Coord. Chem. Rev., 2022, 470, 214729 CrossRef.
- C. Cesari, C. Femoni, F. Forti, M. C. Iapalucci, G. Scorzoni and S. Zacchini, Isomerism in Molecular Metal Carbonyl Clusters, Eur. J. Inorg. Chem., 2024, 27, e202400220 CrossRef.
- C. Cesari, I. Ciabatti, C. Femoni, M. C. Iapalucci, F. Mancini and S. Zacchini, Heteroleptic Chini-Type Platinum Clusters: Synthesis and Characterization of Bis-Phosphine Derivatives of [Pt3n(CO)6n]2− (n = 2–4), Inorg. Chem., 2017, 56, 1655–1668 CrossRef.
- M.-C. Hsu, R. Y. Lin, T.-Y. Sun, Y.-X. Huang, M.-S. Li, Y.-H. Li, H.-L. Chen and M. Shieh, Inorganic-organic hybrid Cu-dipyridyl semiconducting polymers based on the redox-active cluster [SFe3(CO)9]2−: filling the gap in iron carbonyl chalcogenide polymers, Dalton Trans., 2024, 53m, 7303–7314 RSC.
- X. Zhao, W.-J. Chen, Q.-M. Liang, S.-K. Chen, J. Xun, B.-J. Geng, H.-F. Su and Y. Yang, Ag+-Induced Assembly of Pt Clusters for Photocatalytic Hydrogen Production, Inorg. Chem., 2024, 63, 17672–17680 CrossRef PubMed.
- K. L. Kollmannsberger, Poonam, C. Cesari, R. Khare, T. Kratky, M. Boniface, O. Tomanec, J. Michalička, E. Mosconi, A. Gagliardi, S. Günther, W. Kaiser, T. Lunkenbein, S. Zacchini, J. Warnan and R. A. Fischer, Mechanistic Insights into ZIF-8 Encapsulation of Atom-Precise Pt(M) Carbonyl Clusters, Chem. Mater., 2023, 35, 5475–5486 CrossRef.
- P. M. Schneider, K. L. Kollmannsberger, C. Cesari, R. Khare, M. Boniface, B. Roldán Cuenya, T. Lunkenbein, M. Elsner, S. Zacchini, A. S. Bandarenka, J. Warnan and R. A. Fischer, Engineering ORR Electrocatalysts from Co8Pt4 Carbonyl Clusters via ZIF-8 Templating, ChemElectroChem, 2024, 11, e202300476 CrossRef.
- D. Bonincontro, A. Lolli, A. Storione, A. Gasparotto, B. Berti, S. Zacchini, N. Dimitratos and S. Albonetti, Pt and Pt/Sn carbonyl clusters as precursors for the synthesis of supported metal catalysts for the base-free oxidation of HMF, Appl. Catal., A, 2019, 588, 117279 CrossRef.
- F. Bertolotti, D. Moscheni, A. Migliori, S. Zacchini, A. Cervellino, A. Guagliardi and N. Masciocchi, A total scattering Debye function analysis study of faulted Pt nanocrystals embedded in a porous matrix, Acta Crystallogr., 2016, A72, 632–644 CrossRef PubMed.
- I. Robinson, S. Zacchini, L. D. Tung, S. Maenosono and N. T. K. Thanh, Synthesis and Characterization of Magnetic Nanoalloys from Bimetallic Carbonyl Clusters, Chem. Mater., 2009, 21, 3021–3026 CrossRef.
- D. A. Serban, P. Greco, S. Melinte, A. Vlad, C. A. Dutu, S. Zacchini, M. C. Iapalucci, F. Biscarini and M. Cavallini, Towards All-Organic Field-Effect Transistors by Additive Soft Lithography, Small, 2009, 10, 1117–1122 CrossRef.
- P. Greco, M. Cavallini, P. Stoliar, S. D. Quiroga, S. Dutta, S. Zacchini, M. C. Iapalucci, V. Morandi, S. Milita, P. G. Merli and F. Biscarini, Conductive Sub-micrometric Wires of Platinum-Carbonyl Clusters Fabricated by Soft-Lithography, J. Am. Chem. Soc., 2008, 130, 1177–1182 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |