Open Access Article
Open Access ArticleSupramolecular helix of an oligomeric azapeptide building block containing four β-turn structures†
Yingdan
Zhao
 a,
Xiaosheng
Yan
a,
Xiaosheng
Yan
 ab and
Yun-Bao
Jiang
ab and
Yun-Bao
Jiang
 *a
*a
aDepartment of Chemistry, College of Chemistry and Chemical Engineering, the MOE Key Laboratory of Spectrochemical Analysis and Instrumentation, Xiamen University, Xiamen 361005, China. E-mail: ybjiang@xmu.edu.cn
bFujian Provincial Key Laboratory of Innovative Drug Target Research and State Key Laboratory of Cellular Stress Biology, School of Pharmaceutical Sciences, Xiamen University, Xiamen, Fujian 361102, China
First published on 11th March 2024
Abstract
Oligomers of benzoylalanine-based amidothioureas containing four β-turn structures spaced by meta-substituted benzenes were shown to undergo assembly in dilute CH3CN solution into supramolecular helices of enhanced supramolecular helicity, whereas those spaced by para-substituted benzene spacer(s) or those spaced by meta-substituted benzenes but with one or two β-turns exhibit a substantially decreased tendency of assembling.
Helical structures such as the protein α-helix and DNA double helix are of great significance in life sciences. Scientists have thus been exploring the design and development of diverse biomimetic artificial helices of varying functionalities. Supramolecular helical structures have attracted considerable attention also because of their adaptable cavity size, processability, flexibility, and ease of modification and functionalization.1 One strategy for constructing supramolecular helices is to elongate short building blocks in a helically folded conformation, through end-to-end noncovalent interactions.1–4 Among them, aromatic foldamers of good predictability have been widely employed.5–9 Those helices can be applied to the recognition, encapsulation and transport of a variety of ions and neutral molecules,6,10–12 and the properties of the supramolecular helices can be altered by structural adjustments thus to achieve regulated selectivity and activity.13,14
Yet, the design of novel building blocks remains challenging. We have recently built single- and double-stranded supramolecular helices in the solid phase and in dilute solution, using a bis(N-amidothiourea) motif that contains two β-turns in the terminal or central parts of the molecule as the building block, such that the intermolecular halogen bonding bridges molecules into helices.15,16 With these short building blocks, their concentrations might need to be high to allow a long enough helix to be well characterized, which may become hard as their solubility could be a limiting factor. The minimal optimal number of turn structures and the way of their linkage shall then be critical for their successful assembly into supramolecular helices.
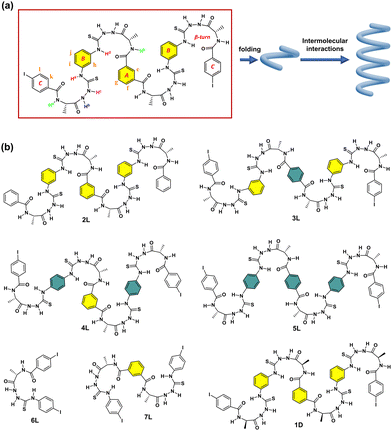
Reported herein are our efforts to achieve amidothiourea-based oligomeric building blocks that contain four β-turn structures spaced by meta-substituted benzenes, 1L and 1D, taking, respectively, L- and D-alanine as the chiral source (Fig. 1a and b). Two iodine atoms are introduced at the para-positions of the two terminal phenyl rings to possibly afford intermolecular halogen bonding. Experiments show that these two oligomeric compounds function to assemble into stable supramolecular helices in dilute CH3CN solution, via halogen bonding. The para-substituted benzene-spaced counterparts do not assemble well (one of the four being para-substituted) or do not assemble at all (more than one) (3L, 4L and 5L, Fig. 1b), and those meta-substituted but containing one or two β-turns (6L and 7L, Fig. 1b) do not assemble either.
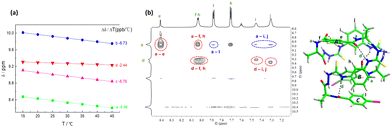
The β-turn structure in N-amidothiourea derivatives provides helical fragments. 1H NMR spectra show that with increasing solution temperature, the chemical shift of each –NH proton in 1L moves downfield, at different rates (Fig. S1, ESI†). Notably, the resonance of the –NHd proton exhibits the smallest temperature coefficient (Δδ/ΔT = −2.44 ppb °C versus –NHa: −8.96, –NHb: −8.73 and –NHc: −8.76, Fig. 2a), indicating that it is less influenced by solution temperature. This means that it is protected, to some extent, agreeing with its taking part in an intramolecular hydrogen bond. Furthermore, the chemical shift of –NHd remains unchanged upon changing the volume ratio of DMSO-d6 to CD3CN in their binary mixtures (Fig. S2 and S3, ESI†), providing further evidence for the presence of the hydrogen bonded β-turn.
Repetition of the β-turn structure, due to its helical character, may facilitate the elongation of the oligomeric building block. This will result in a folding propensity of the formed oligomer, indicated by the DFT-optimized structure of 1L in the gas phase (Fig. 2b). The 2D NOESY spectrum of 1L in DMSO-d6 also reveals the folded conformation of 1L (Fig. 2b and Fig. S4, ESI†). NOE coupling signals between Ha–He and Ha–Hf indicate that the β-turns on two sides of benzene ring A are oriented in opposite directions. Similarly, the β-turns on two sides of benzene ring B also exhibit different orientations, as evidenced by the NOE signals between Hd–Hh, Hd–Hi, and Hd–Hj. Furthermore, the NOE couplings between Ha–Hi/Hj and Ha–Hl further proved that 1L is folded, which brings NHa and benzene rings B and C into close proximity, in line with the calculated monomeric structure of 1L.
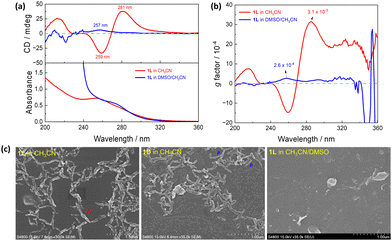
1L in the folded conformation may facilitate its supramolecular assembly, as a basic building block. We did observe such supramolecular assembly in CH3CN. In DMSO, a highly polar aprotic solvent, assembly of it would thus be much less, if any. Experiments in both pure CH3CN and DMSO/CH3CN binary solvents were carried out in detail. In CH3CN, the absorption spectrum of 1L displays a band at 255 nm and a shoulder at 275 nm, accompanied by prominent Cotton effects at 281 nm, 259 nm, and 213 nm in the CD spectrum; in CH3CN containing 0.5% DMSO by volume, however, only a faint CD signal at 257 nm was observed (Fig. 3a). Furthermore, the anisotropic factor, g, in CH3CN (3.1 × 10−3, Fig. 3b) is significantly higher, ca. 12 times that in DMSO/CH3CN (2.6 × 10−4, Fig. 3b). Dynamic light scattering (DLS, Fig. S5, ESI†) allows access to the size distributions of 1L in different solvents. The diameter of the 1L at the same concentration in DMSO/CH3CN was measured as 3.2 nm, whereas in CH3CN it reached 22.8 nm. This further substantiates the assumption that 1L forms assemblies in CH3CN, whereas in DMSO/CH3CN it remains as a monomer. Meanwhile, SEM images of air-dried samples from CH3CN solutions of 1L reveal the presence of ordered short M-helical assemblies and those of 1D the P-helical structures, whereas no regular morphology is observed from the samples prepared in DMSO/CH3CN (Fig. 3c). These findings provide compelling evidence that 1L and 1D assemble into supramolecular helices in CH3CN.
Supramolecular assembly occurs when the dimensionless concentration exceeds unity (cTKe > 1), where cT represents the total concentration and Ke refers to the equilibrium constant. Hence, a critical concentration, corresponding to Ke−1, is required for the helical assembly of 1L to occur.17 Indeed, CD signals of 1L in CH3CN measured at different concentrations indicate a remarkably low critical assembly concentration, ca. 1 μM (Fig. S6, ESI†), through which a high Ke value of 1.0 × 106 M−1 is estimated. Investigation into the concentration-dependent g factor of 1L reveals an increase in g with increasing concentration from 1 to 5 μM (Fig. S7, ESI†) after which it levels off. However, at a concentration below 1 μM, the g factor varies irregularly. Based on these observations, it is inferred that 1L exhibits a state of assembly instability at concentrations close to or below the critical assembly concentration, that the monomer and aggregates may exchange rapidly. As the concentration increases beyond this critical concentration, the monomer molecules assemble to lead to helical chain extension, indicated by an increase in the g factor. As the concentration approaches 5 μM, the chain ceases to propagate, such that a stable assembly state is reached, which the g factor does not change any more. The concentration-dependent DLS data of 1L in CH3CN corroborate this pattern of variation in g factor (Fig. S8 vs. S7, ESI†). Consequently, it is concluded that the supramolecular helix of 1L in CH3CN possesses a finite size range, rather than an infinite extension.
