Open Access Article
Open Access ArticleCoupling deep eutectic solvents with innovative extraction techniques towards plant derived bioactive compositions
Anastasia
Kyriakoudi
a,
Ivana
Radojčić Redovniković
b,
Senka
Vidović
c,
Kristina
Radošević
b,
Thanos
Andreou
 d,
Ioannis
Mourtzinos
d,
Ioannis
Mourtzinos
 *a and
Marina
Cvjetko Bubalo
*a and
Marina
Cvjetko Bubalo
 *b
*b
aLaboratory of Food Chemistry and Biochemistry, Department of Food Science and Technology, School of Agriculture, Faculty of Agriculture, Forestry and Natural Environment, Aristotle University of Thessaloniki (AUTH), 54 124, Thessaloniki, Greece. E-mail: mourtzinos@agro.auth.gr
bLaboratory for Cell Technology, Application and Biotransformations, Department of Biochemical Engineering, Faculty of Food Technology and Biotechnology, University of Zagreb, 10 000, Zagreb, Croatia. E-mail: mcvjetko@pbf.hr
cDepartment of Biotechnology and Pharmaceutical Engineering, Faculty of Technology, University Novi Sad, 21000, Novi Sad, Serbia
dVIO Chemicals, 57001, Thessaloniki, Greece
First published on 26th April 2024
Abstract
The steadily increasing interest of food, cosmetics and pharmaceutical industries for bioactive compounds of natural origin has resulted in a growing demand for the development of novel and effective approaches for their isolation from various plant matrices. In this view, deep eutectic solvents (DESs) constitute an alternative to conventional organic ones, based on their green nature, biodegradability and their adjustable physicochemical properties depending on the application. The combination of DESs with innovative extraction techniques, such as ultrasound- and microwave-assisted extraction, super- and sub-critical fluid extraction, as well as other non-thermal technologies, is a promising approach for the recovery of bioactive compounds from plants. This coupling offers several advantages including improved selectivity as well as increased extraction yields with a concomitant reduced extraction duration. The present review gives an overview of the evolution, the current status and future trends regarding the use of DESs as extraction solvents in combination with innovative extraction techniques towards the recovery of valuable compounds from natural sources for novel laboratory and industrial applications. The challenges regarding the use of DESs as well as the sustainability of the DES-innovative extraction processes are also presented and critically discussed.
Sustainability spotlightOur work introduces a sustainable methodology using Deep Eutectic Solvents (DESs) for extracting bioactive compounds, directly supporting the United Nations Sustainable Development Goals, particularly SDG 12 (Responsible Consumption and Production) and SDG 13 (Climate Action). The use of DESs, characterized by their green chemistry credentials, biodegradability, and low toxicity, marks a sustainable advance in extraction technologies. By reducing the environmental footprint and enhancing the efficiency of natural compound extraction, our approach promotes sustainable industrial practices and contributes to the mitigation of climate change. This initiative not only emphasizes the critical role of sustainable solvents in advancing green technologies but also demonstrates a commitment to environmental sustainability and responsible resource management in line with global sustainability efforts. |
1. Introduction
Plants offer an abundant reservoir of bioactive compounds, encompassing a diverse array of chemical classes like alkaloids, terpenoids, flavonoids, phenolics, and lignans. These compounds exhibit a wide range of biological activities, notably including antioxidant, antimicrobial, anticancer, and anti-inflammatory properties. In recent times, there has been a remarkable surge in the attention devoted to the extraction of bioactive compounds from plants, particularly within the realms of the food, pharmaceutical, and cosmetic industries. The extraction process itself is challenging, as it necessitates the separation and purification of these bioactive compounds from the intricate matrix of the plant.1Bioactive compounds are traditionally extracted from natural sources using established methods such as maceration, steam or hydro-distillation, pressing, decoction, infusion, percolation, and Soxhlet extraction, followed by chemical treatments to isolate the desired compounds. However, these conventional processes are time-consuming and require large amounts of solvents, leading to low yield and degradation of target molecules. In recent years, there has been a growing interest in developing safer and more efficient extraction techniques that balance economic, social, and environmental factors. Accordingly, traditional extraction methods are being replaced by alternative green approaches based on the principles of green chemistry, which aim to reduce or eliminate the use of harmful solvents, reagents, and products that pollute the environment.2 These approaches involve the use of alternative solvents, environmentally friendly raw materials and alternative energy sources. Accordingly, a variety of green extraction techniques have been developed, ranging from maceration to contemporary techniques such as pressurized extraction, microwave-assisted extraction, ultrasound-assisted extraction, and nonthermal techniques.3
A significant focus regarding green extraction techniques has been made on the development of novel environmentally friendly and customizable solvents. This objective aligns with the priorities set by the European Union's environmental policy and legislation for the period from 2010 to 2050, which emphasize the reduction of hazardous solvents in the industrial processes.4 According to the principles of green chemistry, the selection of an appropriate solvent is based on several key considerations, such as the safety of workers, process safety factors, the environmental impact and the sustainability of the process. In fact, a green solvent should exhibit chemical and physical stability, low volatility, user-friendliness, low environmental impact and ease of recycling and reuse.5 A group of alternative green solvents that fit these criteria are the Deep Eutectic Solvents (DESs). These solvents are characterized by their sustainability as well as their tunable physicochemical properties, such as pH value, polarity, hydrophilicity/hydrophobicity and viscosity, based on the desired application. Consequently, DESs emerge as highly desirable green candidates for a wide range of purposes including the extraction of diverse compounds from plant matrixes.6 The academic community has shown significant interest in these innovative solvents for the preparation of green natural extracts, as evidenced by the increasing number of publications during the last decade.
In this context, the present review gives an overview of the evolution, the current status and future trends regarding the use of DESs as extraction solvents in combination with innovative extraction techniques towards the recovery of valuable compounds from natural sources for novel laboratory and industrial food, cosmetics and pharmaceutical applications. The challenges regarding the use of DESs as well as the sustainability of DES-innovative extraction processes are also critically discussed.
2. Evolution of DESs
Eutectic mixtures, defined as homogeneous mixtures of substances that melt at a single temperature which is lower than the melting point of any of its individual constituents, have been known for more than a century.7 However, in 2004, Abbott et al.8 reported mixtures of quaternary ammonium salts (e.g., choline chloride, m.p. 302 °C) and urea (m.p. 133 °C) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio with a substantial (“deep”) decrease in the solid–liquid phase transition temperature than the one expected for an ideal mixture that these compositions remained liquid even at room temperature. These extraordinary findings persuaded Abbott's group to claim a novel class of such mixtures: Deep Eutectic Solvents (DESs). As initially suggested, such significant depression of the melting point is attributed to the charge delocalization due to the formation of strong intermolecular interactions (e.g., hydrogen, ionic and external van der Waals bonds) in a certain range of component ratios. Since then, hundreds of structurally different DESs have been reported, and in that time the initial definition of these solvents became more flexible as most of them have not been properly characterized by solid–liquid phase diagrams, namely, in the past 10 years researchers have been more focused on obtaining mixtures that are liquids at the room temperature (for use in a certain process or product) than trying to obey the strict definition given by Abbott's group. Coutinho's group9 stated that “due to the absence of a strict and clear definition of what a ‘deep eutectic solvent’ is, this term is often abused”, and most recently, they proposed a new definition: “A eutectic solvent is a eutectic-type system that is a liquid at a given desired temperature where at least one of its components would, otherwise, be a solid unfit to be applied as a solvent. A DES is a eutectic solvent whose components present enthalpic-driven negative deviations from thermodynamic ideality” (Abranches and Coutinho, 2023).10 This practically means that any mixture with a certain composition that is in the liquid state at that temperature could be used and defined as a DES.
2 molar ratio with a substantial (“deep”) decrease in the solid–liquid phase transition temperature than the one expected for an ideal mixture that these compositions remained liquid even at room temperature. These extraordinary findings persuaded Abbott's group to claim a novel class of such mixtures: Deep Eutectic Solvents (DESs). As initially suggested, such significant depression of the melting point is attributed to the charge delocalization due to the formation of strong intermolecular interactions (e.g., hydrogen, ionic and external van der Waals bonds) in a certain range of component ratios. Since then, hundreds of structurally different DESs have been reported, and in that time the initial definition of these solvents became more flexible as most of them have not been properly characterized by solid–liquid phase diagrams, namely, in the past 10 years researchers have been more focused on obtaining mixtures that are liquids at the room temperature (for use in a certain process or product) than trying to obey the strict definition given by Abbott's group. Coutinho's group9 stated that “due to the absence of a strict and clear definition of what a ‘deep eutectic solvent’ is, this term is often abused”, and most recently, they proposed a new definition: “A eutectic solvent is a eutectic-type system that is a liquid at a given desired temperature where at least one of its components would, otherwise, be a solid unfit to be applied as a solvent. A DES is a eutectic solvent whose components present enthalpic-driven negative deviations from thermodynamic ideality” (Abranches and Coutinho, 2023).10 This practically means that any mixture with a certain composition that is in the liquid state at that temperature could be used and defined as a DES.
DESs are traditionally divided into four types: type I (quaternary salt Cat+X− and metal halide such as Zn, Sn, Fe), type II (quaternary salt Cat+X− and metal halide such as Cr, Co, Fe), type III (quaternary salt Cat+X− and hydrogen bond donor such as amide, acid and alcohol) and type IV (metal halide and hydrogen bond donor such as amides and alcohols). Recently, a fifth class of DESs has been integrated in the general classification including all the DESs composed of only non-ionic, molecular HBAs (e.g., ternary amines) and HBDs (e.g., alcohols, acids, amines, cyclic and linear terpenes).11 Most of the DESs reported are of the type III and V with quaternary ammonium salts choline chloride or methylamine betaine as cheap, easily available and non-toxic hydrogen bond acceptors, and sugars (e.g., glucose, xylose and sucrose), polyols (e.g., xylitol, sorbitol, ethylene glycol and glycerol), amides (e.g., urea) and organic acids (e.g., lactic acid and malic acid) as uncharged hydrogen bond donors. Furthermore, in addition to the described hydrogen bond donors and acceptors, water is frequently used as a DES component, since it has been shown to play a key role in the formation of DESs by modifying the physicochemical properties of the corresponding DES supramolecular network, and consequently reduces the melting point, as well as the viscosity and solvent density, which is extremely important for its application.12 Moreover, some studies have shown that water acts as a second small HBD in aqueous mixtures of choline chloride-based DESs.13 The point of transition from the water-in-DES to the DES-in-water regime, at which the mixture behaves as a simple aqueous solution of the individual components, is considered to be about 40–50 wt% of water, while at water contents above those values the network is disrupted, leading to a mixture that exhibits behavior closer to that of the individual components in an aqueous solution.14
In the first decade, the DESs prepared were of types I–IV with application in organic synthesis, catalysis and electrochemistry (source: Web of Science). Those solvents were miscible with aqueous media as well as hydrophilic organic solvents, which restricted their applications in water-containing or lipophilic samples.15 Thus, to create hydrophobic phases that could be used in analytical chemistry as hydrophobic extraction media, in 2015, Van Osch et al.16 presented a new subclass of DESs with hydrophobic character named hydrophobic DESs (HDESs). The first HDES was prepared by mixing various long chain quaternary ammonium salts with poorly water-soluble decanoic acid. At the same time, Ribeiro et al.17 reported another hydrophobic DES consisting of DL-menthol and natural acids (pyruvic acid, acetic acid, L-lactic acid, and lauric acid). The hydrophobicity of HDESs lies in the presence of long alkyl chains or cycloalkyl groups, which decrease the importance of hydrophilic domains (charges of the salts) and hydrophilic groups (i.e., carboxylate and hydroxyl groups).18 Accordingly, since then the most reported HDESs are essentially composed of typical long alkyl chain quaternary salts (e.g., tetrabutylammonium, tricaprylylmethylammonium, tetraoctylammonium, tetraheptylammonium, and trihexyltetradecylphosphonium salt), and various hydrophobic HBDs such as carboxylic acids or alcohols with long alkyl chain acids.19 In general, these hydrophobic solvents have been shown useful in applications related to liquid–liquid (micro)extractions, gas–liquid extractions, removal of components from plant material, formation of hydrogels, membrane formation, centrifugal partition chromatography, coating, photoluminescence, dye-sensitized solar cells, and catalysis.20
Apart from the traditional DES classification, these solvents are also categorized into specific (sub)groups according to the origin of their constituents or their purpose. A particular subgroup of DESs, natural DESs (NADESs), was proposed by Choi et al.21 NADESs consist only of compounds that occur in nature (cellular metabolites) such as polyols, sugars, organic acids, amino acids and fatty acids, and thus it is assumed that they are of low toxicity, which is for most prepared NADESs proven so far.22 Choi's group,21 based on the observation that plant secretions, such as sap and nectar, accumulate primary metabolites which are known DES components (choline, betaine, proline, organic acids such as malic, succinic and citric) in molar ratios typical of DES formation, have also suggested the presence of DESs in vivo, namely, the authors went on to propose that plant cells contain a third type of medium, which plays a vital role in solubilizing, storing, and transporting poorly water-soluble metabolites, adjusting the water content of plants, and protecting cells when under harsh conditions. Most recently, inspired by osmolyte distribution across all kingdoms of life, Cvjetko Bubalo et al.23 reported new two-, three-and multicomponent osmolyte-based NADESs with natural methylamines (trimethylamine N-oxide, sarcosine and glycerophosphocholine) as HBAs. In general, the natural origin of NADESs enables a nature-like environment for various biomolecules, such as secondary plant metabolites, proteins, and enzymes, and thus retain their full biological identity and performance. Precisely because of that, these solvents have been successfully applied in areas such as extraction of plant metabolites, biocatalysis, product formulation technology, and biomedical applications.24
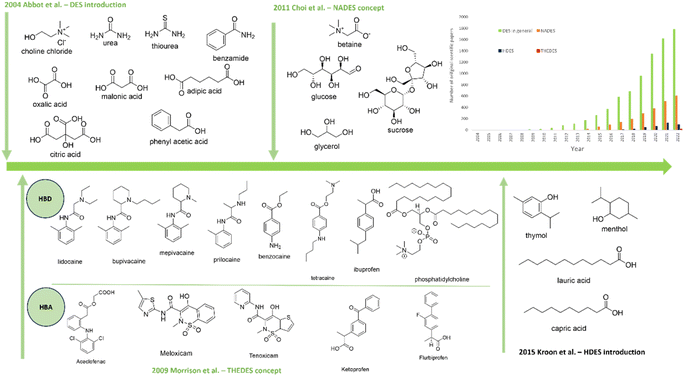
Another DES subgroup, therapeutic deep eutectic systems (THEDESs), is described as eutectic systems in which one of the components is an active pharmaceutical ingredient (API), or a system where an API is dispersed.25 In the latter case, all building units of such a solvent with mutual hydrogens or with ionic bonds, achieve a low-temperature structure of the eutectic solvent, and thus API's solubility, bioavailability and controlled release of the active substance can be improved.26 Morrison et al.27 were first to show that DESs can be extremely efficient in solubilizing poorly soluble compounds (5 to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fold more than that in water) and thus enhance drug bioavailability. Since then, DESs have been widely studied as alternative solvents for APIs' (e.g., non-steroidal anti-inflammatory drugs, antibiotics, and anesthetics) solubilization, particularly for topical formulations.26 The evolution of DESs and distribution of original scientific papers dealing with each DES subgroup are shown in Fig. 1.
000 fold more than that in water) and thus enhance drug bioavailability. Since then, DESs have been widely studied as alternative solvents for APIs' (e.g., non-steroidal anti-inflammatory drugs, antibiotics, and anesthetics) solubilization, particularly for topical formulations.26 The evolution of DESs and distribution of original scientific papers dealing with each DES subgroup are shown in Fig. 1.
3. Trends in the design, preparation and the physicochemical characterization of DESs
The most significant feature of DESs is the ability to design an optimal one for a particular purpose, however huge possibilities of DES structure that govern their properties make the selection of DESs difficult and time-consuming. This search for so-called ideal DESs is usually governed by experimental screening of numerous DES candidates (trial and error approach) previously narrowed down based on experience and data available in the literature or with the aid of computational tools. This is the case of the COnductor-like Screening Model for Real Solvents (COSMO-RS), a thermodynamic model based on quantum chemistry. COSMO-RS is a useful tool for a priori design of a great number of DESs, as the model predicts the solubility of different molecules in them. So far, COSMO-RS has been applied for the extraction of polyphenols from grape pomace,28 carnosic acid and carnosol from rosemary,29 hydroxytyrosol from olive leaves,30 limonene from orange peel waste31 and terpenoids from citrus essential oil.32One of the main advantages of DESs is their easy preparation. Various methodologies have been reported in the literature so far for this purpose. DESs can be usually prepared simply by mixing and heating the individual starting components, until a transparent homogeneous liquid is formed. Typically, the mixing is carried out at elevated temperatures (up to 80 °C) (e.g., (ref. 31,33–35)). If a DES system is too viscous, its viscosity can be easily adjusted by adding water19 (up to about 40–50%, w/w). One should keep in mind that viscosity is one of the most important factors regarding the use of DESs both for laboratory and industrial applications, since it not only affects its extraction efficiency due to low mass transfer but also increases the energy requirements for stirring.
Even though the heating and mixing method is an easy procedure, it has certain limitations, especially in the case of thermolabile starting materials (e.g., organic-acid based DESs). In order to avoid high temperatures, other approaches have been proposed. For example, a grinding method to prepare choline chloride:organic acid DESs has been proposed by Florindo et al.36 The grinding method, which has been widely explored till now for the preparation of DESs for pharmaceutical applications, includes the mixing of two components and their grinding in a mortar with a pestle at room temperature until a homogeneous liquid is formed. Additionally, another method for the synthesis of DESs that has been reported in the literature is freeze-drying.37 In particular, Zurob et al.30 prepared various choline chloride-based NADESs, by mixing the HBD and HBA with the aid of a rotary evaporator under a reducing atmosphere (250 bar) at 50 °C for ca. 30–60 min, until obtaining clear liquids. Moreover, the evaporation method is also useful in the case of highly viscous DESs (e.g., sugar-based ones) that are difficult to stir and usually water is added to the mixture to overcome this drawback. In this case, water can then be evaporated with the aid of a rotary evaporator to obtain the DES.38 This approach has the advantage of being carried out at relatively low temperatures, however the complete removal of water can be time-consuming. Regarding the freeze-drying method, it can result in high purity DESs and is suitable for the preparation of DESs composed of thermally unstable compounds. However, it is not suitable for large-scale production due to energy and time cost.39
Recently, focus has been paid on the use of advanced techniques of limited sample preparation requirements, which are used for the verification of the formation of hydrogen bonds among the individual starting materials. In this frame, Fourier Transform-Infrared (FT-IR) spectroscopy is a useful technique which, in the field of DESs, is used to examine the molecular interactions in order to confirm the formation of hydrogen bonds between an HBD and an HBA. Recently, an increasing number of publications employ FT-IR spectroscopy in the frame of the characterization of the prepared DES. For example, Kyriakoudi et al.34 employed FT-IR spectroscopy in order to examine the successful formation of different hydrophobic NADESs based on terpenes and fatty acids that were used to extract lycopene from tomatoes. Lanjekar and Rathod35 prepared and characterized different choline chloride-based NADESs for the extraction of glycyrrhizic acid from Glycyrrhiza glabra. The authors suggested that the observation of shifts of the stretching vibration of the OH functional groups indicates the successful formation of the eutectic mixture due to intermolecular interactions. In the same frame, Türker and Dogan40 used FT-IR spectroscopy for the molecular characterization of choline chloride-based DESs which were used for the extraction of anthocyanin from black carrots.
Moreover, Raman spectroscopy is a non-destructive, non-invasive analytical tool that provides information about the molecular composition of samples with minimum or no sample preparation. The advantage of Raman over IR spectra is often due to the complexity of overlapping bands in IR spectra that are difficult to deconvolve compared to the peaks of Raman spectra that can be easily observed and assigned. To the best of our knowledge, till now Raman spectroscopy has been rarely employed in DES systems and relevant publications are extremely limited. In particular, Macchioni et al.41 employed Raman spectroscopy in order to investigate the supramolecular interactions in the case of lactic acid-based NADESs hydrated with 20% of water, which were used for the extraction of bioactive metabolites of Humulus lupulus L. The authors recorded the Raman spectra of pure lactic acid, sucrose, urea and glycine as well as their eutectic mixtures and they suggested that a high structural organization appeared in the examined DES, in which water could act as a critical component in the supramolecular assembly. In another study, three typical DESs, namely choline chloride:glycerol, choline chloride:acetic acid and choline chloride:urea, were characterized with the aid of Raman spectroscopy.42 The authors observed redshifts that were assigned to the formation of hydrogen bonds between the quaternary ammonium salt, i.e., choline chloride, and the HBD.
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful technique that gives insight regarding which functional groups are present but also how they rearrange and interact within a structure. To the best of our knowledge, only a few published articles in the field of DESs have exploited the NMR spectroscopy. In particular, Rodrigues et al.43 employed NMR spectroscopy in order to confirm the formation of different terpene-based NADESs that were used as extraction solvents for the recovery of astaxanthin from brown crab shell residues. In particular, the authors recorded the 1H-NMR spectra of each NADES at different temperatures since an increase in temperature is considered to result in an upfield shift of the H-bonded proton chemical shifts due to a decrease of the intermolecular hydrogen bonding. Moreover, the authors recorded the two-dimensional nuclear Overhauser effect spectroscopy (NOESY) spectra in order to study correlations among protons, considering that if any intermolecular correlation is detected, a spatial proximity among the atoms can be verified, indicating the successful formation of a NADES. In another study,441H NMR and 13C NMR spectra were recorded in order to characterize DESs that were prepared using tetramethylguanidine and menthol at different molar ratios as alternative solvents for lipid extraction from the microalgae Nannochloropsis sp. The acquisition of 1H–1H NOESY spectra of nonionic surfactant-based hydrophobic DESs for the liquid–liquid microextraction of Sudan dyes in tomato chili sauces has also been reported.45 The authors observed interactions among hydrogens of the starting materials which was considered an indication of formation of hydrogen bonds. Wu et al.46 also employed NMR spectroscopy in order to characterize various menthol-based hydrophobic DESs that were used for the extraction of coumarins from different plant materials. In particular, the authors recorded the 1H NMR and 13C NMR spectra of the prepared DES in order to evaluate their purity and to verify their successful formation. It is worth mentioning that FT-IR, Raman and NMR techniques can be used complementarily to verify the existence of hydrogen bond-donor supramolecular complexes as they offer information about chemical shifts related to the formation of the bonds.
