Circularly polarized luminescence: a themed collection
Jeanne
Crassous
 *a,
Lorenzo
Di Bari
*a,
Lorenzo
Di Bari
 *b,
Wai-Yeung
Wong
*b,
Wai-Yeung
Wong
 *c and
You-Xuan
Zheng
*c and
You-Xuan
Zheng
 *d
*d
aUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes) – UMR 6226, Rennes, France. E-mail: jeanne.crassous@univ-rennes1.fr
bDipartimento di Chimica e Chimica Industriale, Università di Pisa, via Moruzzi 13, 56124, Pisa, Italy. E-mail: lorenzo.dibari@unipi.it
cDepartment of Applied Biology and Chemical Technology, Research Institute for Smart Energy, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China. E-mail: wai-yeung.wong@polyu.edu.hk
dState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, P. R. China. E-mail: yxzheng@nju.edu.cn
Circularly polarized luminescence (CPL), a fascinating property discovered in the 1970s,1 has recently received great attention thanks to (i) the very recent availability of commercial CPL apparatus operating from the far-UV to the infrared domains and (ii) the availability of a diversity of molecules exhibiting both chirality and emission properties.
CPL intensity is determined by the dissymmetry factor glum, which represents the degree of circular polarization of emitted light and is defined as 2ΔI/I = 2(IL − IR)/(IL + IR), where IL and IR denote the left- and right-handed circularly polarized emission intensities, respectively. From a theoretical point of view, the glum dissymmetry factor varies like 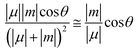 , where m and μ are the magnetic and electric transition moments, respectively, and θ is the angle between them. The approximate equality holds when |μ| ≫ |m|, which is a common situation, and thus magnetically-allowed and electrically-forbidden transitions with well-aligned directions are sought. In an analogy with the brightness of fluorescent emitters, which takes into account the chromophore extinction coefficient, ε, and the emission quantum yield, ϕ, the CPL brightness BCPL = ε × Φ × glum/2 was recently proposed as a good figure of merit.2
, where m and μ are the magnetic and electric transition moments, respectively, and θ is the angle between them. The approximate equality holds when |μ| ≫ |m|, which is a common situation, and thus magnetically-allowed and electrically-forbidden transitions with well-aligned directions are sought. In an analogy with the brightness of fluorescent emitters, which takes into account the chromophore extinction coefficient, ε, and the emission quantum yield, ϕ, the CPL brightness BCPL = ε × Φ × glum/2 was recently proposed as a good figure of merit.2
Extreme glum values of glum = ±2 arise when either pure left- or right-polarized light is emitted after standard excitation, although for the majority of cases glum spans 10−4–10−1 and rarely exceeds 1. All types of stereogenic elements (central, planar, axial, helical, or inherent chirality) are currently being introduced into efficient emitters and structure–property relationships are progressively being established.3 Typical examples are biarylic systems, paracyclophanes, or helicenes. At least in the form of isolated molecules, these organic fluorescent molecules are known to display glum values on the order of 10−4–10−2 in the wavelength domains from the far-UV to the infrared. Introducing donor and acceptor units enables one to tune the emission wavelength and reach challenging domains of great interest, such as deep-blue and infrared chiral emitters, while open-shell systems may contribute to an increase in the magnetic contribution. Furthermore, the combination of CPL properties with other phenomena, such as Förster energy transfer, exciplex formation, triplet–triplet annihilation (TTA), thermally-activated delayed fluorescence (TADF), phosphorescence, aggregation-induced emission, chiral-induced spin selectivity, or implementation into strongly chiral media, are currently closely examined.
The present dedicated issue spans a large variety of topics, witnessing the diverse directions of a fertile research area.
On a dimensional order, we can start with several instances of small organic molecules, composed by one twisted chromophore, or by systems with strongly interacting chromophores, held together by stereodefined frameworks. In these cases, the inequality |μ| ≫ |m| holds, and considerable CPL arises from the combined effect of large |m| and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (i.e. small θ). Helicenes can be excellent examples of this strategy: the distribution of aromatic rings along a well-defined arch determines a considerable rotation of charge during an electronic transition, which means large |m| perpendicular to the helicene pseudoplane. Along the same direction (θ ≈ 0), there is also a significant charge displacement. Going one step further, X.-Y. Wang et al. developed push–pull systems from aza[7]helicene as the chiral donor and triazine as the acceptor and showed that the well-engineered dihedral angle between the two units can give amplified CPL activity (https://doi.org/10.1039/D2TC04848E). Paracyclophanes represent the extreme case of another interesting strategy: bringing two more or less identical chromophores into very close proximity, to the point that there is a very strong orbital interaction between them. Here the approximation of dealing with two (or sometimes more) isolated systems must be released and, at least to some extent, one must switch to a diffuse chromophore description, reminiscent of that for exciplexes (see the review on compact chiral paracyclophanes by E. Benedetti et al., https://doi.org/10.1039/D2TC04885J). Interestingly, heteroatoms can be widely integrated into organic scaffolds, and generate a rich diversity of original architectures with tuned chiroptical activities.