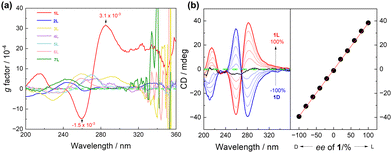
According to our previous research,15,16 it is assumed that the supramolecular helix of 1L is probably driven by intermolecular halogen bonding. Thus, control compound 2L (Fig. 1b), without I-substituents, was examined. First, in CH3CN the CD intensity of 2L is significantly weaker than that of 1L, with no discernible CD signal associated with the assembly (Fig. S9, ESI†). Second, upon increasing the concentration of 2L, no obvious changes were observed (Fig. S10, ESI†). Third, the g factor of 2L reaches a maximum value of 3.1 × 10−4 at 244 nm (Fig. 4a and Fig. S11, ESI†), one tenth that of the maximum value of 1L (3.1 × 10−3 at 285 nm). It is reasonable to conclude that 2L is unable to assemble into supramolecular species in CH3CN. Indeed, DLS experiments reveal that the diameters of 2L in CH3CN are around 3.3 nm (Fig. S12, ESI†), approximately 7 times smaller than those of 1L. No assembly morphology was observed in the SEM images of 2L (Fig. S13, ESI†). Taken together, it is concluded that control compound 2L does not undergo assembly in CH3CN, suggesting thus the role of I-substituents in 1L in driving its assembly into supramolecular helices.
Halogen bonding was further supported by examining in solvents of different alkalinity. On addition of a 20% volume fraction of H2O into CH3CN, the CD spectrum of 1L experiences a significant change and a weakening of the intensity. In contrast, addition of 20% by volume THF, a solvent of weaker electron donating capacity, results in a minor change in the CD spectrum. These observations support the hypothesis that halogen bonding serves as an interaction force between molecules (Fig. S14–S16, ESI†).18 Cl−, Br−, and I− anions can act as halogen bond receptors, and their ability to destroy halogen bonds follows the order of I− > Br− > Cl−.19 On addition of halogen anions at a high concentration of 100 equivalents into CH3CN solution of 1L, the CD signal of the supramolecular species of 1L gradually decreases (Fig. S17, ESI†), and the order of intensity decline is the same as their destructive ability towards halogen bonds, i.e., I− > Br− > Cl− (Fig. S18, ESI†).
NMR spectra also support the existence of intermolecular halogen bonding. In comparison to the 1H NMR spectrum of monomeric 1L in pure DMSO-d6, we observed additional weak 1H NMR signals of 1L in CD3CN/DMSO-d6 (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. S19, ESI†), which were assigned to the assembled oligomers, as evidenced by the COSY couplings of Hk′–Hl′, Hf–Hg′ and Hf′–Hg′ (Fig. S20, ESI†). Interestingly, clear NOESY coupling signals are shown for He–Hk, He–Hl, Hf–Hk, Hf–Hl and Hj–Hl in CD3CN/DMSO-d6 (200
1, v/v) (Fig. S19, ESI†), which were assigned to the assembled oligomers, as evidenced by the COSY couplings of Hk′–Hl′, Hf–Hg′ and Hf′–Hg′ (Fig. S20, ESI†). Interestingly, clear NOESY coupling signals are shown for He–Hk, He–Hl, Hf–Hk, Hf–Hl and Hj–Hl in CD3CN/DMSO-d6 (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), but no such couplings are observed in DMSO-d6 at the same concentration. According to the calculated structure of monomeric 1L, pairs of protons He–Hk, He–Hl, Hf–Hk, and Hf–Hl are too far apart to be intramolecularly coupled. Only when the intermolecular C–I⋯O bonds link adjacent 1L molecules, benzene ring C in which Hk and Hl are located will be brought into close proximity to benzene ring A from another 1L molecule that contains He and Hf, resulting in clear intermolecular coupling signals (Fig. S21, ESI†). We thus proposed that the formation of supramolecular assemblies of 1L is driven by intermolecular C–I⋯O halogen bonding.