4. Innovative extraction techniques coupled to DESs
Different extraction techniques using DESs as the extraction solvents have been reported in the literature. Most of the common emerging and innovative processes that can be coupled to DESs are ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) as well as supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE). A literature review dedicated to such studies is presented below.4.1. Ultrasound-assisted extraction coupled to DESs
Ultrasound-assisted extraction is an innovative extraction technique of reduced energy and solvent consumption that minimizes extraction duration with a concomitant increase of the extraction yield, compared to conventional approaches.47 The enhanced mass transfer is attributed to cavitation phenomena according to which bubbles of air are formed between pulses, they grow, and when their size becomes critical, they collapse and cause cell wall rupture. Ultrasound-assisted extraction, either with a sonication probe or a bath, is widely employed for the extraction of bioactive compounds (e.g., phenolic compounds, carotenoids) from various plant materials, e.g., pumpkin,33 ginseng,48 wine lees,49 mango peels,50 apple pomace,51 mangoes,52Cosmos sulphureus,53 orange peels,54Cinnamomum cassia bark55 and blueberry leaves,56 whereas the number of studies that use UAE coupled to DESs for such purposes has been steadily increasing. An overview of representative studies is presented in Table 1.| Aim of the study | Matrix | DES | Ultrasound-assisted extraction parameters | Analytical techniques | Reference |
|---|---|---|---|---|---|
| Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents | Pumpkin (Cucurbita maxima) |
DL-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) decanoic acid (2 decanoic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DL-menthol 1), DL-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoic acid (1 octanoic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DL-menthol 1), DL-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecanoic acid (2 dodecanoic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), decanoic acid 1), decanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecanoic acid (2 dodecanoic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), octanoic acid 1), octanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) decanoic acid (2 decanoic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), octanoic acid 1), octanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecanoic acid (3 dodecanoic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Ultrasonic bath (37 kHz) | Carotenoid analysis | 33 |
| The tailoring of DESs and optimization of extraction conditions for ginsenoside | Ginseng powder | Citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) adonitol (1 adonitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), citric acid 1), citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-(+)-glucose (2 D-(+)-glucose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), citric acid 1), citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-(+)-galactose (1 D-(+)-galactose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), citric acid 1), citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline (2 proline (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), citric acid:betaine (1 1), citric acid:betaine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DL-malic acid (1 DL-malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylitol (5 xylitol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-(–)-fructose (1 D-(–)-fructose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride:glycerol (1 1), choline chloride:glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Ultrasonic bath, 500 W, 45 min (uncontrolled temperature) | LC-UV, UHPLC-Q-TOF-MS | 48 |
| Extraction of wine lees anthocyanins | Wine lees from merlot grapes | Choline chloride:xylose, choline chloride:fructose, choline chloride:glycerol, choline chloride:malic acid, choline chloride:citric acid, choline chloride:oxalic acid | Ultrasonic bath (power 380 W, frequency 37 kHz), 35 °C | HPLC | 49 |
| Extraction of phenolic compounds from mango by-products using deep eutectic solvents | Mango (Mangifera indica) peels | DESs based on choline chloride, citric acid, ethylene glycol and malic acid | Ultrasound probe system (500 W–20 kHz) | Folin-Ciocalteu assay, DPPH and ABTS radical scavenging activity assays, flavonoid content, RP-HPLC-DAD | 50 |
| Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction | Apple pomace (sp. Red delicious) | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1 lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), choline chloride 3), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-glucose (3 D-glucose (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (1 citric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), urea 1), urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), tartaric acid 2), tartaric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1 glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Lab-scale ultrasound probe sonicator; frequency 20 kHz, power 400 W, amplitude 70% | RP-HPLC-DAD | 51 |
| Utilization of waste mango peels for extraction of polyphenolic antioxidants | Alphonso mangoes (Mangifera indica) | Lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (5 glucose (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Ultrasound probe system with a 12 mm tip diameter, 22 kHz, and 0–200 W output power (pulsed mode) | RP-HPLC-DAD | 52 |
| Deep eutectic solvent-based ultrasound-assisted extraction of polyphenols from Cosmos sulphureus | Cosmos sulphureus seeds | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1 glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sucrose (1 sucrose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (1 ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1 lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Ultrasonic probe system, 25 kHz (pulsed mode: 2 s on, 1 s off) | Folin-Ciocalteu assay, DPPH radical scavenging activity assay | 53 |
| Improvement of carotenoid extraction from orange peels | Orange peels | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), proline 2), proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), L-menthol 1), L-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, L-camphor (1 D, L-camphor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), L-menthol 1), L-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eucalyptol (1 eucalyptol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), lauric acid 1), lauric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanoid acid (1 octanoid acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Ultrasound probe system 50 W (40 kHz) | Total carotenoid content (spectrophotometrically) | 54 |
| Sustainable ultrasound-assisted extraction of polyphenols from Cinnamomum cassia bark | Cinnamomum cassia bark | DES based on choline chloride | Ultrasonic bath (45 kHz) | Ultra-fast LC | 55 |
| Extraction of phenolic compounds from blueberry leaves for the valorization of agrifood wastes | Blueberry leaves | NADES consisting of amines, organic acids and aminoacids as HBAs and sugars, alcohols and urea as HBDs | Ultrasonic bath (350 W, 40 kHz) | Folin-Ciocalteu assay, DPPH radical scavenging activity assay, RP-HPLC-DAD | 56 |
4.2. Microwave-assisted extraction coupled to DESs
Promoting extraction using microwaves has been recognized as an innovative technology that meets the principles of green chemistry. Microwave-assisted extraction (MAE) uses the direct interaction of microwaves with water molecules in plant cells causing a temperature increase. Evaporation and high pressure occur inside the cell weakening the cell wall and making ruptures thus releasing the phytochemicals from the plant matrix into the solvent.57 MAE gives an even higher yield when increasing the extraction temperature due to the faster inflow of the solvent into the cell wall. Solvents used in MAE should have a high dielectric constant so that absorption of microwave energy can occur since the dielectric constant and dissipation factor determine the amount of power that interacts with the sample.58 The chemical properties of DESs enable the electromagnetic field to induce a heating effect, ensuring a homogeneous temperature profile throughout the sample.59 Heating of DESs during microwave irradiation affects the fluidity and viscosity of the solvent as well as the solubility of the targeted compound which can be seen through the achieved high-extraction efficiency at relatively low temperatures.60 Since the nature of HBD used in the composition of a DES dictates its properties, the ability to absorb microwaves is also dependent on the HBD in the system. Wang et al.60 tested the influence of different HBD types (alcohols, amines and acids) in DESs on microwave heating absorption. According to their results, for the alcohol-based DESs, solvent temperature increased with prolonged irradiation time, but overall microwave heating efficiency was reduced. As explained, lower energy absorption by the solvent molecules in the process was probably caused by the decrease in viscosity. However, heating rates are higher if HBA to HBD molar ratio goes in favour of HBD (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) since the polarity of molecules increases which in turn increases the microwave absorption capacity of the DES. For amine-based DESs the heating rate is elevated along with temperature increase when the HBD content is higher, except if the used amine molecule possesses week polarity. The behaviour of the acid-based DES on heating rates was similar to that of the previously explained HBD. The polarity of the system can also be increased with the addition of water molecules. While single DES components behave as MW-transparent materials, MW absorption and heating response change once those components are in the form of a DES.59 This is probably due to the newly arranged hydrogen bond network which can be promoted by the presence of water in the DES but also disrupted if water content reaches a certain proportion that exceeds the definition of DESs. As can be seen from Table 2, there is an increasing number of MAE–DES that are being used to obtain a vast variety of different phytochemicals due to maximized total extraction yields. For instance, MAE in combination with an organic acid-based DES had 2.6 times higher yield (wt%) of extracted polyphenols from chestnut shell waste than pure water when used as the solvent under the same extraction conditions.59 Process-intensification capabilities of MW have been confirmed in the work of Grillo et al.61 where they compared the MAE efficiency of anthocyanin extraction from blueberry peel. Not only did the optimized MAE–DES approach give a much higher yield than the conventional extractions that were carried out in acidified ethanol but also the overall process time was reduced. Yu et al.62 used microwave irradiation with choline chloride:glycerol DES for the extraction of flavonoids which resulted in an 83% higher total extraction yield with the DES when compared to using a conventional solvent (60% of aqueous ethanol solution). When applying microwave irradiation, it is important to reach an equilibrium between applied extraction time and temperature to avoid degradation of the sample if it is exposed to microwaves for a long time due to preheating.58 Panić et al.63 extracted anthocyanins from grape pomace using MW irradiation with process parameters optimized so that degradation of anthocyanin is prevented while keeping low energy consumption. Results showed that the total anthocyanin content increased along with MW power, but extraction time was a limiting factor due to the fact that prolonged heating increased degradation. Similar results were presented in Lasunon and Sengkhamparn's work64 where pectin yield from tomato fruit increased with higher microwave power, but with longer time exposure decrease in yield was detected. A. Meindl et al.65 extracted lignin from larch bark within only 30 min of microwave irradiation using a choline chloride:acetic acid DES in yields of up to 96%. Compared to traditional DES-assisted extraction by conventional heating, the reaction time was cut by 87% and the energy costs were reduced by 93.5%. In conclusion, DESs can be efficiently used for the MAE of biologically active compounds. Combined chemical and thermal approaches have the best impact when organic-acid-based DESs are used due to their highest polarities.66 However, the extraction efficiency of MAE depends on several factors such as the particle size of the sample, the solvent/solid ratio, the applied microwave power, the irradiation time, etc. and no general conclusion can be made. Process optimisation should be made for each plant matrix as well as for each applied DES.67
3) since the polarity of molecules increases which in turn increases the microwave absorption capacity of the DES. For amine-based DESs the heating rate is elevated along with temperature increase when the HBD content is higher, except if the used amine molecule possesses week polarity. The behaviour of the acid-based DES on heating rates was similar to that of the previously explained HBD. The polarity of the system can also be increased with the addition of water molecules. While single DES components behave as MW-transparent materials, MW absorption and heating response change once those components are in the form of a DES.59 This is probably due to the newly arranged hydrogen bond network which can be promoted by the presence of water in the DES but also disrupted if water content reaches a certain proportion that exceeds the definition of DESs. As can be seen from Table 2, there is an increasing number of MAE–DES that are being used to obtain a vast variety of different phytochemicals due to maximized total extraction yields. For instance, MAE in combination with an organic acid-based DES had 2.6 times higher yield (wt%) of extracted polyphenols from chestnut shell waste than pure water when used as the solvent under the same extraction conditions.59 Process-intensification capabilities of MW have been confirmed in the work of Grillo et al.61 where they compared the MAE efficiency of anthocyanin extraction from blueberry peel. Not only did the optimized MAE–DES approach give a much higher yield than the conventional extractions that were carried out in acidified ethanol but also the overall process time was reduced. Yu et al.62 used microwave irradiation with choline chloride:glycerol DES for the extraction of flavonoids which resulted in an 83% higher total extraction yield with the DES when compared to using a conventional solvent (60% of aqueous ethanol solution). When applying microwave irradiation, it is important to reach an equilibrium between applied extraction time and temperature to avoid degradation of the sample if it is exposed to microwaves for a long time due to preheating.58 Panić et al.63 extracted anthocyanins from grape pomace using MW irradiation with process parameters optimized so that degradation of anthocyanin is prevented while keeping low energy consumption. Results showed that the total anthocyanin content increased along with MW power, but extraction time was a limiting factor due to the fact that prolonged heating increased degradation. Similar results were presented in Lasunon and Sengkhamparn's work64 where pectin yield from tomato fruit increased with higher microwave power, but with longer time exposure decrease in yield was detected. A. Meindl et al.65 extracted lignin from larch bark within only 30 min of microwave irradiation using a choline chloride:acetic acid DES in yields of up to 96%. Compared to traditional DES-assisted extraction by conventional heating, the reaction time was cut by 87% and the energy costs were reduced by 93.5%. In conclusion, DESs can be efficiently used for the MAE of biologically active compounds. Combined chemical and thermal approaches have the best impact when organic-acid-based DESs are used due to their highest polarities.66 However, the extraction efficiency of MAE depends on several factors such as the particle size of the sample, the solvent/solid ratio, the applied microwave power, the irradiation time, etc. and no general conclusion can be made. Process optimisation should be made for each plant matrix as well as for each applied DES.67
| Aim of the study | Matrix | DES | Microwave-assisted extraction parameters | Analytical techniques | Reference |
|---|---|---|---|---|---|
| a C DES — concentration of deep eutectic solvents. | |||||
| Chestnut shell waste valorization | Chesnut shell | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid (1 oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (1 citric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid (1 levulinic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid dihydrate (1 oxalic acid dihydrate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 °C, 75 °C or 85 °C; 5, 15, 30 or 60 min, 10–30 W, CDES = 75% (w/w) | HPLC-DAD, FT-IR, SEM | 59 |
| Coupling of MAE to DES for the extraction of anthraquinones | Rheum palmatum | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol, choline chloride glycerol, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycol, choline chloride glycol, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-butanediol, choline chloride 1,4-butanediol, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methylurea, choline chloride methylurea, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetamide, choline chloride acetamide, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, choline chloride urea, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malonic acid, choline chloride malonic acid, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid, choline chloride citric acid, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid; all in molar ratio of 1 malic acid; all in molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
80 W, 16.5 min, CDES = 80% (w/w) | UPLC-DAD, LC-MS, SEM | 60 |
| Development of non-conventional technologies for blueberry-peel extraction | Blueberry peel | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1.5 malic acid (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (2 citric acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1 lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1 glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C, 15 min, 500 W, CDES = 78% (w/w) | UV-vis spectrophotometry | 61 |
| Eco-friendly extraction method for grape pomace anthocyanins on a larger scale | Grape pomace | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (2 citric acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), proline 1), proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), betaine 1), betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), betaine 1), betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (1 citric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), malic acid 1), malic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), malic acid 1), malic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1 glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 °C, 10 min, 100 W, CDES = 75% (w/w) | HPLC | 63 |
| Green extraction of phenolic compounds | Grape skin | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), choline chloride 2), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid (1 oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1.5 malic acid (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbose (1 sorbose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid (1 malic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
65 °C, 100 W, 50 min, CDES = 75% (w/w) | HPLC | 68 |
| Extraction of lignin oligomers from wood biomass | Poplar wood flour | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid dihydrate (1 oxalic acid dihydrate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), choline chloride 1), choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 °C, 3 min, 800 W | NMR, FTIR, GC-MS | 69 |
4.3. Supercritical carbon dioxide extraction coupled to DESs
The information on the application of DESs and/or NADESs combined with supercritical CO2, applied for the extraction/isolation of different compounds, is rather limited in the scientific literature. Actually, CO2 in the supercritical state is mostly applied in the extraction of various natural matrices as the pre-extraction or post-extraction phase to DES or NADES extraction. Thus, in one of the latest studies70 this system was applied for hydroxytyrosol, tyrosol and oleuropein recovery from the waste produced during olive oil production, namely, in their study, Plaza et al.70 extracted olive mill waste and olive leaf samples firstly using the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (at a molar ratio of 1
ethylene glycol (at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and choline chloride
2) and choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (at a molar ratio of 1
citric acid (at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in an ultrasonic bath of 40 kHz of power at a process temperature of 30 °C. Supercritical CO2 was used as a stripping phase to recover hydroxytyrosol, tyrosol and oleuropein from the obtained DES extracts. Studies confirmed that DES extraction processes gave high re-extraction capacities when supercritical CO2 was used as a stripping phase. The hydroxytyrosol recovery yields were highest when the DES choline chloride:ethylene glycol was used as the extraction solvent (81.1% from olive mill waste extracts and 57% from olive leaf extracts), followed by a final purification step using supercritical CO2 at 35 °C and 100 bar. According to the authors of the study, this supercritical fluid process, in addition to recovering the compounds of interest, is capable of regenerating the DESs, thus allowing the possible reuse of DESs to extract another sample, which is one of the advantages of combining these two techniques.
1) in an ultrasonic bath of 40 kHz of power at a process temperature of 30 °C. Supercritical CO2 was used as a stripping phase to recover hydroxytyrosol, tyrosol and oleuropein from the obtained DES extracts. Studies confirmed that DES extraction processes gave high re-extraction capacities when supercritical CO2 was used as a stripping phase. The hydroxytyrosol recovery yields were highest when the DES choline chloride:ethylene glycol was used as the extraction solvent (81.1% from olive mill waste extracts and 57% from olive leaf extracts), followed by a final purification step using supercritical CO2 at 35 °C and 100 bar. According to the authors of the study, this supercritical fluid process, in addition to recovering the compounds of interest, is capable of regenerating the DESs, thus allowing the possible reuse of DESs to extract another sample, which is one of the advantages of combining these two techniques.
Besides this, the interaction between supercritical CO2 and DES or NADES was recently studied by Jiang et al.71 for improving the capacity of the analytical procedure – supercritical fluid chromatography (SFC). Here the DESs were utilized as eco-friendly novel mobile phase additives to supercritical CO2 applied for alkaloid separation. The separation potential of this kind of procedure was studied for 10 different isoquinoline alkaloids (IQAs). Studies suggest that the separation of IQAs from complex samples in SFC can be effectively improved using DESs as mobile phase additives. Supercritical CO2 and MeOH–2% H2O–0.5% FA–0.25% ChCl–Gly were used as the optimized mobile phase. The separation mechanism was explained by the fact that DESs were used as silanol blockers to compete for the adsorption sites with IQAs on the silica surface.
The interaction of NADESs and supercritical CO2 was also investigated in the field of foam production by supercritical CO2 where the possibility of NADESs acting as plasticizing agents enhancing the supercritical foaming of nature-based polymers was investigated. According to the study of Martins et al.72 provided from this field, in the gas foaming procedure the polymer is exposed to supercritical CO2 at the saturation pressure and temperature, which plasticizes the polymer and reduces the glass transition temperature. Upon depressurization, thermodynamic instability causes supersaturation of the CO2 dissolved in the polymeric matrix, and hence, nucleation of the cells occurs. The main requirement of the CO2-foaming process is that CO2 can be dissolved in a sufficient amount in the polymer, which excludes the use of polymers which have a very low affinity for CO2.72
Unlike the lack of scientific data on the application of DESs or NADESs with supercritical CO2, adequate data exist on the interaction between DESs and/or NADESs and CO2 in the gas state, especially in the field of CO2 capture applied in removing CO2 from gas mixtures. Until now several studies have demonstrated the potential of DESs and NADESs for CO2 capture.73–75 For this kind of application, according to Krishnan et al.,74 the most influencing factors are: (a) the density of the solvent which has a significant effect on the CO2 uptake, (b) the molar ratios of the mixtures, (c) the molecular properties of the hydrogen-bond donor, which impact the density of the DES or NADES and its variation with temperature, (d) the viscosity of the eutectic solvent, and the (e) eutectic solvent surface tension, which has a large impact on the rate of mass transfer of gas molecules from the gas phase to the liquid phase.
One of the studies which have evaluated the potential of NADESs for CO2 capture was reported by Ren et al.73 In this study, a series of hydrophilic NADESs and their hydrates based on L-arginine and glycerol (L-Arg/Gly) was investigated. It was shown that in the gas–liquid absorption process, the hydrophilic properties of the L-Arg/Gly DES and its hydrate greatly reduced the transfer barrier and accelerated the phase transfer by increasing the accessibility of active amine sites on NADES and CO2. Besides, it was found that the L-Arg/Gly DES could be reused 5 times without obvious inactivation. Therefore, the authors concluded that the L-Arg/Gly DES could be a potential substitute for the traditional volatile amine solvents for CO2 capture.73 Most of the studies investigated the potential of ionic DESs for CO2 capture. But due to the drawbacks of ionic DESs, especially in terms of hygroscopicity, nowadays increased attention for the same application has been directed to hydrophobic DESs. Thus, in one of the latest research studies, a hydrophobic DES containing L-menthol was applied due to its outstanding properties such as low viscosity and eutectic temperatures, especially when L-menthol is mixed with phenolic alcohols, such as thymol or carvacrol.75 The study evaluates the potential of nonionic DESs containing phenolic alcohols (L menthol/thymol in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, and thymol/2,6-xylenol in 1
2 molar ratio, and thymol/2,6-xylenol in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) for CO2 capture, showing that nonionic DESs containing phenolic alcohols can act as excellent, inexpensive, and simple solvents for this kind of application.
1 molar ratio) for CO2 capture, showing that nonionic DESs containing phenolic alcohols can act as excellent, inexpensive, and simple solvents for this kind of application.
4.4. Pressurized liquid extraction including subcritical water extraction coupled to DESs
Studies on the integration of high-pressure processes and eutectic solvents are relatively new, and in comparison to the integration of other extraction techniques information on high-pressure extractions and eutectic system coupling are still limited. The integration of advanced extraction technology, such as pressurized liquid extraction (PLE), with bio-based solvents, such as eutectic systems, makes the whole process of bioactive compound isolation more competitive and more “green”, and this is especially noticeable in the context of process efficiency, energy and solvent consumption, and product stability. Therefore, recently, in order to improve the efficiency of the extraction processes, as well as stability of isolated compounds, PLE was combined with eutectic systems, targeting the isolation of low polar as well as polar constituents from different natural matrices. According to Zielinski et al.76 within the emerging technologies, PLE applies high-pressure to improve the solvent diffusion, promoting high solvation power, besides facilitating the rupture of the cells from the solid matrix, which results in high yields at a low processing time and solvent amount.One of the latest scientific studies, by Sanchez Bragagnolo et al.,77 employed PLE with NADESs for the utilization of bioactives, primarily phenolic ones, from soybean and its by-products. In this study two combinations of PLE with selected eutectic systems were analyzed, where the first eutectic system was used for the defatting process of soybean, while the second one was applied for the extraction of phenolic constituents, such as apigenin, daidzin and genistin from soybean and corresponding by-products. The defatting process of soybeans is usually performed using hexane, which is a toxic organic solvent, therefore in this process new greener bio-based alternatives are needed. In the study of Sanchez Bragagnolo et al.,77 the defatting process of soybeans was performed using n-heptane as the benchmark solvent, which is greener but highly similar to hexane regarding the Hansen solubility parameter. Using n-heptane the maximal extraction yield of 21.14% was obtained after 20 min of extraction at 120 °C. To replace n-heptane, three natural HDESs were selected: (a) eucalyptol/menthol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) camphor/menthol (1
1, (b) camphor/menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and (c) camphor/thymol (3
1), and (c) camphor/thymol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). In the case of HDESs the extraction procedure was performed at three temperatures (40, 80, and 120 °C) for 90 min, while the pressure applied was set to 100 bar. A similar yield and chromatography profile compared to n-heptane were obtained using all three selected eutectic systems, while eucalyptol/menthol 1
2). In the case of HDESs the extraction procedure was performed at three temperatures (40, 80, and 120 °C) for 90 min, while the pressure applied was set to 100 bar. A similar yield and chromatography profile compared to n-heptane were obtained using all three selected eutectic systems, while eucalyptol/menthol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 achieved the highest results. Besides, no statistical differences were observed for the three mixtures at the three temperatures. It means that extractions with the investigated HDESs at 90 °C could be successfully used to replace n-heptane to defat soybean at 120 °C. Therefore, according to the authors of the study this represents a new alternative to conventional soybean oil extraction. In the same study77 the combination of PLE and choline chloride/citric acid/water (1
1 achieved the highest results. Besides, no statistical differences were observed for the three mixtures at the three temperatures. It means that extractions with the investigated HDESs at 90 °C could be successfully used to replace n-heptane to defat soybean at 120 °C. Therefore, according to the authors of the study this represents a new alternative to conventional soybean oil extraction. In the same study77 the combination of PLE and choline chloride/citric acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 molar ratio) was applied for the isolation of phenolic compounds from soybean by-products. The process was performed at a pressure of 100 bar, using three working temperatures: 60, 90, and 120 °C. Using this eutectic system, the highest yields of phenolics and flavonoids were produced at 120 °C (extraction time of 20 min). In the obtained extracts, isoflavonoids such as daidzin and genistin, as well as the flavonoid apigenin, were found. Besides, according to the authors, despite the high temperature applied (120 °C), which could affect the glucoside forms of the flavonoids and isoflavonoids, 6-O-acetyldaidzin, malonyldaidzin, daidzin, genistin, glycitin, and kaempferol 3-rutinoside 4′-glucoside were also identified in the extracts, meaning NADES composition may have interfered with the temperature effect on flavonoids and isoflavonoids. According to the scientific literature, the recovery of polar compounds from a solid matrix is more efficient with DES–water solutions, compared with only DES, since the water can form hydrogen bonds with the target compound, contributing to its dissolution.78 Therefore, just recently, Benvenutti et al.79 carried out an investigation on the possibility of the recovery of anthocyanin rich-fractions from jaboticaba (Brazilian berry) peels by combining aqueous solutions of DES and PLE as the extraction technology. In the study PLE efficiencies with different DESs [choline chloride combined with propylene glycol (ChCl
11 molar ratio) was applied for the isolation of phenolic compounds from soybean by-products. The process was performed at a pressure of 100 bar, using three working temperatures: 60, 90, and 120 °C. Using this eutectic system, the highest yields of phenolics and flavonoids were produced at 120 °C (extraction time of 20 min). In the obtained extracts, isoflavonoids such as daidzin and genistin, as well as the flavonoid apigenin, were found. Besides, according to the authors, despite the high temperature applied (120 °C), which could affect the glucoside forms of the flavonoids and isoflavonoids, 6-O-acetyldaidzin, malonyldaidzin, daidzin, genistin, glycitin, and kaempferol 3-rutinoside 4′-glucoside were also identified in the extracts, meaning NADES composition may have interfered with the temperature effect on flavonoids and isoflavonoids. According to the scientific literature, the recovery of polar compounds from a solid matrix is more efficient with DES–water solutions, compared with only DES, since the water can form hydrogen bonds with the target compound, contributing to its dissolution.78 Therefore, just recently, Benvenutti et al.79 carried out an investigation on the possibility of the recovery of anthocyanin rich-fractions from jaboticaba (Brazilian berry) peels by combining aqueous solutions of DES and PLE as the extraction technology. In the study PLE efficiencies with different DESs [choline chloride combined with propylene glycol (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro) or malic acid (ChCl
Pro) or malic acid (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ma) at a molar ratio of 1
Ma) at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] were compared to the efficiency of conventional solvents (water and acidified water). To decrease the ChCl
1] were compared to the efficiency of conventional solvents (water and acidified water). To decrease the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro and ChCl
Pro and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ma viscosities, enabling handling by the PLE unit and improving dissolution rates, the solvents were diluted in distilled water at concentrations from 5 to 55%. In the study the independent variables were temperature (60, 90, and 120 °C), DES concentration in water (15, 30, and 45%), and flow rate (3, 4, and 5 mL min−1). The pressure and time were set to 100 bar and 12 min. According to the obtained results, DES-assisted extraction resulted in anthocyanin yields up to 50% higher than those of conventional solvents, while the eutectic system ChCl:Ma was found to have the highest potential regarding the recovery capacity as well as the highest anthocyanin stability. In the study of L. Loarce et al.80 (2020) NADESs were applied as modifiers to enhance the efficiency of pressurized hot water extraction (PHWE). Eight choline chloride-based and citric acid-based NADESs composed of acids (oxalic and lactic acids), polyalcohols (ethylene glycol and 1,2-propanediol), sugars (fructose and maltose) and a nitrogen-containing substance (urea) were screened to evaluate their possible application to the extraction of grape pomace phenolic compounds with subcritical water. The selected NADESs were added at different percentages in PHWE (10, 20, 30 and 40%), while the best composition was studied at different temperatures (in the temperature range from 40 to 120 °C). According to the results, choline chloride combined with urea, added in percentage of 20% to PHWE at 80°, was found to be the mixture with the highest power extraction for catechins present in the processed material. But generally, the best results were achieved with the choline chloride/oxalic acid 1
Ma viscosities, enabling handling by the PLE unit and improving dissolution rates, the solvents were diluted in distilled water at concentrations from 5 to 55%. In the study the independent variables were temperature (60, 90, and 120 °C), DES concentration in water (15, 30, and 45%), and flow rate (3, 4, and 5 mL min−1). The pressure and time were set to 100 bar and 12 min. According to the obtained results, DES-assisted extraction resulted in anthocyanin yields up to 50% higher than those of conventional solvents, while the eutectic system ChCl:Ma was found to have the highest potential regarding the recovery capacity as well as the highest anthocyanin stability. In the study of L. Loarce et al.80 (2020) NADESs were applied as modifiers to enhance the efficiency of pressurized hot water extraction (PHWE). Eight choline chloride-based and citric acid-based NADESs composed of acids (oxalic and lactic acids), polyalcohols (ethylene glycol and 1,2-propanediol), sugars (fructose and maltose) and a nitrogen-containing substance (urea) were screened to evaluate their possible application to the extraction of grape pomace phenolic compounds with subcritical water. The selected NADESs were added at different percentages in PHWE (10, 20, 30 and 40%), while the best composition was studied at different temperatures (in the temperature range from 40 to 120 °C). According to the results, choline chloride combined with urea, added in percentage of 20% to PHWE at 80°, was found to be the mixture with the highest power extraction for catechins present in the processed material. But generally, the best results were achieved with the choline chloride/oxalic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio) system, added as a modifier in percentage of 30% to PHWE at the temperature of 100 °C. According to the authors of the study the higher concentrations of NADESs demonstrated a higher content of anthocyanin and pyroanthocyanins isolated. Regarding applied temperatures, no significant differences were found among extractions performed at 100 °C and 120 °C in the case of catechins, tannins and flavonols. Despite the absence of significant differences in almost all phenolic families, values of antioxidant activity increased considerably in those extracts at 120 °C, which was explained with the generation of new antioxidant compounds at elevated temperatures, via Maillard, caramelization and/or thermoxidation reactions. Earlier, DESs were used as modifiers in another study which investigated the application of subcritical water extraction for the isolation of phenolic compounds and xanthone from pericarps of mangosteen.81 Here the subcritical water extraction was investigated in the temperature range from 120 to 160 °C, in batch and semi-batch systems, while the DES was added as a modifier in the concentration from 10 to 30%. The DES was prepared by mixing the crystalline white solid of citric acid and the white powder of alanine, dissolved in deionized water with concentrations of 2.0 and 1.5 mg mL−1, respectively, in a molar ratio 1
1 (molar ratio) system, added as a modifier in percentage of 30% to PHWE at the temperature of 100 °C. According to the authors of the study the higher concentrations of NADESs demonstrated a higher content of anthocyanin and pyroanthocyanins isolated. Regarding applied temperatures, no significant differences were found among extractions performed at 100 °C and 120 °C in the case of catechins, tannins and flavonols. Despite the absence of significant differences in almost all phenolic families, values of antioxidant activity increased considerably in those extracts at 120 °C, which was explained with the generation of new antioxidant compounds at elevated temperatures, via Maillard, caramelization and/or thermoxidation reactions. Earlier, DESs were used as modifiers in another study which investigated the application of subcritical water extraction for the isolation of phenolic compounds and xanthone from pericarps of mangosteen.81 Here the subcritical water extraction was investigated in the temperature range from 120 to 160 °C, in batch and semi-batch systems, while the DES was added as a modifier in the concentration from 10 to 30%. The DES was prepared by mixing the crystalline white solid of citric acid and the white powder of alanine, dissolved in deionized water with concentrations of 2.0 and 1.5 mg mL−1, respectively, in a molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. According to the authors, when mangosteen pericarps are heated under subcritical water conditions, the following occurs: (a) the degradation reaction of their components into their backbone units and production of numerous radicals as a result of thermal cleavage, (b) the radicals can be stabilized by the addition of hydrogen, supplied by a donor solvent, (c) carbon bonds such as C–C and C–O bonds as in ethers and esters and the aliphatic C–H bonds may cleave in subcritical water, (d) hence, physical structure disruption might be found after treatment by subcritical water. Machmudah et al.81 stated that DESs can improve the dissolution capability of water-soluble compounds and macromolecular compounds that are split from the mangosteen pericarps. As a result of this, the highest isolation of xanthone in the study, 27.15 mg g−1 dried sample, was achieved when the extraction was performed in a semi-batch system at a temperature of 160 °C and pressure of 50 bar using the investigated eutectic system as the modifier in a concentration of 10%. In the case of phenolics the highest result 372.69 mg of GAE per g dried sample was obtained again in a semi-batch system at a process temperature of 120 °C and pressure of 10 bar using a higher concentration of the investigated eutectic system as the modifier, 30%. For isolation of baicalin, the active ingredient of Scutellaria baicalensis Georgi which has demonstrated various pharmacological activities, the DES was coupled with ultrahigh pressure extraction (UPE) in the study of Wang et al.82 Here, choline chloride
1. According to the authors, when mangosteen pericarps are heated under subcritical water conditions, the following occurs: (a) the degradation reaction of their components into their backbone units and production of numerous radicals as a result of thermal cleavage, (b) the radicals can be stabilized by the addition of hydrogen, supplied by a donor solvent, (c) carbon bonds such as C–C and C–O bonds as in ethers and esters and the aliphatic C–H bonds may cleave in subcritical water, (d) hence, physical structure disruption might be found after treatment by subcritical water. Machmudah et al.81 stated that DESs can improve the dissolution capability of water-soluble compounds and macromolecular compounds that are split from the mangosteen pericarps. As a result of this, the highest isolation of xanthone in the study, 27.15 mg g−1 dried sample, was achieved when the extraction was performed in a semi-batch system at a temperature of 160 °C and pressure of 50 bar using the investigated eutectic system as the modifier in a concentration of 10%. In the case of phenolics the highest result 372.69 mg of GAE per g dried sample was obtained again in a semi-batch system at a process temperature of 120 °C and pressure of 10 bar using a higher concentration of the investigated eutectic system as the modifier, 30%. For isolation of baicalin, the active ingredient of Scutellaria baicalensis Georgi which has demonstrated various pharmacological activities, the DES was coupled with ultrahigh pressure extraction (UPE) in the study of Wang et al.82 Here, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (ChCl–LA, molar ratio 1
lactic acid (ChCl–LA, molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was selected as the most appropriate DES by comparing the extraction yield of different DESs. According to the authors, under the optimal conditions (40 vol% water content, extraction pressure of 4000 bar, extraction time of 4 min, and a liquid–solid ratio of 110 mL g−1), a maximum yield of 116.8 mg baicalin per g was achieved. The extraction efficiency from DES-based UPE of 116.8 mg g−1 is slightly higher than 110.4 mg g−1 from the pharmacopoeia procedure. However, the extraction time is dramatically shortened from 3 h to 4 min.
1) was selected as the most appropriate DES by comparing the extraction yield of different DESs. According to the authors, under the optimal conditions (40 vol% water content, extraction pressure of 4000 bar, extraction time of 4 min, and a liquid–solid ratio of 110 mL g−1), a maximum yield of 116.8 mg baicalin per g was achieved. The extraction efficiency from DES-based UPE of 116.8 mg g−1 is slightly higher than 110.4 mg g−1 from the pharmacopoeia procedure. However, the extraction time is dramatically shortened from 3 h to 4 min.
4.5. Other non-thermal innovative techniques coupled to DESs
In addition to ultrasound-assisted extraction, negative pressure cavitation,83 high voltage electrical discharges,84 and pulsed electric field85 are other highly popular, innovative, eco-friendly, and efficient methods used for extracting and isolating bioactive compounds from various sources. While these techniques differ in terms of their energy sources and physical mechanisms, they all possess the fundamental ability to induce physical or chemical transformations in the plant matrix under non-thermal conditions. As a result, they enable improved extraction efficiency, reduced extraction time, and the ability to extract a broader range of compounds.86 These non-thermal methods, whether used as a pretreatment or in conjunction with DESs, have been successfully coupled with DESs to extract bioactive compounds from plant matrices, especially for the extraction of thermo-sensitive bioactive compounds under mild processing conditions.In the negative pressure cavitation extraction (NPCE) method, cavitation is generated through negative pressure and continuously introduced into the liquid–solid system to enhance turbulence, collision, and mass transfer between the extracting solvent and the solid matrix (plant material). NPCE has demonstrated several advantages over traditional methods, including shorter extraction time, reduced solvent consumption, superior extraction efficiency, and ease of operation on a large scale.83 Qi et al.87 were the first to showcase a green and efficient alternative to the DES-NPCE method for extracting and separating bioactives, specifically nine target flavonoids, from Equisetum palustre. By optimizing the conditions and using choline chloride:betaine hydrochloride:ethylene glycol as the solvent, they achieved higher extraction yields compared to conventional methods utilizing ethanol. Furthermore, they successfully enriched the nine target flavonoids directly from the DES extraction solution using macroporous resin. Li et al.88 also demonstrated the effectiveness of the DES-NPCE method for extracting four main isoflavonoids (prunetin, tectorigenin, genistein, and biochanin A) from Dalbergia odorifera T. Chen leaves, employing choline chloride:ethylene glycol as the solvent. This method outperformed ultrasound-assisted extraction in terms of the extraction yield of individual isoflavonoids. Subsequently, Kou et al.89 presented a sustainable and efficient process for preparing anthocyanin compounds from blue honeysuckle fruits using DES-NPCE. The extraction yields of total anthocyanins and the main constituent cyanidin-3-glucoside in the DES choline chloride:lactic acid as a 20% aqueous solution, under optimized conditions, were significantly higher compared to those of traditional methods (up to 1.6-fold increase). Acid-based DESs were found to enhance the structural stability of anthocyanin compounds.
High voltage electrical discharge (HVED), another non-thermal technique, utilizes electrical energy to generate an electrical discharge within a gaseous medium. This discharge leads to the formation of a high-intensity plasma, which generates reactive species, electric fields, and shockwaves. These effects can disrupt cell structures, thereby enhancing the release of target compounds, such as bioactive compounds from plants. HVED is commonly employed as a pre-treatment method for biomass, specifically plants, to improve the extraction kinetics and yields in subsequent solid–liquid extractions.90 El Kantar et al.91 were the first to utilize HVED as a pretreatment technique in DES-assisted extraction of polyphenols from plant matrices, specifically grapefruit peels. The results demonstrated that HVED pretreatment of grapefruit peels significantly enhanced both the extraction kinetics and yields of polyphenols during subsequent solid–liquid extraction. Moreover, it was found that the energy input required for HVED could be reduced by up to 6-fold when the subsequent solid–liquid extraction was performed using a 20% (w/v) aqueous glycerol solution or a DES (lactic acid: glucose) instead of water. In a recent study by E. Hernández-Corroto et al.92 HVED and DES were employed for the simultaneous extraction of proteins and polyphenols from pomegranate seeds. The researchers first subjected the pomegranate seeds to HVED pretreatment and then carried out solid–liquid extraction using DESs. A comparison between HVED and ultrasound treatment revealed that HVED showed superior performance by disintegrating 86% of cells, resulting in the release of 70% of proteins and 78% of polyphenols.
Pulsed electric field (PEF) is a technique that involves the application of short, intense electrical pulses to biological materials, resulting in the disruption of cell membranes and the release of intracellular components, including bioactive compounds. PEF has been suggested to enhance (or assist) various processing operations, including solid–liquid extraction (e.g., extracting active ingredients), mechanical extraction (e.g., juicing), pre-treating solid plant matrices before further processing (such as cutting/slicing of potato snacks), or for microbial inactivation (preservation) of liquid food products. Its benefits include improved yield, faster processing, enhanced food quality, reduced impact on sensory properties, and increased cost efficiency.93
Wei et al.94 employed this approach for the extraction of flavonoids from Kapok, a traditional Chinese medicinal plant. Their study demonstrated that using a three-component DES (betaine:glycerol:propylene glycol, containing 50% water) under optimized extraction conditions resulted in high flavonoid yields. Furthermore, the extracts exhibited good stability without any precipitation or discoloration for up to six months. In another study, Atanasov et al.95 successfully demonstrated the combination of PEF-DES with low-frequency ultrasound (100 W, 32 kHz) for the extraction of the phenolic compound silymarin from milk thistle.
5. Recovery of bioactive compounds from DES extracts and DES recycling
One of the greatest challenges regarding the industrial use of DESs is to obtain a purified extract without the solvent as well as to be able to recycle the solvent. Considering the low vapor pressure of DESs, they cannot be simply removed by rotary evaporation and therefore other approaches must be followed. Methods including solid–liquid extraction using resin or molecular sieves, liquid–liquid extraction using an anti-solvent, as well as ultrafiltration, have been used so far for purification purposes.96 In particular, Nam et al.97 extracted flavonoids from Japanese acacia using an L-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (2
glycerol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) DES. After the extraction, the solvent was regenerated by liquid–liquid extraction using water as an anti-solvent, with 75% separation efficiency, and by solid-phase extraction in a reverse-phase cartridge (C18) with 92% efficiency. Li et al.98 used an adsorption column with an NKA-9 macroporous resin to recover up to 86.1% of the solute catechin from different DESs. The recovery of flavonoids extracted with the DES based on choline chloride from Hippophae rhamnoides L. with the aid of microporous resins (i.e., NKA-9, AB-8 and D101) has also been reported by Cui et al.99 Stupar et al.33 managed to recover 90% of pumpkin carotenoids from a hydrophobic fatty acid-based NADES extract by switching its polarity using water and a weak base such as NH4OH. Using the same methodology, tomato carotenoids (∼37%) were also recovered from a hydrophobic fatty acid-based NADES.34 Moreover, a recovery of ∼42% has been reported for curcuminoids from a choline chloride-based DES turmeric extract employing an anti-solvent precipitation technique (Patil et al., 2021).100 A 75% recovery of sunflower oil and algae oil has been achieved by Lo et al.101 by shifting the hydrophobicity spectrum of a semi-hydrophobic solvent consisting of the hydrophilic imidazole and the hydrophobic hexanoic acid. Polarity switch of a hydrophobic DES synthesized from N,N-dimethylcyclohexylamine and n-butanol in order to recover (91%) β-carotene from millets has also been reported.102
5) DES. After the extraction, the solvent was regenerated by liquid–liquid extraction using water as an anti-solvent, with 75% separation efficiency, and by solid-phase extraction in a reverse-phase cartridge (C18) with 92% efficiency. Li et al.98 used an adsorption column with an NKA-9 macroporous resin to recover up to 86.1% of the solute catechin from different DESs. The recovery of flavonoids extracted with the DES based on choline chloride from Hippophae rhamnoides L. with the aid of microporous resins (i.e., NKA-9, AB-8 and D101) has also been reported by Cui et al.99 Stupar et al.33 managed to recover 90% of pumpkin carotenoids from a hydrophobic fatty acid-based NADES extract by switching its polarity using water and a weak base such as NH4OH. Using the same methodology, tomato carotenoids (∼37%) were also recovered from a hydrophobic fatty acid-based NADES.34 Moreover, a recovery of ∼42% has been reported for curcuminoids from a choline chloride-based DES turmeric extract employing an anti-solvent precipitation technique (Patil et al., 2021).100 A 75% recovery of sunflower oil and algae oil has been achieved by Lo et al.101 by shifting the hydrophobicity spectrum of a semi-hydrophobic solvent consisting of the hydrophilic imidazole and the hydrophobic hexanoic acid. Polarity switch of a hydrophobic DES synthesized from N,N-dimethylcyclohexylamine and n-butanol in order to recover (91%) β-carotene from millets has also been reported.102
Regarding the recyclability of DES, a limited number of papers have reported successful recycling and reuse following the extraction process. One of the pioneering studies in this area was published by Panić et al.,63 detailing the extraction of grape-pomace anthocyanins using a NADES in batches of up to half a liter. The potential for reusing the NADES with a high yield (approximately 10% lower than that of the freshly prepared NADES) for at least three cycles, along with reported recycling yields of the NADES (ranging from approximately 60% to 90%, depending on the recycling method employed), was documented.
Furthermore, Anstiss et al.103 explored twenty-two hydrophobic DESs for fatty acid extraction, achieving efficient extraction in five consecutive cycles without removing the DES from the extracts. Lanjekar et al.104 utilized macroporous resin to isolate glycyrrhizin acid from extracts, reusing the DES for two cycles with over 90% extraction efficiency. Tang et al.105 developed hydrophobic temperature-switchable DESs for Lycium barbarum polysaccharide extraction, achieving 80.2% recovery after five cycles. In the aforementioned paper by Zhang et al.102 dealing with the extraction of β-carotene from millets using a hydrophobic DES, the authors suggested that this switchable DES could be recycled multiple times for successive β-carotene extraction.
Since NADESs are prepared using starting materials of natural origin that are included in humans' daily diet (e.g., sugars, organic acids, amino acids, etc.), the obtained extracts could be used directly in food, pharmaceutical and cosmeceutical applications as ready-to-use extracts.106 This view is supported by a steadily increasing number of publications. In particular, Jeong et al.107 suggested that a DES prepared using betaine, glycerol and D-(+)-glucose (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be used as a multi-functional medium to produce a catechin-rich tea extract that could be directly applied to cosmetic and pharmaceutical formulations for the skin. In the same frame, da Silva et al.108 comparatively examined the bioavailability of blueberry anthocyanins from an organic solvent extract and a choline chloride
1) could be used as a multi-functional medium to produce a catechin-rich tea extract that could be directly applied to cosmetic and pharmaceutical formulations for the skin. In the same frame, da Silva et al.108 comparatively examined the bioavailability of blueberry anthocyanins from an organic solvent extract and a choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol
glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (0.5
citric acid (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 molar ratio) NADES extract. The authors concluded that in the latter case, the bioavailability of anthocyanins was increased by 140% compared to the former case, as the NADES increased the stability of the phenolic compounds upon in vitro digestion probably by delaying gastric chyme neutralization. In this view the authors postulated that this ready-to-use NADES extract could be employed as a vehicle for increasing oral absorption of bioactive compounds, such as anthocyanins and that it could be used for the development of functional foods, nutraceuticals and pharmacological formulations. Furthermore, when assessing ready-to-use DES extracts of bioactive compounds, it is crucial to consider their role in maintaining the extract's stability and biological effectiveness.109 For instance, organic acid-based DESs with acidic properties can aid in stabilizing anthocyanins, possibly through hydrogen bond interactions.63 Moreover, DES-based plant extracts typically demonstrate higher biological activity than those obtained through conventional methods, owing to their distinct profile of bioactive compounds.61 DESs also reinforce the antioxidant properties of these extracts, potentially outperforming traditional antioxidants.96 Additionally, considering the documented antibacterial effects of DESs,110 DES-based ready-to-use extracts may exhibit reduced susceptibility to microbial contamination.
0.5 molar ratio) NADES extract. The authors concluded that in the latter case, the bioavailability of anthocyanins was increased by 140% compared to the former case, as the NADES increased the stability of the phenolic compounds upon in vitro digestion probably by delaying gastric chyme neutralization. In this view the authors postulated that this ready-to-use NADES extract could be employed as a vehicle for increasing oral absorption of bioactive compounds, such as anthocyanins and that it could be used for the development of functional foods, nutraceuticals and pharmacological formulations. Furthermore, when assessing ready-to-use DES extracts of bioactive compounds, it is crucial to consider their role in maintaining the extract's stability and biological effectiveness.109 For instance, organic acid-based DESs with acidic properties can aid in stabilizing anthocyanins, possibly through hydrogen bond interactions.63 Moreover, DES-based plant extracts typically demonstrate higher biological activity than those obtained through conventional methods, owing to their distinct profile of bioactive compounds.61 DESs also reinforce the antioxidant properties of these extracts, potentially outperforming traditional antioxidants.96 Additionally, considering the documented antibacterial effects of DESs,110 DES-based ready-to-use extracts may exhibit reduced susceptibility to microbial contamination.
6. Sustainability of the DES-innovative extraction processes
DESs are widely recognized for their ability to enhance the environmental performance and sustainability of specific processes due to their eco-friendly characteristics, such as non-volatility, non-flammability, non-toxicity, biodegradability, and their solvent-free preparation from readily available natural raw materials. As a result, the combination of DESs with innovative extraction techniques, as discussed in this paper, can be considered as a green alternative to conventional procedures for extracting biologically active molecules. The synergistic effect of DESs and these innovative techniques is promising thanks to the eco-friendly nature of DESs, the reduced solvent usage facilitated by methods like ultrasound- and microwave-assisted extraction, the energy efficiency of non-thermal techniques, and the potential for higher extraction yields and improved extract quality. However, a significant hurdle in gaining investor confidence for establishing commercial industrial processes based on DES-innovative extraction techniques lies, among other ends, in the lack of comprehensive understanding of DES process parameters. To promote the adoption of these solvents in new or existing industrial processes, a more comprehensive approach is required. This approach should also encompass economic considerations such as DES cost, capital investment, and utility expenses, as well as the need for specialized equipment to handle viscous fluids during pumping and mixing. Moreover, the complexity of product isolation, particularly due to the non-volatility of DESs, and the potential for DES reusability should also be taken into account to ensure a holistic evaluation.Since 2004, when Abbot et al.8 introduced DESs, there has been a notable increase in published manuscripts exploring newly prepared DESs and their toxicity. However, despite this growing body of literature, the impact of these green solvents on the environment and humans remains uncertain. DESs generally exhibit low to moderate toxicity towards various organisms, with the pH value being a crucial factor affecting their toxicity. DESs containing organic acids as hydrogen bond donors tend to be moderately toxic, while those comprising choline, sugars, and polyols are less toxic, possibly due to higher cellular tolerance. Some DESs produced from natural metabolites are considered “biodegradable” according to OECD guidelines, but their biodegradability can vary depending on the components used.111–113
The sustainability of DESs, in the context of sustainability of their preparation, has only been recently discussed by Nejrotti et al.114 where it was pointed out that the commonly used HBA, choline chloride, is currently dependent on non-renewable sources, and thus it is crucial to replace choline chloride with some other equally efficient HBA, such as betaine or some other natural methylamines, as recently demonstrated by Cvjetko Bubalo et al.23 Still, it's essential to note that during DES preparation, the mixing of HBA and HBD results in a 100% yield: all reactants are fully incorporated into the solvent (atom economy = 100%), with no waste generated (E-factor = 0). Economically, DES prices vary significantly based on their primary components, ranging from €7 to €100 kg−1, which is comparable to conventional organic solvents.102
To the best of our knowledge, only one paper has addressed the economic and environmental assessment of DES-assisted extraction of bioactives from plants, utilizing ultrasound as an innovative extraction technique.102 It was shown that the DES-assisted extraction of anthocyanin from grape pomace resulted in slightly increased ecological impacts, higher capital investments, and operating costs at a larger scale, compared to that using ethanol as a conventional extraction solvent . The authors calculated E-factors for both DES- and ethanol-assisted processes at a scale of 0.1 L, and determined that in DES-assisted extraction, the E-factor was 3.5 times higher compared to the conventional method. This increase was attributed to the more complex purification steps inherent in DES-assisted processes, particularly the significant water consumption during adsorption chromatography. It's notable that the calculated E-factors were several orders of magnitude higher than typical values in the chemical industry (ranging from 0.1 to 100, depending on the industrial sector). However, transitioning from a process requiring product isolation to one that yields ready-to-use extracts based on DESs (extracts without further purification or isolation of the target components) positively impacted process sustainability in terms of all factors describing the sustainability (E-factor = 0). The lowest environmental impact of the process yielding ready-to-use extracts was further confirmed by using the Complex GAPI tool, which considers factors such as process yield, energy consumed, reagent and solvent usage, instrumentation, and purification complexity. The authors concluded that, considering the benefits of ready-to-use DES-based extracts, such as product stability, enhanced biological activity, and absence of microbial contamination, this approach presents a significant opportunity for producing high-quality plant extracts. In the same frame, during the evaluation of the environmental impact of DES-assisted extraction of bioactive compounds from Dacryodes rostrata using Life Cycle Assessment (LCA), the authors found that the well-established ethanol extraction method resulted in lower impact and energy consumption compared to that using DES.115 Additionally, transport activities and electricity consumption during the extraction process were identified as the primary contributors to the overall environmental impact.