θ (i.e. small θ). Helicenes can be excellent examples of this strategy: the distribution of aromatic rings along a well-defined arch determines a considerable rotation of charge during an electronic transition, which means large |m| perpendicular to the helicene pseudoplane. Along the same direction (θ ≈ 0), there is also a significant charge displacement. Going one step further, X.-Y. Wang et al. developed push–pull systems from aza[7]helicene as the chiral donor and triazine as the acceptor and showed that the well-engineered dihedral angle between the two units can give amplified CPL activity (https://doi.org/10.1039/D2TC04848E). Paracyclophanes represent the extreme case of another interesting strategy: bringing two more or less identical chromophores into very close proximity, to the point that there is a very strong orbital interaction between them. Here the approximation of dealing with two (or sometimes more) isolated systems must be released and, at least to some extent, one must switch to a diffuse chromophore description, reminiscent of that for exciplexes (see the review on compact chiral paracyclophanes by E. Benedetti et al., https://doi.org/10.1039/D2TC04885J). Interestingly, heteroatoms can be widely integrated into organic scaffolds, and generate a rich diversity of original architectures with tuned chiroptical activities.
Going one step further, purely organic molecules have been integrated with a variety of transition metals. These coordination compounds join the pliancy of organic frameworks with the spectroscopic properties of transition metals. Firstly, these compounds take advantage of long-lived emission from triplet states, i.e. phosphorescence, thanks to the strong spin–orbit coupling of the heavy metal. Secondly, they take advantage of low energy (vis-NIR) electronic transition with considerable magnetic-dipole character and, finally, they display very low propensity to photobleaching. A particular case that deserves a separate mention is provided by lanthanide systems. Their peculiarity resides in the poor covalency of the bonds they establish, which ensures that f → f transitions maintain most of their intraconfigurational character: they exhibit sharp, pure-color emission lines, often with extraordinarily high glum values. Interestingly, all these organometallic systems span a very wide range of emission wavelengths (https://doi.org/10.1039/D3TC00034F) and are amenable to two-photon excitation (https://doi.org/10.1039/D2TC05362D).
Chiral organic polymers have also revealed appealing CPL activity. In the polymeric systems described herein (see for instance the advances and perspectives reviewed by Z.-Q. Wu et al.; https://doi.org/10.1039/D2TC04715B), the backbone can be helical, thus ensuring a long-range chiral environment. Thanks to the ordered repetition of chiral elements, glum can attain values often above 0.1, i.e. much larger than for discrete molecules. When the helical backbone itself is a conjugated chain, it must be considered an intrinsically chiral fluorophore, which may be responsible for the observed chiroptical properties. Interestingly, these conditions can be associated with applications in optoelectronic devices, thanks to the possibility that these systems act as (semi)conductors.
At a still further level of complexity, we find aggregated systems endowed with supramolecular chiral order. This can give rise to liquid-crystalline phases, like chiral nematic, where molecules gather in domains characterized by high local order (see the work of H.-L. Xie et al. on the circularly polarized (CP) organic room-temperature phosphorescence activated by liquid-crystalline polymer networks; https://doi.org/10.1039/D2TC04829A). In the absence of external stimuli, these domains remain uncorrelated, which ensures that, overall, the local linear anisotropies average to 0. Still, if the chiral order remains the same for each domain, common chiroptical features appear. Mesogens can be either small molecules or even polymeric systems. The interesting feature of liquid-crystalline phases arises from their dynamical behaviour, which may react to external stimuli of various types. In these systems, CPL provides a very sensitive readout of the functional responses. In general, molecular aggregates can give rise to conspicuous natural and magnetic CPL (https://doi.org/10.1039/D2TC05006D and https://doi.org/10.1039/D2TC04841H).
We next consider more rigid organizations, like in gels or in completely solid (thin) films. Here, the chiroptical properties can report the pathway through which aggregation is achieved. With proper transduction technologies, where circular polarization of light is exploited, these systems may find applications in the development of sensors. Furthermore, we should not forget that in the vast majority of applications in devices, one must deal with solid-state films of suitable materials. As a result, CP emitters have been incorporated into optoelectronic devices (such as in white CP OLEDs described by W.-Y. Wong et al.; https://doi.org/10.1039/D2TC04802G), in biological or other imaging techniques, or in cryptography and counterfeiting systems.
A special mention must be made of chiral perovskites (https://doi.org/10.1039/D2TC04825F and https://doi.org/10.1039/D2TC03810B). The excellent emitting properties of these intrinsically hybrid systems and the possibility to incorporate chiral molecules, in particular chiral cations, has drawn considerable interest, which is likely to grow in the near future.
Finally, we would note the successful modulation of CPL though the control of geometrical parameters, achieved, for example, in the works of de la Moya (https://doi.org/10.1039/D2TC04793D) and Ono (https://doi.org/10.1039/D2TC04636A).
In conclusion, this themed collection gives an effective, integrated and multidisciplinary representation of the state of the art of CPL investigation.
References
- (a) J. P. Riehl and G. Muller, Circularly polarized luminescence spectroscopy and emission-detected circular dichroism, in Comprehensive chiroptical spectroscopy, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, vol. 1, Wiley, Hoboken, NJ, 2012 Search PubMed; (b) H. P. J. M. Dekkers, Circularly Polarized Luminescence: A Probe For Chirality in The Excited State, in Circular dichroism: principles and applications, ed. N. Berova, K. Nakanishi and R. W. Woody, Wiley-VCH, New York, 2000, pp. 185–215 Search PubMed.
- L. Arrico, L. Di Bari and F. Zinna, Quantifying the Overall Efficiency of Circularly Polarized Emitters, Chem. – Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- H. Tanaka, Y. Inoue and T. Mori, Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation Between Excitation and Emission Dissymmetry Factors, ChemPhotoChem, 2018, 2, 386–402 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |