Polystyrene nanoplastics alter the ecotoxicological effects of diclofenac on freshwater microalgae Scenedesmus obliquus†
Abisha Christy
Christudoss
,
Natarajan
Chandrasekaran
 and
Amitava
Mukherjee
and
Amitava
Mukherjee
 *
*
Centre for Nanobiotechnology, Vellore Institute of Technology, Vellore, Tamil Nadu, India. E-mail: amit.mookerjea@gmail.com; amitav@vit.ac.in; Fax: +91-416-2243; Tel: +91 416 2202620
First published on 2nd November 2023
Abstract
Due to the escalating risk of plastic pollution, nanoplastics have attracted considerable attention in the recent past. They can co-exist and interact with other contaminants like pharmaceuticals in the aquatic environment. Therefore, it is pertinent to understand how these pollutants interact with one another in the ecosystem. The current study examined the individual and combined effects of fluorescent polystyrene nanoplastics (FNPs) and diclofenac (DCF) on Scenedesmus obliquus using a full factorial design. The toxicity of S. obliquus significantly increased in a dose-dependent manner upon exposure to pristine forms of DCF and FNPs. The major cause of individual toxicity of DCF and FNPs in S. obliquus was oxidative stress. In the combined toxicity tests when FNPs (0.01, 0.1, and 1 mg L−1) and DCF (1 mg L−1) were mixed, a synergistic effect was noted compared to the respective pristine FNPs. However, when the DCF concentration in the mixture was decreased to 0.25 mg L−1, the combined toxicity with FNPs (0.01, 0.1, and 1 mg L−1) reduced indicating an antagonistic effect. The independent action model also showed an antagonistic effect for low-dose combinations of DCF and a synergistic effect for high-dose combinations. The estimation of oxidative stress parameters, antioxidant enzyme activity, and photosynthetic pigment content in the algae further validated the cytotoxicity data. The mean hydrodynamic diameter and surface charge analyses further indicated that the colloidal stability of the FNPs in the medium was affected when they were combined with DCF. The key reason for differences in the cytotoxicity of combinations could be observed variations in the aggregation of FNPs and differential adsorption patterns of DCF on the FNPs. These factors efficiently altered cell–particle interactions in the mixture demonstrating a hormesis effect. Thus, this current study highlighted the hazardous nature of the nanoplastics and their co-exposure risks with pharmaceuticals on microalgae in freshwater environments.
Environmental significanceThe substantial risk posed by emerging pollutants like nanoplastics and pharmaceuticals to the aquatic biota is quite well established through recent studies. However, the combined toxicity of nanoplastics and their remarkable carrier effects for other co-pollutants in the aquatic environment like pharmaceuticals need to be explored. The current study focuses on the individual and combined effects of a nonsteroidal anti-inflammatory drug, diclofenac, and fluorescent polystyrene nanoplastics on freshwater microalgae Scenedesmus obliquus. This study seeks to understand how the mutual interactions between pharmaceuticals and nanoplastics would affect the primary producers (i.e., microalgae) occupying the base of the food chain. |
1. Introduction
Plastic materials have extensive applications in various sectors, including domestic goods, packaging, engineering and construction, electronics, agriculture, healthcare, pharmaceuticals, etc., due to their high durability and convenient production.1 The total production of virgin plastics is estimated to be 8300 million metric tonnes as of today.2 The environmental concentration of plastic particles in the aquatic environment was between 0.01 and 100 mg L−1.3 Through mechanical abrasion and weathering in the water bodies, bigger plastic particles can fragment into micro- and nano-sized pieces.4 Nanoplastics (NPs) (1–1000 nm) are significantly smaller than microplastics (MPs) (1–1000 μm) and possess higher surface area-to-volume ratio.5 NPs intentionally or accidentally spill into freshwater systems, which contributes to their contamination. During global plastic surveys, polystyrene (PS) has been identified as one of the most prevalent plastic polymers detected in litter.6 As primary producers microalgae play a critical role in freshwater ecosystems. Consequently, microalgae serve as a biological indicator for assessing the toxicity of emerging pollutants in the aquatic environment.7 A previous report on the toxicity of PS NPs (200 nm) in Chlorella vulgaris showed a significant decrease in the cell biomass and chlorophyll content.8 Also, it was reported that the PS NPs (60 nm) had a dose-dependent inhibitory effect on the growth rate of the freshwater microalga Microcystis aeruginosa.9 A recent report revealed that PS-MPs (between 1 and 12 μm) reduced the growth rates, as well as chlorophyll and esterase activities, and increased the production of oxygen species and related antioxidant enzyme activity in S. obliquus in a concentration dependent manner.10NPs frequently coexist in aquatic environments with other contaminants, such as heavy metals, pesticides, pharmaceuticals, and other nanoparticles.11 Among them, pharmaceuticals from diverse categories are used to treat and maintain the health of both humans and animals.12 NPs are characterized by their small size, which results in a significantly larger specific surface area as compared to MPs. This unique feature allows NPs to adsorb and accumulate other contaminants, including pharmaceuticals, present in the aquatic environment. As a result, NPs can act as carriers, promoting the interaction between pharmaceuticals and aquatic organisms. When ingested, NPs have the ability to release the sorbed pharmaceuticals, which enhances their bioavailability. This process can lead to an increase in bioaccumulation and biomagnification of pharmaceuticals in the food chain, as well as alterations in their toxic effects.13 Among various pharmaceuticals, diclofenac (DCF) is a highly utilized nonsteroidal anti-inflammatory drug (NSAID) used to treat painful and inflammatory disorders. An estimated 940 tonnes of DCF are consumed annually globally, with an average of 65% of the oral dosage of this medication being excreted with its active metabolites into the environment. The occurrence of DCF in surface water was said to be in the range of 0.1–75 μg L−1.14 Due to the availability and persistence in the environment, these NPs and DCF can be co-exposed in the freshwater environment through indiscriminate disposal of pharmaceuticals and personal care products (PPCPs) and may have a detrimental impact on the organisms.15 A recent study on the toxicity of DCF on Phaeodactylum tricornutum and Euglena sp. highlighted a significant dose-dependent growth inhibition effect.15 In another study, the growth inhibition of Parachlorella kessleri by DCF (0.1 and 0.5 mg L−1) was attributed to enhanced oxidative stress.16
These previous published studies are mostly restricted to risk evaluations of NPs and pharmaceuticals separately in microalgae. Only a handful of studies evaluated the combined toxicity of NPs and pharmaceuticals. Combined toxicity caused by the adsorption of co-contaminants on the NP surfaces may have various implications, such as additive, antagonistic, or synergistic effects.17 For example, in a combined toxicity test with ciprofloxacin (CIP) and PS NPs on cyanobacterium Synechocystis sp., PS NPs reduced the toxicity of CIP by lowering its availability in the medium through adsorption.18 In another study, the cell membrane damage due to the combined effects of tetracycline-saturated PS-SO3H and PS increased by 11.5% and 53.5% in Skeletonema costatum compared to single PS-SO3H and PS systems. On the other hand, tetracycline enhanced the surface hydrophobicity of positively charged PS-NH2 particles leading to an antagonistic effect on their joint toxicity in S. costatum.17 Another recent report on the combined toxicity of DCF and PS MPs showed that DCF toxicity on Phaeodactylum tricornutum and Euglena sp. decreased significantly when combined with larger-sized MPs.15 These results indicate that the adsorption of drugs and physicochemical characteristics (i.e., size, hydrophobicity, and surface charges) of the particles were the major factors that decided the combined toxicity.18
Despite these recent studies, it is unclear how the interactions between pharmaceuticals and NPs would impact the combined toxicity of freshwater algae. Hence, we hypothesized that the combined toxic effects of NPs and pharmaceuticals like DCF would vary significantly from their individual effects on freshwater organisms and these variations can be correlated with their physicochemical interactions in the environment. The primary objective of this research was to perform a comprehensive evaluation of the effects of DCF and FNPs, both individually and in combination, on freshwater microalgae Scenedesmus obliquus. To our knowledge, this work is the first to investigate how fluorescent polystyrene nanoplastics (FNPs) influenced DCF toxicity in freshwater alga S. obliquus. The experimental design comprised analyses of the colloidal stability of both DCF and FNPs, determination of adsorption of DCF over FNPs, and the individual and combined toxicity analyses of DCF and FNPs on the microalgae. For analyzing the combined toxicity, a complete factorial design was applied to determine the toxic potential of both DCF and FNPs in the maximum possible combinations. The oxidative stress generation in the algae was evaluated through total reactive oxygen species (ROS) analysis, malondialdehyde content analysis (MDA), and antioxidant enzyme activities. Independent action mathematical modeling (IA model) was used to examine the nature of the interactions between DCFs and FNPs. All the results were compared for individual and combined exposure to DCF and FNPs to help better understand the significance of combined effects in the freshwater medium.
2. Materials and methods
2.1 Materials required
The chemicals used for conducting the experiments in this study include diclofenac sodium (DCF), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), thiobarbituric acid (TBA), sodium carbonate (Na2CO3), and Triton X-100, which were acquired from Sigma Aldrich. Fluorescent polystyrene nanoplastics (FNPs) (100–200 nm) were procured from Corpuscular, Inc., USA. Hydrogen peroxide (H2O2, 30% w/v), acetonitrile (HPLC grade), and nitroblue tetrazolium chloride (NBT) were bought from SDFCL in Mumbai, India. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), trichloroacetic acid (TCA), hydroxylamine hydrochloride, and dimethyl sulfoxide (DMSO) were obtained from Hi-Media Pvt. Ltd in Mumbai, India.Freshwater was drawn from a lake inside the Vellore Institute of Technology (VIT) campus in Vellore, Tamil Nadu, India. A filtration process was carried out to obtain particle-free lake water. For filtration, blotting paper was used to eliminate bigger particles, followed by Whatman no. 1 filter paper (pore size: 11 μm) being used to get rid of any colloidal particles that might have remained in the lake water. In order to preserve its physicochemical properties, any further filtration procedures were omitted. Then, the water was autoclaved at 121 °C for 15 min.19 The sterilized environmental water (devoid of other contaminants) will hereafter be referred to as ‘lake water medium’. This lake water medium was used for interactions to mimic the aquatic environment which supports algal cell growth without altering the physicochemical composition of the water as explained in our previous report.20
2.2 Preparation of stock solutions
A stock suspension of FNPs (50 mg L−1) was dispersed in Milli-Q water. Using a bath sonicator, the suspension was then sonicated for 15 min to get a uniform particle distribution. DCF (100 mg L−1) was freshly mixed in Milli-Q water to prepare the stock solution.2.3 Physicochemical characterization of FNPs, DCF, and their combinations
Field-emission scanning electron microscopy (FE-SEM) was performed to determine the size and shape of the FNPs. FNP aliquots were placed on a glass slide, air-dried, and gold-coated using a sputtering technique, and then observed under an electron microscope (Thermo Fisher FEI Quanta 250 FEG). Through dynamic light scattering (DLS) combined with a zeta potential analyzer (90 Plus Particle Size Analyzer, Brookhaven Instruments Corp., USA), the mean hydrodynamic diameter (MHD) and the surface charge of FNPs were analyzed. The FNPs and DCF were mixed in lake water. Then, the aggregation patterns and surface potentials were analyzed in both pristine FNPs and DCF–FNP combinations at 0 h and 72 h.Chromatographic techniques were performed using ultra-performance liquid chromatography (UPLC system, Waters Inc., Bedford, MA, US) with an Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 150 mm) to analyze the available concentration of DCF in the sterilized environmental water medium at 0 h and 72 h (i.e., before and after interaction under abiotic conditions without algal cells).21 Reverse-phase isocratic elution was used for the separation, and the mobile phase was composed of 0.05 M acetate buffer (pH 2.5) and acetonitrile (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v), which was run at 1 mL min−1 flow rate and 20 μL injection volume. The 2D channel was set to record at 254 nm while the photodiode array (PDA) detector was configured to gather data from 210 to 280 nm. The sample temperature was held at 10 °C, while the column temperature was maintained at 50 °C. From the stock solution of 100 mg L−1 DCF, working standard solutions were prepared in the range of 0.2–2 mg L−1. This wide concentration range was designed to encompass different DCF doses used in the present study. Calibration curves were obtained by plotting the peak area against standard DCF concentration, and regression equations were computed. For analysis, three doses of DCF (0.25, 0.5, and 1 mg L−1) alone and its combination with three different doses of FNPs (0.01, 0.1, and 1 mg L−1) were dispersed in a sterilized environmental water medium. Then, the FNPs were removed using a 0.1 μM syringe filter immediately (0 h) and after 72 h, and finally, the residual DCF solution was taken for analysis. From the known standards, the residual concentrations of all the combinations were determined. Following that, the residual concentration was subtracted from the actual concentration of DCF to calculate the adsorption percentage for each group.
50, v/v), which was run at 1 mL min−1 flow rate and 20 μL injection volume. The 2D channel was set to record at 254 nm while the photodiode array (PDA) detector was configured to gather data from 210 to 280 nm. The sample temperature was held at 10 °C, while the column temperature was maintained at 50 °C. From the stock solution of 100 mg L−1 DCF, working standard solutions were prepared in the range of 0.2–2 mg L−1. This wide concentration range was designed to encompass different DCF doses used in the present study. Calibration curves were obtained by plotting the peak area against standard DCF concentration, and regression equations were computed. For analysis, three doses of DCF (0.25, 0.5, and 1 mg L−1) alone and its combination with three different doses of FNPs (0.01, 0.1, and 1 mg L−1) were dispersed in a sterilized environmental water medium. Then, the FNPs were removed using a 0.1 μM syringe filter immediately (0 h) and after 72 h, and finally, the residual DCF solution was taken for analysis. From the known standards, the residual concentrations of all the combinations were determined. Following that, the residual concentration was subtracted from the actual concentration of DCF to calculate the adsorption percentage for each group.
2.4 Test organism and experimental setup
A green freshwater microalga, Scenedesmus obliquus, was used as an environmentally abundant and easily grown model in this study. This alga was isolated from the VIT lake, Vellore (12°58′ 10′′ N, 79°9′ 37′′ E) and characterized as previously described.22 The isolated culture was maintained in the BG-11 medium at 23 ± 2 °C under a Philips fluorescent lamp (3000 lux) with a photoperiod of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h of light and dark. For the experiment, a 15-day culture (at the log phase) was used for all the toxicity endpoints, and the same was sub-cultured for maintaining the culture. To perform the experiments, OECD guidelines were followed.23
8 h of light and dark. For the experiment, a 15-day culture (at the log phase) was used for all the toxicity endpoints, and the same was sub-cultured for maintaining the culture. To perform the experiments, OECD guidelines were followed.23
2.5 Experimental setup for toxicity assessment
During the exponential phase, algae cells were collected and centrifuged for 10 min at 7000 rpm (4 °C). The collected pellet was cleansed twice with a lake water medium. Algal cells with 0.5 optical density (OD) containing 4 × 103 cells mL−1 were incubated in a glass beaker (50 mL) with various doses of DCF (0.25, 0.5, and 1 mg L−1) and FNPs (0.01, 0.1, and 1 mg L−1) and kept under 3000 lux light for 72 hours at a stable temperature of 23 °C.24 The DCF and FNP concentrations were selected based on the measured EC50 values (0.8 mg L−1 and 1 mg L−1, respectively) as detailed in the ESI.† One concentration above the EC50 value and two concentrations below/near the EC50 value were selected. Each DCF concentration was combined with all FNP concentrations using the full factorial approach (i.e., a total of 9 combinations).25 As a negative control, the algal suspensions without DCF and FNP treatment were used. To keep the cells from adhering to the beaker's bottom or walls, intermittent agitation of cells was provided at equal time intervals. The tests were performed in triplicate (n = 3) to assess the statistical significance of the results.2.6 Cell viability assessment
The detailed procedure for the assessment of cell viability19 of the algal cells interacting with both the pristine form and combinations of DCF and FNPs is explained in the ESI† (Methods section).2.7 Assessment of oxidative stress
2.8 Antioxidant enzyme assessment
As a scavenging mechanism, antioxidant enzymes can be generated inside cells during oxidative stress. The methods to assess the antioxidant enzymes (superoxide dismutase27 and catalase19) are mentioned in the ESI† (Methods section).2.9 Photosynthetic pigments assessment
The content of photosynthetic pigments (chlorophyll A, chlorophyll B, and carotenoids) in the algal cells under treatment was measured spectrophotometrically.28 The detailed procedure is provided in the ESI† (Methods section).2.10 Statistical analysis
The independent action (IA) model was used to examine the influence of FNPs on DCF toxicity because DCF and FNPs may lead to different outcomes on targets.29 We identified the nature of the interaction that takes place between DCF and FNPs. Based on the toxicity (%) brought about by pristine DCF and FNPs, eqn (1) was utilized to determine the predicted toxicity (CExp) of the mixture.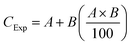 | (1) |
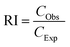 | (2) |
According to Zhu et al.,30 an interaction is identified to be additive when RI = 1, synergistic when RI > 1, and antagonistic when RI < 1 between two pollutants. A statistical study was done to compare the measured and predicted toxicity using the two-way ANOVA method. Regardless of the RI value determined, the nature of the interaction between the pollutants is considered for all the combinations.
In this work, each experiment was run in triplicate (n = 3). The information is displayed as mean ± SD (standard deviation). A two-way ANOVA test with a Bonferroni post-test was employed in Graph Pad Prism 5 to examine the statistical significance of various test samples compared to controls. At the 95% confidence level, data with a p-value of 0.05 or lower were estimated to be statistically significant.
3. Results
3.1 Physical and chemical characterization of FNPs, DCF, and their combinations
The FE-SEM image of FNPs exhibited a spherical morphology of particles with an average diameter of 120 nm (Fig. 1). The colloidal stability of FNPs was investigated using the mean hydrodynamic diameter (MHD) and zeta potential analyses individually and in the presence of DCF in suspensions. The MHD and zeta potential of FNPs in a Milli-Q dispersion were found to be 118.39 ± 3.26 nm and −34.32 ± 1.08 mV, respectively. The MHD values of pristine FNPs and their combinations with DCF dispersed in lake water medium for 0 h and 72 h are presented in Table 1. The MHD of the three doses (0.01, 0.1, and 1 mg L−1) of pristine FNPs at 0 h (107.01 ± 4.85, 115.22 ± 6.87, and 132.96 ± 10.04 nm, respectively) increased significantly (p < 0.001) to (138.60 ± 12.98, 446.78 ± 31.99, and 633.06 ± 41.92 nm, respectively) within 72 h in the lake water medium.| Concentrations of the FNPs (mg L−1) | Concentrations of DCF (mg L−1) | MHD at 0 h (nm) | MHD at 72 h (nm) |
|---|---|---|---|
| 0.01 | 0 | 107.01 ± 4.85 | 138.60 ± 12.98 |
| 0.25 | 127.12 ± 2.64 | 534.07 ± 20.61 | |
| 0.5 | 174.08 ± 5.71 | 563.96 ± 31.70 | |
| 1 | 202.82 ± 11.97 | 589.36 ± 34.60 | |
| 0.1 | 0 | 115.22 ± 6.87 | 446.78 ± 31.99 |
| 0.25 | 185.15 ± 19.41 | 639.5 ± 22.88 | |
| 0.5 | 242.68 ± 13.87 | 673.90 ± 10.94 | |
| 1 | 314.92 ± 12.68 | 725.84 ± 13.81 | |
| 1 | 0 | 132.96 ± 10.04 | 633.06 ± 41.92 |
| 0.25 | 220.85 ± 6.90 | 749.99 ± 44.18 | |
| 0.5 | 255.75 ± 13.75 | 759.59 ± 28.30 | |
| 1 | 366.11 ± 68.89 | 864.76 ± 41.33 |
In the same way, the MHD values of the combinations displayed a significant increment (p < 0.001) compared to their pristine FNP concentrations in a dose and time-dependent manner. In lower-concentration combinations of FNPs (0.01 mg L−1) with three DCF concentrations (0.25, 0.5, and 1 mg L−1), the MHD was enhanced by 42, 32, and 29%, respectively at 72 h. Meanwhile, in higher-concentration combinations (1 mg L−1), the size difference was less (33, 29, and 23%, respectively). The overall MHD analysis in combinations showed that the size increase in lower-concentration combinations was higher than in the higher-concentration combinations. The alterations in the zeta potential values of pristine FNPs and their combinations with DCF dispersed in lake water for 0 h and 72 h are illustrated in Table 2. The surface potentials showed a minor decline for all the concentrations at 72 h when compared to 0 h values.
| Concentrations of the FNPs (mg L−1) | Concentrations of DCF (mg L−1) | Zeta potential at 0 h (mV) | Zeta potential at 72 h (mV) |
|---|---|---|---|
| 0.01 | 0 | −13.38 ± 1.36 | −11.26 ± 1.33 |
| 0.25 | −15.84 ± 1.01 | −10.66 ± 0.32 | |
| 0.5 | −15.97 ± 0.20 | −11.93 ± 0.50 | |
| 1 | −16.46 ± 0.76 | −12.1 ± 1.72 | |
| 0.1 | 0 | −15.3 ± 2.63 | −13.11 ± 1.94 |
| 0.25 | −16.54 ± 1.45 | −11.68 ± 0.44 | |
| 0.5 | −16.7 ± 1.14 | −13.02 ± 1.59 | |
| 1 | −16.86 ± 0.60 | −12.72 ± 1.40 | |
| 1 | 0 | −15.49 ± 1.5 | −14.01 ± 0.70 |
| 0.25 | −15.6 ± 1.49 | −11.29 ± 0.54 | |
| 0.5 | −15.68 ± 0.76 | −11.54 ± 2.04 | |
| 1 | −17.19 ± 0.95 | −11.86 ± 0.86 |
Table 3 depicts the available concentrations of DCF and percentage adsorption on FNPs at 0 h and 72 h in the lake water medium. Considering the available concentrations of DCF in the medium, the percentage adsorption of DCF on FNPs was determined for all the combinations. After 72 h of interactions, the percentage adsorption was found to be maximum for the combinations of the lowest concentration of DCF (0.25 mg L−1) with the FNPs (0.01, 0.1, and 1 mg L−1) at 48, 52, and 60%, respectively. In the case of higher concentrations of DCF (1 mg L−1), the percentage adsorption was however reduced (up to 20, 41, and 44%, respectively). The results demonstrated that the adsorption decreased as the concentration of DCF increased in a dose-dependent manner.
| Added concentration of the FNPs (mg L−1) | Added concentration of DCF (mg L−1) | 0 h | 72 h | ||||
|---|---|---|---|---|---|---|---|
| Available concentration of DCF (mg L−1) | Accuracy (%) | Percentage adsorption of DCF on FNPs (%) | Available concentration of DCF (mg L−1) | Accuracy (%) | Percentage adsorption of DCF on FNPs (%) | ||
| 0 | 0.25 | 0.28 | 112 | 0 | 0.24 | 96 | 4 |
| 0.01 | 0.25 | 0.24 | 96 | 4 | 0.13 | 52 | 48 |
| 0.1 | 0.25 | 0.25 | 100 | 0 | 0.12 | 48 | 52 |
| 1 | 0.25 | 0.23 | 92 | 8 | 0.1 | 40 | 60 |
| 0 | 0.5 | 0.47 | 94 | 6 | 0.46 | 92 | 8 |
| 0.01 | 0.5 | 0.43 | 86 | 14 | 0.38 | 76 | 24 |
| 0.1 | 0.5 | 0.4 | 80 | 20 | 0.29 | 58 | 42 |
| 1 | 0.5 | 0.35 | 70 | 30 | 0.27 | 54 | 46 |
| 0 | 1 | 1.04 | 104 | 0 | 0.98 | 98 | 2 |
| 0.01 | 1 | 0.88 | 88 | 12 | 0.8 | 80 | 20 |
| 0.1 | 1 | 0.79 | 79 | 21 | 0.59 | 59 | 41 |
| 1 | 1 | 0.72 | 72 | 28 | 0.56 | 56 | 44 |
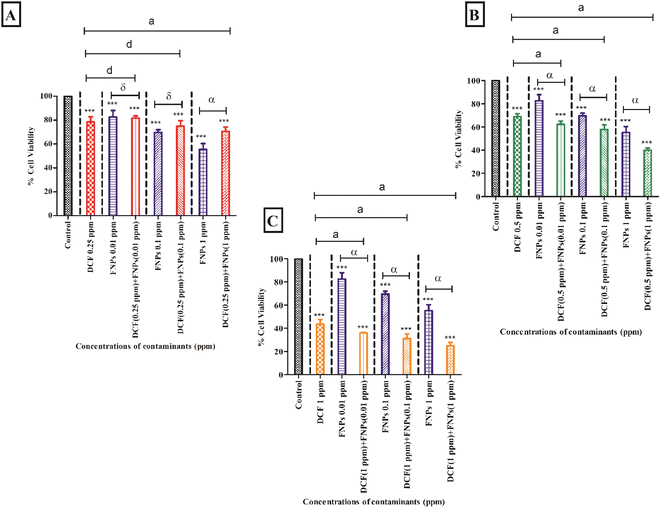
3.2 Effect of FNPs on the cell viability of DCF
Fig. 2 shows the individual and combined effects of the target contaminants (DCF and FNPs) on the viability of the algal cells.Fig. 2A demonstrates the viability of algal cells when exposed to 0.25 mg L−1 DCF and its combinations with three doses of FNPs (0.01 mg L−1, 0.1 mg L−1, and 1 mg L−1, respectively). With respect to the control, there was a significant reduction of 22% in cell viability (p < 0.001) at 0.25 mg L−1 concentration of DCF. The pristine FNPs (0.01 mg L−1, 0.1 mg L−1, and 1 mg L−1) demonstrated a significant (p < 0.001) dose-dependent decrease in viability when compared to the control.
A concentration-dependent reduction in cell viability was observed for all three combinations of 0.25 mg L−1 DCF with FNPs compared to the control. When comparing the combined effects with the effects of different concentrations of pristine FNPs, interestingly a hormesis effect was observed. An enhancement in the viability was observed for the combinations with a low concentration of DCF (0.25 mg L−1 DCF + 0.01 mg L−1, 0.1 mg L−1, and 1 mg L−1 FNPs, respectively) in a dose-dependent manner where the 0.25 mg L−1 DCF + 1 mg L−1 FNPs combination showed a significant (p < 0.001) increase in cell viability compared to the pristine FNPs.
Fig. 2B shows the cell viability at the concentration of 0.5 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant reduction (32%) in cell viability (p < 0.001) at 0.5 mg L−1 DCF. A concentration-dependent decline in viability for all three combinations of 0.5 mg L−1 DCF with the FNPs was observed compared to the control. All the combinations resulted in a highly significant (p < 0.001) difference in viability compared with the control. Interestingly, when compared to the effects of pristine FNPs, all three combinations showed a significant concentration-wise decline (p < 0.001) in cell viability.
Fig. 2C depicts the decrease in cell viability at 1 mg L−1 concentration of DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant reduction (57%) in cell viability (p < 0.001) at 1 mg L−1 DCF. A concentration-dependent reduction was observed in the combinations of 1 mg L−1 DCF with the FNPs where all combinations resulted in a highly significant decrease (p < 0.001) compared with the control. When compared to the effects of pristine FNPs, all the combinations showed a significant concentration-wise reduction (p < 0.001) in cell viability.
Table 4 demonstrates the IA modelling carried out to provide the nature of the interaction of DCF while interacting with FNPs. When the concentration of DCF increased, the ratio of inhibition (RI) also increased. All the combinations of 0.25 and 0.5 mg L−1 DCF with 0.01 and 0.1 mg L−1 FNPs showed an antagonistic effect and the 1 mg L−1 FNPs with 0.5 mg L−1 DCF combination showed an additive effect whereas the other higher concentration combinations of DCF (1 mg L−1) and FNPs showed a synergistic effect.
| Concentrations of DCF (mg L−1) | Concentrations of the FNPs (mg L−1) | Expected toxicity (%) | Observed toxicity (%) | Ratio of inhibition (RI) | p-Value | Nature of interaction |
|---|---|---|---|---|---|---|
| 0.25 | 0.01 | 35.24 | 18.43 | 0.52 | <0.001 | Antagonistic |
| 0.1 | 45.43 | 25.14 | 0.55 | <0.001 | Antagonistic | |
| 1 | 56.64 | 29.50 | 0.52 | <0.001 | Antagonistic | |
| 0.5 | 0.01 | 43.17 | 37.73 | 0.87 | <0.001 | Antagonistic |
| 0.1 | 52.11 | 42.07 | 0.81 | <0.001 | Antagonistic | |
| 1 | 61.95 | 59.95 | 0.97 | >0.05 | Additive | |
| 1 | 0.01 | 63.98 | 70.97 | 1.12 | >0.01 | Synergistic |
| 0.1 | 69.65 | 76.69 | 1.13 | >0.01 | Synergistic | |
| 1 | 75.88 | 83.91 | 1.10 | >0.01 | Synergistic |
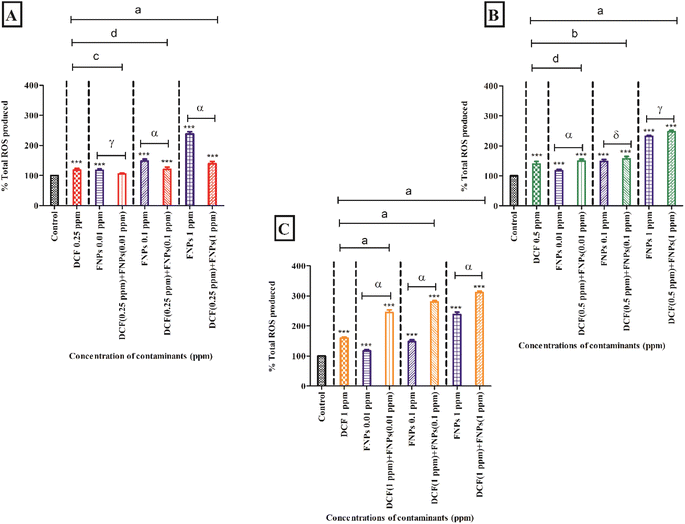
3.3 Oxidative stress assessment
Fig. 3A demonstrates the ROS production when the algae were exposed to 0.25 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant increase (p < 0.001) in ROS production by 18% at 0.25 mg L−1 concentration of DCF. There was a dose-dependent increase (p < 0.001) in the observed ROS when the algal cells interacted with pristine FNPs at all three concentrations compared to the control. A concentration-wise increase in radical production was observed for all three combinations of 0.25 mg L−1 DCF with FNPs compared to the control. Among the three treatments, except 0.25 mg L−1 DCF + 0.01 mg L−1 FNPs all the other combinations showed a highly significant (p < 0.001) increase in ROS production compared with the control. When comparing the combined effects with the effects of pristine FNPs, interestingly, a hormesis effect was observed as found in cell viability. Interestingly, there was a dose-dependent decline in the low-concentration combinations of DCF (0.25 mg L−1 DCF + 0.01 mg L−1, 0.1 mg L−1, and 1 mg L−1 FNPs, respectively) where 0.25 mg L−1 DCF + 0.1 and 1 mg L−1 FNPs combinations showed a highly significant (p < 0.001) decline.
Fig. 3B shows the ROS production at the concentration of 0.5 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant increase (40%) in ROS production (p < 0.001) at 0.5 mg L−1 DCF. There was a concentration-dependent increase in radical generation for all three combinations of 0.5 mg L−1 DCF with the FNPs compared to the control. All the combinations resulted in a highly significant (p < 0.001) increase in ROS production with respect to the control. Interestingly, when compared to the effects of pristine FNPs, all three combinations showed a concentration-wise increase in ROS activity where 0.5 mg L−1 DCF + 0.01 mg L−1 FNPs combination showed a highly significant (p < 0.001) increase.