1, v/v), but no such couplings are observed in DMSO-d6 at the same concentration. According to the calculated structure of monomeric 1L, pairs of protons He–Hk, He–Hl, Hf–Hk, and Hf–Hl are too far apart to be intramolecularly coupled. Only when the intermolecular C–I⋯O bonds link adjacent 1L molecules, benzene ring C in which Hk and Hl are located will be brought into close proximity to benzene ring A from another 1L molecule that contains He and Hf, resulting in clear intermolecular coupling signals (Fig. S21, ESI†). We thus proposed that the formation of supramolecular assemblies of 1L is driven by intermolecular C–I⋯O halogen bonding.
In 1L, the β-turn provides the helical conformation, four of which are spaced by meta-substituted benzene rings, which is more conducive to the propagation of the helicity of the turn structure. We thus developed control compounds 3L, 4L and 5L, containing, respectively, 1, 2, and 3 para-substituted benzene spacers (Fig. 1b). CD spectra in CH3CN show that these three control compounds exhibit weaker CD intensities than 1, and the signal peaks appear at shorter wavelengths (Fig. S22, ESI†). The g factor values of 3L, 4L, and 5L are significantly smaller than that of 1L (Fig. 4a). This finding further supports the superior propagation of the helicity of 1L and highlights the synergy of the meta-substitution at the benzene spacers in facilitating the transmission of homochirality. In addition, we compared the spectra of the control compounds containing different numbers of β-turns, 6L containing only one and 7L containing two (Fig. 1b). The CD spectra show that at the same concentration of the β-turn in 1L, 6L, and 7L, 1L in CH3CN exhibits a much higher CD intensity and a larger g factor than those of 6L and 7L (Fig. 4a and Fig. S23, ESI†). Even at increased concentrations of 60 μM for 6L and 200 μM for 7L, no assembly was observed (Fig. S24 and S25, ESI†). This suggests that the size of the oligomeric building block is important too, and smaller does not guarantee a stronger tendency of assembly, despite being less flexible. In view of the SEM images, none of the control compounds (2L, 3L, 4L, 5L, 6L and 7L) were found to assemble into helical aggregates in CH3CN (Fig. S26, ESI†). It is of interest to recall that the para-substituted benzene spaced counterpart of 7L containing two β-turns undergoes assembly into helices in dilute CH3CN,15 again highlighting the critical role of the spacer that links the β-turn structures.
Temperature-dependent CD signals of 1L in CH3CN show that the helix is highly heat stable. The CD intensity was only slightly reduced upon heating the solution up to 70 °C and it can be completely restored after cooling (Fig. S27 and S28, ESI†). The enantiomeric mixtures of 1L and 1D of varying ratios in CH3CN show a linear-dependence of the CD signals on the enantiomeric excess (ee) (Fig. 4b), suggesting that the enantiomers of 1L and 1D are self-sorting in their assemblies.20,21 This is different from the single-stranded helix from the building blocks via C–I⋯π halogen bonding (S-shaped CD-ee dependence),15 but similar to that of the double helix driven by two crossed C–I⋯S halogen bonds.16
In summary, an oligomeric building block that contains four β-turns using a benzoylalanine-based amidothiourea motif is shown to be of optimal minimum length to allow efficient assembly into a supramolecular helix. 1L or 1D in which four β-turn structures are spaced by meta-substituted benzene rings undergoes assembly in CH3CN at a μM-level concentration, via intermolecular halogen bonding. The formed helix exhibits strong CD signals of a high g factor of 3.1 × 10−3 at 285 nm for instance and a linear CD-ee dependence, together with an excellent thermal stability. DLS data show that the length of the formed supramolecular helix is finite. Replacing the meta-substituted benzene spacers with para-substituted one(s) substantially decreases the tendency of assembling, highlighting the critical role of helicity propagation in facilitating the helical assembly, and neither does a decrease in the number of β-turns spaced by meta-substituted benzenes. We therefore show that with oligomeric helical building blocks, an optimal number of turns and the way they are spaced are important factors dictating their assembly into helices.
This work has been supported by the National Science Foundation of China (Grants 21820102006, 22101240, 22241503 and 92356308), the Fundamental Research Funds for the Central Universities (Grants 20720220005 and 20720220121), and the Natural Science Foundation of Fujian Province of China (No. 2023J01038).
Conflicts of interest
The authors declare no conflicts of interest.Notes and references
- C. Z. Liu, M. Yan, H. Wang, D. W. Zhang and Z. T. Li, ACS Omega, 2018, 3, 5165–5176 CrossRef CAS PubMed.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- M. Gonzalez-Sanchez, M. J. Mayoral, V. Vazquez-Gonzalez, M. Paloncyova, I. Sancho-Casado, F. Aparicio, A. de Juan, G. Longhi, P. Norman, M. Linares and D. Gonzalez-Rodriguez, J. Am. Chem. Soc., 2023, 145, 17805–17818 CrossRef CAS PubMed.
- Q. Gan, Y. Wang and H. Jiang, Chin. J. Chem., 2013, 31, 651–656 CrossRef CAS.
- D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes and J. S. Moore, Chem. Rev., 2001, 101, 3893–4012 CrossRef CAS PubMed.
- H. Juwarker, J. M. Suk and K. S. Jeong, Chem. Soc. Rev., 2009, 38, 3316–3325 RSC.
- D. W. Zhang, X. Zhao, J. L. Hou and Z. T. Li, Chem. Rev., 2012, 112, 5271–5316 CrossRef CAS PubMed.
- D. W. Zhang, W. K. Wang and Z. T. Li, Chem. Rec., 2015, 15, 233–251 CrossRef CAS PubMed.
- C. Z. Liu, S. Koppireddi, H. Wang, D. W. Zhang and Z. T. Li, Angew. Chem., Int. Ed., 2019, 58, 226–230 CrossRef CAS PubMed.
- T. Yan, F. Yang, S. Qi, X. Fan, S. Liu, N. Ma, Q. Luo, Z. Dong and J. Liu, Chem. Commun., 2019, 55, 2509–2512 RSC.
- Y. Ferrand and I. Huc, Acc. Chem. Res., 2018, 51, 970–977 CrossRef CAS PubMed.
- C. Zhang, X. Deng, C. Wang, C. Bao, B. Yang, H. Zhang, S. Qi and Z. Dong, Chem. Sci., 2019, 10, 8648–8653 RSC.
- X. Yan, P. Weng, D. Shi and Y.-B. Jiang, Chem. Commun., 2021, 57, 12562–12574 RSC.
- D. Bindl, P. K. Mandal and I. Huc, Chem. – Eur. J., 2022, 28, e202200538 CrossRef CAS PubMed.
- J. Cao, X. Yan, W. He, X. Li, Z. Li, Y. Mo, M. Liu and Y.-B. Jiang, J. Am. Chem. Soc., 2017, 139, 6605–6610 CrossRef CAS PubMed.
- X. Yan, K. Zou, J. Cao, X. Li, Z. Zhao, Z. Li, A. Wu, W. Liang, Y. Mo and Y.-B. Jiang, Nat. Commun., 2019, 10, 3610 CrossRef PubMed.
- M. M. Smulders, M. M. Nieuwenhuizen, T. F. de Greef, P. van der Schoot, A. P. Schenning and E. W. Meijer, Chem. – Eur. J., 2010, 16, 362–367 CrossRef CAS PubMed.
- C. Laurence, M. Queignec-Cabanetos, T. Dziembowska, R. Queignec and B. Wojtkowiak, J. Am. Chem. Soc., 2002, 103, 2567–2573 CrossRef.
- A. Mele, P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, J. Am. Chem. Soc., 2005, 127, 14972–14973 CrossRef CAS PubMed.
- X. Yan, Q. Wang, X. Chen and Y.-B. Jiang, Adv. Mater., 2020, 32, e1905667 CrossRef PubMed.
- C. Roche, H. J. Sun, M. E. Prendergast, P. Leowanawat, B. E. Partridge, P. A. Heiney, F. Araoka, R. Graf, H. W. Spiess, X. Zeng, G. Ungar and V. Percec, J. Am. Chem. Soc., 2014, 136, 7169–7185 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04859d |
| This journal is © The Royal Society of Chemistry 2024 |