In summary, despite the progress made in understanding DESs, there are still challenges to overcome, such as the sustainability of their preparation and the dependence on non-renewable sources for certain components, as well as addressing their environmental impact during large-scale extraction processes. This means, while DESs offer promising opportunities for green extraction methods, further research and comprehensive assessments are still needed to ensure that their full potential is realized in sustainable and efficient industrial processes.
7. Concluding remarks and future perspectives
The combination of DESs with innovative extraction techniques, such as ultrasound- and microwave-assisted extraction, super- and sub-critical fluid extraction, as well as other non-thermal technologies, presents a promising approach for the extraction of bioactive compounds from plants. The coupling of DESs to innovative extraction techniques offers several advantages, including the eco-friendly nature of the DESs, their biodegradability, the possibility to easily adjust their properties based on the potent application, improved selectivity and higher extraction yields at reduced extraction times. In this view, the combination of DESs and novel extraction techniques could be a promising alternative towards the production of high-quality plant extracts. Further research is necessary regarding the toxicity of such DES extracts, the bioaccessibility/bioavailability of the extracted compound(s) as well as their effects on human health.Throughout the manufacturing industry landscape, the overall sustainability metrics are quickly becoming critical criteria for a wide range of decisions: raw material sourcing, procurement and supply chain choices increasingly depend on environment-sensitive practices, manufacturing processes strive to achieve lower energy consumption and circular predispositions towards what was once called “waste”, while marketing and Corporate Social Responsibility strategies are heavily leaning towards end-to-end ownership of consumer products, advocating cradle-to-cradle mentalities to match the growing public awareness on climate change and related hazards. The latest research findings in the field of DESs, and especially NADESs, provide additional and unprecedented affordances with respect to what may be achieved to make manufacturing more sustainable. The agency of the scientific community to expand the use of DESs in a multitude of fields and disciplines is omnipresent, as reflected in the exponentially increasing number of related research papers and publications. What has been, however, missing is an effective assemblage:116 a coherent, factual and overall advantageous narrative which will streamline the resources needed in order to scale and maximize impact. We believe that evolving NADESs beyond their function as a “medium” and consolidating them as multifunctional platforms of co-formulation can act as a constructor to eventually forge such a narrative and help promote manufacturing sustainability “by design” in a wide variety of industries and market sectors.
Author contributions
Conceptualization, all authors; writing—original draft preparation, all authors; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This review article is the outcome of a network collaboration in the frame of the COST Action “Green Chemical Engineering Network towards upscaling sustainable processes – GREENERING”, CA18224 (https://www.greenering.eu/), supported by COST (European Cooperation in Science and Technology) (http://www.cost.eu/). The work also received funding from a Croatian national funding body, namely the Croatian Science Foundation (Grants IPS-2022-02-3938 and IP-2019-04-7712).References
- M. Cvjetko Bubalo, S. Vidović, I. Radojčić Redovniković and S. Jokić, Food Bioproc. Tech., 2018, 109, 52–73 CAS.
- F. Chemat, M. A. Vian and G. Cravotto, Int. J. Mol. Sci., 2012, 13, 8615–8627 CrossRef CAS PubMed.
- C. Picot-Allain, M. F. Mahomoodally, G. Ak and G. Zengin, Curr. Opin. Food Sci., 2021, 40, 144–156 CrossRef CAS.
- M. Cvjetko Bubalo, S. Vidović, I. Radojčić Redovniković and S. Jokić, J. Chem. Technol., 2015, 90, 1631–1639 CAS.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, J. M., A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- F. Guthrie, London, Edinburgh Dublin Philos. Mag. J. Sci., 2010, 17, 462–482 CrossRef.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, J. Solution Chem., 2019, 48, 962–982 CrossRef CAS.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2019, 55, 10253–10256 RSC.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- O. S. Hammond, D. T. Bowron, A. J. Jackson, T. Arnold, A. Sanchez-Fernandez, N. Tsapatsaris, V. Garcia Sakai and K. J. Edler, J. Phys. Chem. B, 2017, 121, 7473–7483 CrossRef CAS PubMed.
- A. Gutiérrez, M. Atilhan and S. Aparicio, J. Mol. Liq., 2021, 339, 116758 CrossRef.
- J. Cao and E. Su, J. Clean. Prod., 2021, 314, 127965 CrossRef CAS.
- D. J. G. P. Van Osch, L. F. Zubeir, A. Van Den Bruinhorst, M. A. A. Rocha and M. C. Kroon, Green Chem., 2015, 17, 4518–4521 RSC.
- B. D. Ribeiro, C. Florindo, L. C. Iff, M. A. Z. Coelho and I. M. Marrucho, ACS Sustain. Chem. Eng., 2015, 3, 2469–2477 CrossRef CAS.
- C. Florindo Branco and I. M. Marrucho, ChemSusChem, 2019, 12, 1549–1559 CrossRef PubMed.
- W. Tang, Y. An and K. H. Row, TrAC, Trends Anal. Chem., 2021, 136, 116187 CrossRef CAS.
- D. J. G. P. Van Osch, C. H. J. T. Dietz, S. E. E. Warrag and M. C. Kroon, ACS Sustain. Chem. Eng., 2020, 8(29), 10591–10612 CAS.
- Y. H. Choi, J. Van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. C. E. Arends, G. J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed.
- G. M. Martínez, G. G. Townley and R. M. Martínez-Espinosa, Heliyon, 2022, 8, 12567 CrossRef PubMed.
- M. Cvjetko Bubalo, T. Andreou, M. Panić, M. Radović, K. Radošević and I. Radojčić Redovniković, Green Chem., 2023, 25, 3398 RSC.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S. N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- A. R. C. Duarte, A. S. D. Ferreira, S. Barreiros, E. Cabrita, R. L. Reis and A. Paiva, Eur. J. Pharm. Biopharm., 2017, 114, 296–304 CrossRef CAS PubMed.
- F. Oliveira, F. Santos and A. R. C. Duarte, Molecules, 2021, 26, 7022 CrossRef CAS PubMed.
- H. G. Morrison, C. C. Sun and S. Neervannan, Int. J. Pharm., 2009, 378, 136–139 CrossRef CAS PubMed.
- M. Panić, V. Gunjević, K. Radošević, M. Cvjetko Bubalo, K. K. Ganić and I. R. Redovniković, Molecules, 2021, 26, 4722 CrossRef PubMed.
- J. P. Wojeicchowski, A. M. Ferreira, D. O. Abranches, J. P. Marcos, R. Mafra and J. A. P. Coutinho, ACS Sustain. Chem. Eng., 2020, 8, 12132–12141 CrossRef CAS.
- E. Zurob, R. Cabezas, E. Villarroel, N. Rosas, G. Merlet, E. Quijada-Maldonado, J. Romero and A. Plaza, Sep. Purif. Technol., 2020, 248, 117054 CrossRef CAS.
- B. Ozturk, J. Winterburn and M. Gonzalez-Miquel, Biochem. Eng. J., 2019, 151, 107298 CrossRef CAS.
- B. Ozturk and M. Gonzalez-Miquel, Sep. Purif. Technol., 2019, 227, 115707 CrossRef CAS.
- A. Stupar, V. Šeregelj, B. D. Ribeiro, L. Pezo, A. Cvetanović, A. Mišan and I. Marrucho, Ultrason. Sonochem., 2021, 76, 105638 CrossRef CAS PubMed.
- A. Kyriakoudi, A. Tsiouras and I. Mourtzinos, Foods, 2022, 11, 2645 CrossRef CAS PubMed.
- K. J. Lanjekar and V. K. Rathod, Process Biochem., 2021, 102, 22–32 CrossRef CAS.
- C. Florindo, F. S. Oliveira, L. P. N. Rebelo, A. M. Fernandes and I. M. Marrucho, ACS Sustain. Chem. Eng., 2014, 2, 2416–2425 CrossRef CAS.
- M. C. Gutiérrez, M. L. Ferrer, C. R. Mateo and F. Del Monte, Langmuir, 2009, 25(10), 5509–5515 CrossRef PubMed.
- T. R. Sekharan, M. Chandira, C. S. Rajesh, S. Tamilvanan, C. T. Vijayakumar and B. S. Venkateswarlu, Biointerface Res. Appl. Chem., 2021, 11, 14620–14633 CAS.
- H. Zhang, W. Zhao, L. Liu, W. Wen, X. Jing and X. Wang, Microchem. J., 2023, 187, 108369 CrossRef CAS.
- A. D. Türker and M. Doğan, LWT, 2021, 138, 110775 CrossRef.
- V. Macchioni, K. Carbone, A. Cataldo, R. Fraschini and S. Bellucci, Sep. Purif. Technol., 2021, 264, 118039 CrossRef CAS.
- S. Zhu, H. Li, W. Zhu, W. Jiang, C. Wang, P. Wu, Q. Zhang and H. Li, J. Mol. Graph. Model., 2016, 68, 158–175 CrossRef CAS PubMed.
- L. A. Rodrigues, C. V. Pereira, I. C. Leonardo, N. Fernández, F. B. Gaspar, J. M. Silva, R. L. Reis, A. R. C. Duarte, A. Paiva and A. A. Matias, ACS Sustain. Chem. Eng., 2020, 8(5), 2246–2259 CrossRef CAS.
- C. Cai, X. Chen, F. Li and Z. Tan, Sep. Purif. Technol., 2021, 279, 119685 CrossRef CAS.
- J. Chen, X. Li, A. Huang, W. Deng and Y. Xiao, Food Chem., 2021, 364, 130373 CrossRef CAS PubMed.
- R. Wu, X. Wu, J. Wu, G. Liu, X. Chen, Z. Wang, Z. Dong and N. Tan, Microchem. J., 2022, 183, 107883 CrossRef CAS.
- F. Chemat, N. Rombaut, A. G. Sicaire, A. Meullemiestre, A. S. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- K. M. Jeong, M. S. Lee, M. W. Nam, J. Zhao, Y. Jin, D. K. Lee, S. W. Kwon, J. H. Jeong and J. Lee, J. Chromatogr. A, 2015, 424, 10–17 CrossRef PubMed.
- T. Bosiljkov, F. Dujmić, M. Cvjetko Bubalo, J. Hribar, R. Vidrih, M. Brnčić, E. Zlatic, I. Radojčić Redovniković and S. Jokić, Food Bioproc. Tech., 2017, 102, 195–203 CAS.
- G. A. Ojeda, M. M. Vallejos, S. C. Sgroppo, C. Sánchez-Moreno and B. De Ancos, Heliyon, 2023, 9, 16912 CrossRef PubMed.
- R. Rashid, S. M. Wani, S. Manzoor, F. A. Masoodi and M. M. Dar, Food Chem., 2023, 398, 133871 CrossRef CAS PubMed.
- K. J. Lanjekar, S. Gokhale and V. K. Rathod, Bioresour. Technol., 2022, 18, 101074 CrossRef CAS.
- X. Y. Liu, H. Ou, H. Gregersen and J. Zuo, J. Appl. Res. Med. Aromat. Plants, 2023, 32, 100444 CAS.
- A. Viñas-Ospino, M. Panić, I. Radojčić- Redovniković, J. Blesa and M. J. Esteve, Food Biosci., 2023, 53, 102570 CrossRef.
- S. M. Hong, A. H. Kamaruddin and M. M. Nadzir, Process Biochem., 2023, 132, 323–336 CrossRef CAS.
- M. Santos-Martín, J. Cubero-Cardoso, R. González-Domínguez, E. Cortés-Triviño, A. Sayago, J. Urbano and A. Fernández-Recamales, Biomass Bioenergy, 2023, 175, 106882 CrossRef.
- S. B. Bagade and M. Patil, Crit. Rev. Anal. Chem., 2021, 51, 138–149 CrossRef CAS PubMed.
- S. Pimentel-Moral, I. Borrás-Linares, J. Lozano-Sánchez, D. Arráez-Román, A. Martínez-Férez and A. Segura-Carretero, J. Pharm. Biomed. Anal., 2018, 156, 313–322 CrossRef CAS PubMed.
- J. González-Rivera, A. Mero, E. Husanu, A. Mezzetta, C. Ferrari, F. D'Andrea, E. Bramanti, C. S. Pomelli and L. Guazzelli, Green Chem., 2021, 23(24), 10101–10115 RSC.
- J. Wang, J. Wenqiang, T. Haiyuan, M. Liu, H. Yan, W. Bi and D. Da Yong Chen, ACS Sustain. Chem. Eng., 2020, 8, 12080–12088 CrossRef CAS.
- G. Grillo, V. Gunjević, K. Radošević, I. R. Redovniković and G. Cravotto, Antioxidants, 2020, 9, 1069 CrossRef CAS PubMed.
- Q. Yu, F. Wang, Y. Jian, V. M. Chernyshev, Y. Zhang, Z. Wang, Z. Yuan and X. Chen, J. Mol. Liq., 2022, 363, 119848 CrossRef CAS.
- M. Panić, V. Gunjević, G. Cravotto and I. R. Redovniković, Food Chem., 2019, 300, 125185 CrossRef PubMed.
- P. Lasunon and N. Sengkhamparn, Molecules, 2022, 27, 1157 CrossRef CAS PubMed.
- A. Meindl, A. Petutschnigg and T. Schnabel, Polymers, 2022, 14, 4319 CrossRef CAS PubMed.
- E. Tommasi, C. Giancarlo, P. Galletti, G. Grillo, M. Mazzotti, G. Sacchetti, C. Samorì, S. Tabasso, M. Tacchini and E. Tagliavini, ACS Sustain. Chem. Eng., 2017, 5, 8316–8322 CrossRef CAS.
- M. Ivanović, M. Islamčević Razboršek and M. Kolar, Plants, 2020, 9, 1428 CrossRef PubMed.
- M. Cvjetko Bubalo, N. Ćurko, M. Tomašević, K. Kovačević Ganić and I. Radojčić Redovniković, Food Chem., 2016, 200, 159–166 CrossRef CAS PubMed.
- Y. Liu, W. Chen, Q. Xia, B. Guo, Q. Wang, S. Liu, Y. Liu, J. Li and H. Yu, ChemSusChem, 2017, 10, 1692–1700 CrossRef CAS PubMed.
- A. Plaza, X. Tapia, C. Yañez, F. Vilches, O. Candia, R. Cabezas and J. Romero, Waste Biomass Valorization, 2019, 11, 6273–6284 CrossRef.
- Z. M. Jiang, W. J. Liu, Y. Li, J. Liu, H. Y. Wnag, P. Li and E. H. Liu, ACS Sustain. Chem. Eng., 2020, 8, 13777–13783 CrossRef CAS.
- M. Martins, I. Aroso, R. Craveiro, R. L. Reis, A. Paiva and A. R. Duarte, AIChE J., 2014, 60, 3701–3706 CrossRef CAS.
- H. Ren, S. Lian, X. Wang, Y. Zhang and E. Duan, J. Clean. Prod., 2018, 193, 802–810 CrossRef CAS.
- A. Krishnan, K. P. Gopinath, D. V. N. Vo, R. Malolan, V. M. Nagarajan and J. Arun, Environ. Chem. Lett., 2022, 18, 2031–2054 CrossRef.
- A. Alhadid, J. Safarov, L. Mokrushina, K. Muller and M. Minceva, Front. Chem., 2022, 10, 864663 CrossRef CAS PubMed.
- A. A. F. Zielinski, A. P. del Sanchez-Camargo, L. Benvenutti, D. M. Ferro, J. L. Dias and S. R. S. Ferreira, Trends Food Sci. Technol., 2021, 118, 850–86911 CrossRef CAS.
- F. Sanchez Bragagnolo, B. Socas-Rodríguez, J. A. Mendiola, A. Cifuentes, C. Soleo Funari and H. Ibáñez, Front. Nutr., 2022, 9, 953169 CrossRef PubMed.
- A. Shishov, I. Dubrovsky, S. Kirichenko and A. Bulatov, J. Mol. Liq., 2022, 347, 117987 CrossRef CAS.
- L. Benvenutti, A. A. F. Zielinski and S. R. S. Ferreira, Food Chem., 2022, 13, 100236 CAS.
- L. Loarce, R. Oliver-Simancas, L. Marchante, M. C. Díaz-Maroto and M. E. Alañón, LWT, 2021, 149, 111889 CrossRef CAS.
- S. Machmudah, S. D. Lestari, W. Widiyastuti, H. Kanda, S. Winardi and M. Goto, J. Supercrit. Fluids, 2018, 133, 615–624 CrossRef CAS.
- H. Wang, X. Ma, Q. Cheng, L. Wang and L. Zhang, Molecules, 2018, 23, 3233 CrossRef PubMed.
- S. Roohinejad, M. Koubaa, F. J. Barba, R. Greiner, V. Orlien and N. I. Lebovka, Trends Food Sci. Technol., 2016, 52, 98–108 CrossRef CAS.
- M. Nutrizio, G. Pataro, D. Carullo, S. Carpentieri, L. Mazza, G. Ferrari, F. Chemat, M. Banović and A. Režek Jambrak, Molecules, 2020, 25, 4131 CrossRef CAS PubMed.
- R. Bocker and E. K. Silva, Food Chem.: X, 2022, 15, 100398 CAS.
- F. Chemat, M. Abert Vian, A. S. Fabiano-Tixier, M. Nutrizio, M. R. A. Jambrak, P. E. S. Munekata, J. M. Lorenzo, F. J. Barba, A. Binello and G. Cravotto, Green Chem., 2020, 22, 2325–2353 RSC.
- X. L. Qi, X. Peng, Y. Y. Huang, L. Li, Z. F. Wei, Y. G. Zu and Y. J. Fu, Ind. Crops Prod., 2015, 70, 142–148 CrossRef CAS.
- L. Li, J. Z. Liu, M. Luo, W. Wang, Y. Y. Huang, T. Efferth, H. M. Wang and Y. J. Fu, J. Chromatogr. B, 2016, 1033–1034, 40–48 CrossRef CAS PubMed.
- P. Kou, N. Wan, L. T. Wang, H. Y. Pan, J. Jiao, C. J. Zhao, Z. G. Liu, X. Q. Wang and Y. J. Fu, J. Taiwan Inst. Chem. Eng., 2020, 116, 3–10 CrossRef CAS.
- H. N. Rajha, N. Boussetta, N. Louka, R. G. Maroun and E. Vorobiev, Food Res. Int., 2014, 65, 462–468 CrossRef CAS.
- S. El Kantar, H. N. Rajha, N. Boussetta, E. Vorobiev, R. G. Maroun and N. Louka, Food Chem., 2019, 295, 165–171 CrossRef CAS PubMed.
- E. Hernández-Corroto, N. Boussetta, M. L. Marina, M. C. García and E. Vorobiev, Innovative Food Sci. Emerging Technol., 2022, 79, 103055 CrossRef.
- Handbook of Electroporation, E. Puértolas, G. Saldaña and J. Raso and Miklavcic, D., ed. Springer, Cham, 2016 Search PubMed.
- R. Wei, L. Hu, L. Wang, P. Yan, T. Lin, N. Wang, H. Sun, B. Zheng and C. Guo, RSC Adv., 2022, 12, 25025–25034 RSC.
- S. Atanasov, B. L. Stoylov, I. Saykova and S. T. Tchaoushev, Global NEST J., 2018, 21, 30–36 Search PubMed.
- L. Benvenutti, A. A. F. Zielinski and S. R. S. Ferreira, Trends Food Sci. Technol., 2019, 90, 133–146 CrossRef CAS.
- M. W. Nam, J. Zhao, M. S. Lee, J. H. Jeong and J. Lee, Green Chem., 2015, 17, 1718–1727 RSC.
- J. Li, Z. Han, Y. Zou and B. Yu, RSC Adv., 2015, 5, 93937–93944 RSC.
- Q. Cui, J. Z. Liu, L. T. Wang, Y. F. Kang, Y. Meng, J. Jiao and Y. J. Fu, J. Clean. Prod., 2018, 184, 826–835 CrossRef CAS.
- S. S. Patil, A. Pathak and V. K. Rathod, Ultrason. Sonochem., 2021, 70, 105267 CrossRef CAS PubMed.
- C. Lo, J. Semerel, C. van den Berg, R. H. Wijffelsab and M. H. M. Eppink, RSC Adv., 2021, 11, 8142–8149 RSC.
- H. Zhang, W. Zhao, L. Liu, W. Wen, X. Jing and X. Wang, Microchem. J., 2023, 187, 108369 CrossRef CAS.
- L. Anstiss, C. C. Weber, S. Baroutian and K. Shahbaz, J. Chem. Technol. Biotechnol., 2023, 98, 1791–1802 CrossRef CAS.
- K. J. Lanjekar and V. K. Rathod, Prep. Biochem. Biotechnol., 2023, 54, 39–48 CrossRef PubMed.
- Z. Tang, Y. Xu, C. Cai and Z. Tan, J. Mol. Liq., 2023, 383, 122063 CrossRef CAS.
- D. Rente, M. Cvjetko Bubalo, M. Panić, A. Paiva, B. Caprin, I. Radojčić Redovniković and A. R. C. Duarte, J. Clean. Prod., 2022, 380, 135147 CrossRef CAS.
- K. M. Jeong, J. Ko, J. Zhao, Y. Jin, D. E. Yoo, S. Y. Han and J. Lee, J. Clean. Prod., 2017, 151, 87–95 CrossRef CAS.
- D. T. da Silva, F. A. Smaniotto, I. F. Costa, J. Baranzelli, A. Muller, S. Somacal, C. S. Monteiro, M. Vizzotto, E. Rodrigues, M. T. Barcia and T. Emanuelli, Food Chem., 2021, 364, 130370 CrossRef CAS PubMed.
- M. Panić, M. Radić Stojković, K. Kraljić, D. Škevin, I. Radojčić Redovniković, V. Gaurina Srček and K. Radošević, Food Chem., 2019, 283, 628–636 CrossRef PubMed.
- K. O. Wikene, H. V. Rukke, E. Bruzell and H. H. Tønnesen, J. Photochem. Photobiol., B, 2017, 171, 27–33 CrossRef CAS PubMed.
- Y. P. Mbous, M. Hayyan, W. F. Wong, C. Y. Looi and M. A. Hashim, Sci. Rep., 2017, 7(1), 41257 CrossRef CAS PubMed.
- K. Radošević, M. Cvjetko Bubalo, V. Gaurina Srček, D. Grgas, T. Landeka Dragičević and I. Radojčić Redovniković, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef PubMed.
- K. Radošević, J. Železnjak, M. Cvjetko Bubalo, I. Radojčić Redovniković, I. Slivac and V. Gaurina Srček, Ecotoxicol. Environ. Saf., 2016, 131, 30–36 CrossRef PubMed.
- S. Nejrotti, A. Antenucci, C. Pontremoli, L. Gontrani, N. Barbero, M. Carbone and M. Bonomo, ACS Omega, 2022, 7, 47449–47461 CrossRef CAS PubMed.
- M. D. Murugan, L. H. Tee and K. S. Oh, J. Phys.: Conf. Ser., 2021, 2120, 012005 CrossRef.
- The Cynefin Co, 2023, https://thecynefin.co/cynefin-ethics-2-of-2/, last access on 17-12-.
| This journal is © The Royal Society of Chemistry 2024 |