Fig. 3C depicts the increase in the production of ROS at 1 mg L−1 concentration of DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant enhancement (60%) in ROS activity (p < 0.001) at 1 mg L−1 DCF. There was a concentration-dependent increase in ROS generation in the combinations of 1 mg L−1 DCF with FNPs (p < 0.001) with respect to the control. When compared to the effects of pristine FNPs, all three combinations showed a significant increase (p < 0.001) in ROS generation.
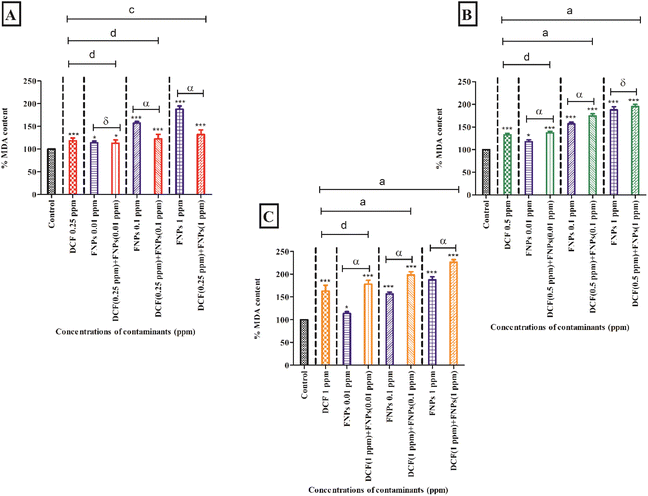
Fig. 4A demonstrates the MDA content after treating the algal cells with 0.25 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant increase (18%) in MDA level (p < 0.001) at the concentration of 0.25 mg L−1 DCF. The pristine FNPs showed a dose-dependent increase in MDA level where 0.1 and 1 mg L−1 concentrations showed high significance (p < 0.001) when compared to the control. A dose-dependent increase in MDA level was observed for all the combinations of 0.5 mg L−1 DCF with FNPs compared with the control. Among the three treatments, except 0.25 mg L−1 DCF + 0.01 mg L−1 FNPs all the other combinations showed a highly significant (p < 0.001) increase in MDA level with respect to the control. When comparing the combined effects with the pristine FNP effects, interestingly there was a decline in MDA content as observed in the ROS profile. A decline in the MDA profile was observed in the combinations with a low concentration of DCF (0.25 mg L−1 DCF + 0.01 mg L−1, 0.1 mg L−1, and 1 mg L−1 FNPs, respectively) in a dose-dependent manner where 0.25 mg L−1 DCF + 0.1 and 1 mg L−1 FNPs combinations showed a highly significant (p < 0.001) decline.
Fig. 4B depicts the MDA level at the concentration of 0.5 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant increase (32%) in MDA level (p < 0.001) at 0.5 mg L−1 DCF. There was a concentration-dependent enhancement in MDA content for all three combinations of 0.5 mg L−1 DCF with the FNPs. All the combinations except 0.5 mg L−1 DCF + 1 mg L−1 FNPs resulted in a highly significant (p < 0.001) difference in MDA level compared with the control. Interestingly, compared to their respective pristine FNPs, all three combinations showed a concentration-wise increase in MDA level where 0.5 mg L−1 DCF + 0.01 and 0.1 mg L−1 FNPs combinations showed a highly significant (p < 0.001) increase.
Fig. 4C depicts the increase in the MDA level at 1 mg L−1 concentration of DCF and its combinations with three concentrations of FNPs. Compared to the control, there was a significant increase (63%) in MDA content (p < 0.001) at 1 mg L−1 DCF. A concentration-dependent increase in the combinations of 1 mg L−1 DCF with FNPs was observed in the MDA level where all combinations resulted in a highly significant increase (p < 0.001) with respect to the control. When compared to the effects of pristine FNPs, all the combinations showed a significant concentration-dependent increase (p < 0.001) in the MDA levels.
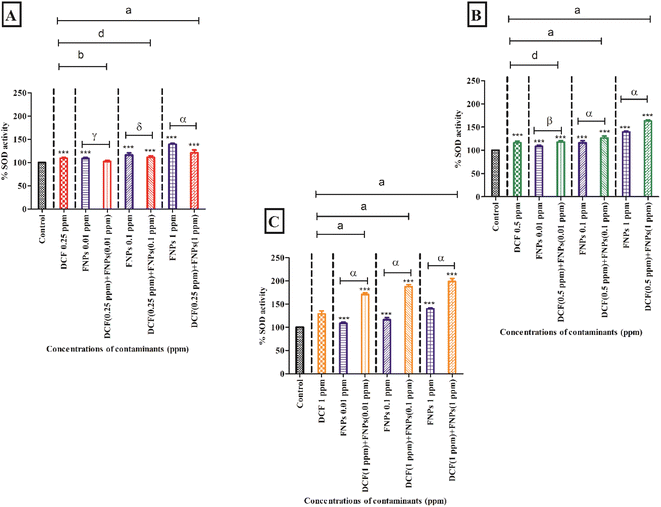
3.4 Antioxidant enzyme activity assessment
Fig. 5 and 6 present the alterations in the antioxidant enzyme activities (superoxide dismutase and catalase) when algae are treated with individual and combined target pollutants (DCF and FNPs).Fig. 5B shows the SOD activity at the concentration of 0.5 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant enhancement (16%) in SOD activity (p < 0.001) at 0.5 mg L−1 DCF. There was a concentration-dependent enhancement in SOD activity for all three combinations of 0.5 mg L−1 DCF with the FNPs. All the combinations resulted in a highly significant (p < 0.001) increase compared with the control. Importantly, when compared to the effects of pristine FNPs, all three combinations showed a concentration-wise increase in SOD activity where 0.5 mg L−1 DCF + 0.1 and 1 mg L−1 FNPs combinations showed a highly significant (p < 0.001) increase.
Fig. 5C depicts the increase in the activity of SOD at 1 mg L−1 concentration of DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant enhancement (28%) in SOD activity (p < 0.001) at 1 mg L−1 DCF. There was a concentration-wise increase in the combinations of 1 mg L−1 DCF with FNPs where all combinations resulted in a highly significant difference (p < 0.001) with respect to the control. When compared to the effects of pristine FNPs, all the combinations showed a significant concentration-wise increase (p < 0.001) in the SOD activity.
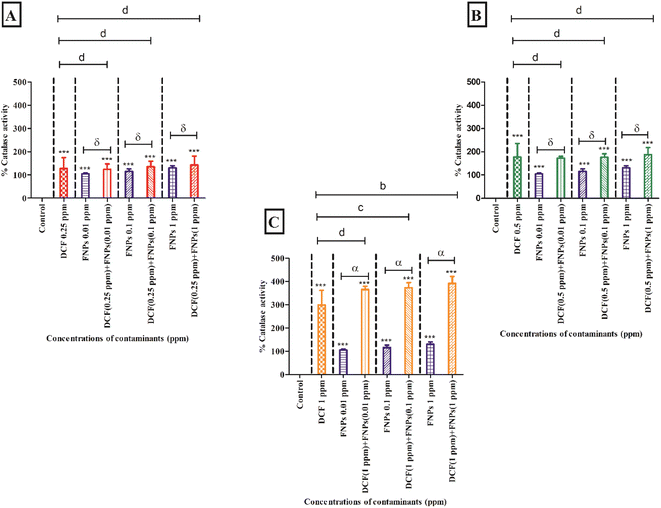
Fig. 6B shows the CAT activity at the concentration of 0.5 mg L−1 DCF and its combinations with three concentrations of FNPs. In comparison to the control, there was a significant increase in CAT activity (p < 0.001) at 0.5 mg L−1 DCF. CAT activity showed a concentration-dependent enhancement for all three combinations of 0.5 mg L−1 DCF with FNPs. All the combinations except 0.5 mg L−1 DCF + 0.01 mg L−1 FNPs showed a highly significant (p < 0.001) increase with respect to the control. Importantly, compared to the effects of pristine FNPs, all combinations showed a concentration-wise increase in CAT activity.
Fig. 6C depicts the increase in the activity of CAT at 1 mg L−1 concentration of DCF and its combinations with different doses of FNPs. Compared to the control, CAT activity was significantly enhanced (p < 0.001) at 1 mg L−1 DCF. There was a concentration-wise increase in the combinations of 1 mg L−1 DCF with FNPs where all combinations demonstrated high significance (p < 0.001) compared with the control. When compared to the pristine FNP effects, a significant dose-wise increase (p < 0.001) in the CAT activity was noticed for all the combinations.
3.5 Effects on photosynthetic pigment content
Fig. S1–S3 (ESI†) demonstrate the alterations in CHL A and B and CAR contents when the algal cells are treated with individual and combined target pollutants.The decrease in the CHL A content when the algal cells were exposed to 0.25, 0.5 and 1 mg L−1 DCF along with three doses of FNPs is illustrated in Fig. S1(A–C), respectively (ESI†). The individual effect of DCF exhibited a significant reduction (p < 0.001) of CHL A content compared to the control. Similarly, the pristine FNPs demonstrated a dose-dependent decrease in CHL A content where 0.1 and 1 mg L−1 concentrations showed a significant reduction (p < 0.001) with respect to the control. A concentration-dependent reduction was noticed for 0.5 and 1 mg L−1 combinations of DCF with FNPs, all showing a highly significant (p < 0.001) difference compared with the control. When comparing the combined effects with the individual effects of the respective concentrations of FNPs, the combinations of 0.5 and 1 mg L−1 DCF showed a concentration-wise reduction (p < 0.001) in CHL A content.
The decrease in the CHL B content when the cells interacted with 0.25, 0.5, and 1 mg L−1 DCF in a mixture with three doses of FNPs is illustrated in Fig. S2(A–C) respectively (ESI†). It is noted that 0.5 and 1 mg L−1 doses of DCF resulted in a highly significant (p < 0.001) decrease in CHL B compared to the control. Like the CHL A content, the pristine FNPs demonstrated a dose-dependent reduction in CHL B content where 1 mg L−1 concentration showed a highly significant reduction (p < 0.001) with respect to the control. A concentration-wise reduction was observed for 0.5 and 1 mg L−1 combinations of DCF with FNPs (p < 0.001) with respect to the control. When comparing the combined effects with their respective FNP effects, the combinations containing 1 mg L−1 DCF with FNPs showed a significant reduction (p < 0.001) in CHL B content.
The reduction in the CAR content on exposing the cells to 0.25, 0.5, and 1 mg L−1 DCF combined with three doses of FNPs is illustrated in Fig. S3(A–C) respectively (ESI†). A dose-dependent decline in CAR content was observed in the three doses of DCF and it showed a highly significant (p < 0.001) decrease compared to the control. Similarly, the pristine FNPs exhibited a dose-wise reduction in CAR content where 0.1 and 1 mg L−1 concentrations showed high significance (p < 0.001) compared with the control. With respect to the control, a significant concentration-wise reduction (p < 0.001) was observed for 0.5 and 1 mg L−1 combinations of DCF with FNPs. When comparing the combined effects with the pristine FNP effects, the combinations of 0.5 and 1 mg L−1 DCF + 0.01 and 0.1 mg L−1 FNPs respectively showed a significant reduction (p < 0.001) in CAR content.
4. Discussion
The FE-SEM image of FNPs (Fig. 1) revealed that the mean size of the particles was nearly 120 nm indicating that they would have a high specific surface area and sorption capacity.31 The observed increase in the MHDs of the pristine FNPs confirmed the aggregation of particles. The dose-dependent aggregation was possibly facilitated by the small size of FNPs. Generally, smaller-sized particles are more amenable to aggregation.32 Also, at higher concentrations of particles, the aggregation was considerably enhanced. Aggregation of the particles would reduce their specific surface area and diminish their sorption capacity. This would in effect decrease their bioavailability to the organisms being exposed.33 Similarly, the zeta potential values supported the aggregation of FNPs in the combinations with a slight decline in the values at 72 h (Table 2). Reportedly, the sorption of DCF through hydrogen bonding might reduce the electrostatic repulsion between the NPs.34 This reduction in the electrostatic barrier may increase cell–particle interactions, which would enhance the toxicity of combinations.17NPs can interact well with environmental contaminants due to their increased surface area and availability of more sorption sites.35 Through hydrophobic and π–π interactions, they can bind to organic pollutants.31 Being hydrophobic, DCF would mainly interact through hydrophobic interactions.36 Previous studies suggest that DCF has a high sorption capacity towards NPs through chemisorption mode among other NSAIDs due to the presence of an amine group and aromatic rings in its structure. The degree of chemisorption would depend on the extent of surface area available on the adsorbent. Hence, an increase in the specific surface area of the adsorbent leads to a corresponding increase in the sorption capacity.31 The increased adsorption observed for the combinations with lower concentrations of DCF was possibly due to the availability of numerous sorption sites. The comparative reduction in the adsorption at higher concentrations of DCF indicated the possibility of saturation once the available sorption sites on FNPs were already occupied. These data are consistent with a previous study on the adsorption of bisphenol-A and ciprofloxacin on the MPs, suggesting that the adsorption capacity of MPs reduced once it reached saturation point.37 Also, the reduction in adsorption was consistent with the concentration- and time-dependent aggregation of the FNPs in the medium as observed in MHD analysis. This aggregation may reduce the sorption capacity of FNPs at higher concentrations.33
With increasing concentrations of both DCF and FNPs in their pristine forms, the cell viability of S. obliquus decreased in a dose-dependent manner. Similar dose-dependent growth inhibition was observed in Phaeodactylum tricornutum and Euglena sp. when they were exposed to DCF (up to 10 mg L−1 and 50 mg L−1, respectively) for 1–2 days.15 The growth inhibition by the PS NPs on microalgal cells has been reported by various researchers. The PS particles showed size and concentration-dependent toxic effects in various algal cells like P. tricornutum, Euglena sp., Chlorella vulgaris, S. obliquus, etc.15,24,38
The combined toxicity results revealed a concentration-wise decrease in cell viability. Interestingly, the hormesis effect was observed when comparing the cell viability of pristine FNPs and their respective combinations with DCF. For the mixtures with low concentrations of DCF, the combinations showed enhanced cell viability compared to their pristine counterparts. However, with increasing concentrations of DCF, the cell viability was again reduced significantly. Previous research suggests that the principal antagonistic mechanism of NPs with their co-pollutants was the adsorption of organic pollutants by NPs.18 As per the literature, NSAIDs like diclofenac, diazepam, paracetamol, ibuprofen, etc. can have an inductive effect on the growth of Chlorella sorokiniana, Spirulina platensis, and diatom Navicula sp.39,40 This biphasic dose response has been noted from primary producers like S. obliquus to higher organisms when exposed to NPs.41 Similar to our study, the combined effect of PS particles and DBP on Chlorella pyrenoidosa was antagonistic at lower doses of DBP while being synergistic at higher doses.42 According to Zhang et al., some pharmaceuticals at lower doses can serve as sources of carbon and phosphorus for the growth of algae and may promote the activity of certain enzymes.43 At higher doses, the potential increase in toxicity of the interacting chemicals is possibly linked to the uptake of residual chemicals in the medium and carrier effects of PS NPs. This would increase the interacting components' bioavailability and accumulation, increasing their potential for toxicity.44
ROS serve as secondary messengers in a variety of cellular activities against environmental stressors.45 These reactive species could severely harm the cell membrane by peroxidation of polyunsaturated fatty acids and proteins.16 This damage is further accelerated by an increase in membrane permeability.46 Upon interaction, both DCF and FNPs individually showed a concentration-dependent rise in total ROS and MDA production. This is consistent with the previous results where DCF alone showed a concentration-wise increase in ROS and MDA levels in Chlorella sorokiniana.39 Similar to our study, pristine NPs showed an increase in ROS and MDA content on S. obliquus.24 In the combinations with the higher DCF concentration, an enhancement in ROS and MDA generation was noticed. This is in line with the mixture toxicity of PS particles and tetracycline on S. costatum.17 Meanwhile, at lower DCF concentrations, the oxidative stress decreased. This may be related to the higher adsorption of DCF on particles that hinders the cell–particle interactions thereby reducing the membrane damage and ROS release.17
As a defence mechanism, microalgae can produce antioxidative enzymes like SOD and CAT to combat the damaging effects of excessive ROS caused by the contaminants.12 Both the individual and combined effects of DCF and FNPs on SOD and CAT production followed a similar trend as noticed in oxidative stress. Similar to our study, a report on the growth inhibition of Parachlorella kessleri by DCF (0.1 and 0.5 mg L−1) stated that the cell damage due to the increased oxidative stress spontaneously produces enhanced SOD and CAT enzymes.16 Also, an earlier study found that SOD and CAT activity in S. obliquus considerably increased when exposed to NPs, supporting the results which supported the data that the SOD and CAT production increased when it interacted with pristine FNPs.19 When observing the combined effects, both SOD and CAT increased extensively in higher-concentration combinations in a dose-dependent way. This is in line with the increased SOD and CAT levels when co-exposed with PS particles and roxithromycin at higher doses in Daphnia magna.47
Algal growth inhibition is typically associated with changes in the chlorophyll pigments, essential for photosynthesis. These pigments also play crucial roles in light gathering, energy transfer, and light energy conversion. The pigmentation systems are considered to be possible defence mechanisms against multiple abiotic stressors.19 In the current study, both DCF and FNPs individually caused a dose-dependent decline in the CHL A and B and CAR contents in a dose-dependent manner. Previous findings demonstrated that NSAID exposure had an impact on the photosynthetic pigment level, and cellular ultrastructure investigations of S. obliquus indicated the altered chlorophyll structure and destroyed thylakoids.48 A similar reduction in the photosynthetic pigments was observed in S. obliquus due to the exposure to PS NPs19 which correlates with the current study. When compared to the pristine FNPs, the combinations showed a hormesis effect as observed in previous parameters. The combined toxicity observed in the current study was supported by a previous report which showed that MP-loaded azithromycin and clarithromycin significantly decreased the chlorophyll pigments of cyanobacterium Anabaena sp. PCC7120.49
5. Conclusion
The findings suggest that while the FNPs enhanced the toxic action of DCF in the higher-concentration combinations, in lower-concentration combinations they caused a decline in toxicity on S. obliquus. This dichotomous trend was correlated with the results of oxidative stress generation and the levels of photosynthetic pigment contents in the treated cells. The variation in the toxic effects could be possibly explained based on the differential adsorption of DCF on the FNPs in the mixtures. Thus, the toxicity of DCF was greatly affected by the physicochemical characteristics of the FNPs. The key results from this study provide insights into the combined ecotoxicity of NPs and pharmaceuticals and highlight the potential implications of co-pollutants at ecologically relevant concentrations. Further studies can be done for various combinations of co-pollutants with NPs to assess how the toxicity potential of both the toxicants may change in the combinations. The eco-toxicity studies with the combinations of pollutants may also highlight the variations in the modes of action of the mixtures compared to the pristine materials. Combined toxicity tests are also encouraged to be taken up with higher-level model organisms to understand the environmental risks posed by the plastics in the presence of various co-pollutants in the aquatic food chain.Author contributions
Abisha Christy Christudoss: investigation, methodology, visualization, formal analysis, writing – original draft. Natarajan Chandrasekaran: formal analysis, resources. Amitava Mukherjee: conceptualization, methodology, supervision, project administration, writing – review and editing.Conflicts of interest
The authors do not have any conflict of interest to declare.Acknowledgements
The authors would like to thank Vellore Institute of Technology (VIT) for assistance with the Field Emission-Scanning Electron Microscopy (FE-SEM) and Ultra-Performance Liquid Chromatography (UPLC) facility used in this study.References
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk, N. Tufenkji, S. Feng and M. Wiesner, Nanoplastics are neither microplastics nor engineered nanoparticles, Nat. Nanotechnol., 2021, 16, 501–507 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- O. Pencik, M. Durdakova, K. Molnarova, A. Kucsera, D. Klofac, M. Kolackova, V. Adam and D. Huska, Microplastics and nanoplastics toxicity assays : A revision towards to environmental-relevance in water environment, J. Hazard. Mater., 2023, 454, 131476 CrossRef CAS PubMed.
- S. Wang, M. Liu, J. Wang, J. Huang and J. Wang, Polystyrene nanoplastics cause growth inhibition, morphological damage and physiological disturbance in the marine microalga Platymonas helgolandica, Mar. Pollut. Bull., 2020, 158, 111403 CrossRef CAS PubMed.
- M. Sendra, E. Sparaventi, B. Novoa and A. Figueras, Science of the Total Environment An overview of the internalization and effects of microplastics and nanoplastics as pollutants of emerging concern in bivalves, Sci. Total Environ., 2021, 753, 142024 CrossRef CAS PubMed.
- M. Sendra, E. Staffieri, M. P. Yeste, I. Moreno-Garrido, J. M. Gatica, I. Corsi and J. Blasco, Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum?, Environ. Pollut., 2019, 249, 610–619 CrossRef CAS PubMed.
- A. Zaghloul, M. Saber, S. Gadow and F. Awad, Biological indicators for pollution detection in terrestrial and aquatic ecosystems, Bull. Natl. Res. Cent., 2020, 44, 1–11 CrossRef.
- M. Khoshnamvand, P. Hanachi, S. Ashtiani and T. R. Walker, Toxic effects of polystyrene nanoplastics on microalgae Chlorella vulgaris: changes in biomass, photosynthetic pigments and morphology, Chemosphere, 2021, 280, 130725 CrossRef CAS PubMed.
- X. Zheng, Y. Yuan, Y. Li, X. Liu, X. Wang and Z. Fan, Polystyrene nanoplastics affect growth and microcystin production of Microcystis aeruginosa, Environ. Sci. Pollut. Res., 2021, 28, 13394–13403 CrossRef CAS PubMed.
- L. Natarajan, D. Soupam, S. Dey, N. Chandrasekaran, R. Kundu, S. Paul and A. Mukherjee, Toxicity of polystyrene microplastics in freshwater algae Scenedesmus obliquus: Effects of particle size and surface charge, Toxicol. Rep., 2022, 9, 1953–1961 CrossRef CAS PubMed.
- V. Thiagarajan, S. A. Alex, R. Seenivasan, N. Chandrasekaran and A. Mukherjee, Interactive effects of micro/nanoplastics and nanomaterials/pharmaceuticals: Their ecotoxicological consequences in the aquatic systems, Aquat. Toxicol., 2021, 232, 105747 CrossRef CAS PubMed.
- M. Hejna, D. Kapuścińska and A. Aksmann, Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae, Int. J. Environ. Res. Public Health, 2022, 19, 7717 CrossRef CAS PubMed.
- L. H. Santos, S. Rodríguez-Mozaz and D. Barceló, Microplastics as vectors of pharmaceuticals in aquatic organisms–an overview of their environmental implications, Case Stud. Chem. Environ. Eng., 2021, 3, 100079 CrossRef CAS.
- I. Alessandretti, C. V. T. Rigueto, M. T. Nazari, M. Rosseto and A. Dettmer, Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques, J. Environ. Chem. Eng., 2021, 9, 106743 CrossRef CAS.
- T. Ding, X. Huang, L. Wei and J. Li, Size-dependent effect of microplastics on toxicity and fate of diclofenac in two algae, J. Hazard. Mater., 2023, 451, 131071 CrossRef CAS PubMed.
- E. M. Jiménez-Bambague, J. S. Florez-Castillo, R. D. Gómez-Angulo, P. A. Morales-Acosta, E. J. Peña-Salamanca, F. Machuca-Martínez and C. A. Madera-Parra, Cell growth and removal capacity of ibuprofen and diclofenac by Parachlorella kessleri at bench scale, J. Chem. Technol. Biotechnol., 2022, 97, 1416–1423 CrossRef.
- L. J. Feng, Y. Shi, X. Y. Li, X. D. Sun, F. Xiao, J. W. Sun, Y. Wang, X. Y. Liu, S. G. Wang and X. Z. Yuan, Behavior of tetracycline and polystyrene nanoparticles in estuaries and their joint toxicity on marine microalgae Skeletonema costatum, Environ. Pollut., 2020, 263, 114453 CrossRef CAS PubMed.
- X. You, X. Cao, X. Zhang, J. Guo and W. Sun, Unraveling individual and combined toxicity of nano/microplastics and ciprofloxacin to Synechocystis sp. at the cellular and molecular levels, Environ. Int., 2021, 157, 106842 CrossRef CAS.
- S. Giri, A. C. Christudoss, N. Chandrasekaran, W. J. G. M. Peijnenburg and A. Mukherjee, The role of algal EPS in reducing the combined toxicity of BPA and polystyrene nanoparticles to the freshwater algae Scenedesmus obliquus, Plant Physiol. Biochem., 2023, 197, 107664 CrossRef CAS.
- S. Das, N. Chandrasekaran and A. Mukherjee, Unmasking effects of masks: Microplastics released from disposable surgical face masks induce toxic effects in microalgae Scenedesmus obliquus and Chlorella sp, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2023, 267, 109587 CAS.
- E. M. Elzayat, M. F. Ibrahim, A. A. Abdel-Rahman, S. M. Ahmed, F. K. Alanazi and W. A. Habib, A validated stability-indicating UPLC method for determination of diclofenac sodium in its pure form and matrix formulations, Arabian J. Chem., 2017, 10, S3245–S3254 CrossRef CAS.
- I. M. Sadiq, S. Pakrashi, N. Chandrasekaran and A. Mukherjee, Studies on toxicity of aluminum oxide (Al 2 O 3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp, J. Nanopart. Res., 2011, 13, 3287–3299 CrossRef CAS.
- OECD, OECD Guidelines for the Testing of Chemicals. Freshwater Alga and Cyanobacteria, Growth Inhibition Test, Organisation for Economic Cooperation and Development Search PubMed.
- S. Das, V. Thiagarajan, N. Chandrasekaran, B. Ravindran and A. Mukherjee, Nanoplastics enhance the toxic effects of titanium dioxide nanoparticle in freshwater algae Scenedesmus obliquus, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2022, 256, 109305 CAS.
- R. C. Gebara, L. de O. G. Alho, G. S. Rocha, A. da Silva Mansano and M. da G. G. Melão, Zinc and aluminum mixtures have synergic effects to the algae Raphidocelis subcapitata at environmental concentrations, Chemosphere, 2020, 242, 125231 CrossRef CAS PubMed.
- A. Piotrowska-Niczyporuk, A. Bajguz, E. Zambrzycka and B. Godlewska-yżłkiewicz, Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae), Plant Physiol. Biochem., 2012, 52, 52–65 CrossRef CAS PubMed.
- Y. Kono, Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase, Arch. Biochem. Biophys., 1978, 186, 189–195 CrossRef CAS PubMed.
- A. Filová, A. Fargašová and M. Molnárová, Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae, Environ. Sci. Pollut. Res., 2021, 28, 58426–58441 CrossRef PubMed.
- W. S. Abbott, A method of computing the effectiveness of an insecticide, J. Econ. Entomol., 1925, 18, 265–267 CrossRef CAS.
- Z. Zhu, S. Wang, F. Zhao, S. Wang, F. Liu and G. Liu, Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum, Environ. Pollut., 2019, 246, 509–517 CrossRef CAS PubMed.
- J. Li, X. Huang, Z. Hou and T. Ding, Sorption of diclofenac by polystyrene microplastics: Kinetics, isotherms and particle size effects, Chemosphere, 2022, 290, 133311 CrossRef CAS.
- Z. Song, X. Yang, F. Chen, F. Zhao, Y. Zhao, L. Ruan, Y. Wang and Y. Yang, Fate and transport of nanoplastics in complex natural aquifer media: Effect of particle size and surface functionalization, Sci. Total Environ., 2019, 669, 120–128 CrossRef CAS PubMed.
- J. Bhagat, N. Nishimura and Y. Shimada, Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives, J. Hazard. Mater., 2021, 405, 123913 CrossRef CAS PubMed.
- T. Atugoda, M. Vithanage, H. Wijesekara, N. Bolan, A. K. Sarmah, M. S. Bank, S. You and Y. S. Ok, Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport, Environ. Int., 2021, 149, 106367 CrossRef CAS PubMed.
- F. Wang, B. Wang, H. Qu, W. Zhao, L. Duan, Y. Zhang, Y. Zhou and G. Yu, The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa, Environ. Pollut., 2020, 263, 114593 CrossRef CAS PubMed.
- Y. Zhang, G. W. Price, R. Jamieson, D. Burton and K. Khosravi, Chemosphere Sorption and desorption of selected non-steroidal anti-in fl ammatory drugs in an agricultural loam-textured soil, Chemosphere, 2017, 174, 628–637 CrossRef CAS PubMed.
- Y. Xiong, J. Zhao, L. Li, Y. Wang, X. Dai, F. Yu and J. Ma, Interfacial interaction between micro/nanoplastics and typical PPCPs and nanoplastics removal via electrosorption from an aqueous solution, Water Res., 2020, 184, 116100 CrossRef CAS PubMed.
- L. J. Hazeem, G. Yesilay, M. Bououdina, S. Perna, D. Cetin, Z. Suludere, A. Barras and R. Boukherroub, Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes, Mar. Pollut. Bull., 2020, 156, 111278 CrossRef CAS.
- J. Sharma, I. Mariam, M. Suresh Kareya, P. Pavan Jutur, M. Joshi, Harish, A. Bhatnagar, A. K. Chaurasia and S. Nigam, Metabolomic response of microalgae towards diclofenac sodium during its removal from water and concomitant recovery of pigments and lipids, Bioresour. Technol., 2023, 371, 128617 CrossRef CAS PubMed.
- T. Ding, M. Yang, J. Zhang, B. Yang, K. Lin, J. Li and J. Gan, Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp, J. Hazard. Mater., 2017, 330, 127–134 CrossRef CAS PubMed.
- A. Rempel, J. P. Gutkoski, M. T. Nazari, G. N. Biolchi, B. Biduski, H. Treichel and L. M. Colla, Microalgae growth with a high concentration of emerging pollutants and phytotoxicity evaluation of cultivation wastewater, J. Water Process. Eng., 2022, 46, 102616 CrossRef.
- Z. Li, X. Yi, H. Zhou, T. Chi, W. Li and K. Yang, Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa, Environ. Pollut., 2020, 257, 113604 CrossRef CAS PubMed.
- Y. Zhang, J. Guo, T. Yao, Y. Zhang, X. Zhou and H. Chu, The influence of four pharmaceuticals on Chlorellapyrenoidosa culture, Sci. Rep., 2019, 9, 1624 CrossRef CAS PubMed.
- E. Agathokleous, D. Barceló, D. Fatta-Kassinos, M. N. Moore and E. J. Calabrese, Contaminants of emerging concern and aquatic organisms: the need to consider hormetic responses in effect evaluations, Water Emerg, Contam. Nanoplast., 2021, 1–9 Search PubMed.
- M. Rezayian, V. Niknam and H. Ebrahimzadeh, Oxidative damage and antioxidative system in algae, Toxicol. Rep., 2019, 6, 1309–1313 CrossRef CAS PubMed.
- R. Feito, Y. Valcárcel and M. Catalá, Biomarker assessment of toxicity with miniaturised bioassays: Diclofenac as a case study, Ecotoxicology, 2012, 21, 289–296 CrossRef CAS PubMed.
- S. Zhang, J. Ding, R. M. Razanajatovo, H. Jiang, H. Zou and W. Zhu, Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus), Sci. Total Environ., 2019, 648, 1431–1439 CrossRef CAS PubMed.
- H. Wang, M. Jin, W. Mao, C. Chen, L. Fu, Z. Li, S. Du and H. Liu, Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus, Sci. Total Environ., 2020, 707, 136176 CrossRef CAS PubMed.
- M. González-Pleiter, A. Pedrouzo-Rodríguez, I. Verdú, F. Leganés, E. Marco, R. Rosal and F. Fernández-Piñas, Microplastics as vectors of the antibiotics azithromycin and clarithromycin: Effects towards freshwater microalgae, Chemosphere, 2021, 268, 128824 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00341h |
| This journal is © The Royal Society of Chemistry 2024 |