Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Using host–guest interactions at the interface of quantum dots to load drug molecules for biocompatible, safe, and effective chemo-photodynamic therapy against cancer†
Xiaoxia
Wu
 ae,
Jinghui
Yang
ab,
Jie
Xing
d,
Yonglei
Lyu
ab,
Ruifen
Zou
d,
Xin
Wang
ab,
Junlie
Yao
d,
Dinghu
Zhang
e,
Dawei
Qi
a,
Guoliang
Shao
e,
Aiguo
Wu
ae,
Jinghui
Yang
ab,
Jie
Xing
d,
Yonglei
Lyu
ab,
Ruifen
Zou
d,
Xin
Wang
ab,
Junlie
Yao
d,
Dinghu
Zhang
e,
Dawei
Qi
a,
Guoliang
Shao
e,
Aiguo
Wu
 *d and
Jianwei
Li
*d and
Jianwei
Li
 *ac
*ac
aMediCity Research Laboratory, University of Turku, Tykistökatu 6, FI-20520 Turku, Finland. E-mail: jianwei.li@utu.fi
bDepartment of Chemistry, University of Turku, Vatselankatu 2, FI-20014 Turku, Finland
cHainan Provincial Key Laboratory of Fine Chem, School of Chemical Engineering and Technology, Hainan University, Haikou 570228, China
dCixi Institute of Biomedical Engineering, International Cooperation Base of Biomedical Materials Technology and Application, Chinese Academy of Science (CAS) Key Laboratory of Magnetic Materials and Devices & Zhejiang Engineering Research Center for Biomedical Materials, Ningbo Institute of Materials Technology and Engineering, CAS, 1219 ZhongGuan West Road, Ningbo 315201, China. E-mail: aiguo@nimte.ac.cn
eDepartment of Interventional Radiology, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences (CAS), Hangzhou 310022, China
First published on 26th April 2023
Abstract
Combining photodynamic therapy (PDT) and chemotherapy (CHT) by loading an anti-cancer drug and a photosensitizer (PS) into the same delivery nanosystem has been proposed as an effective approach to achieve synergistic effects for a safe cancer treatment. However, exploring an ideal delivery nanosystem has been challenging, because the noncovalent interactions must be maintained between the multiple components to produce a stable yet responsive nanostructure that takes into account the encapsulation of drug molecules. We addressed this issue by engineering the interfacial interaction between Ag2S quantum dots (QDs) using a pillararene derivative to direct the co-self-assembly of the entire system. The high surface area-to-volume ratio of the Ag2S QDs provided ample hydrophobic space to accommodate the anti-drug molecule doxrubicine. Moreover, Ag2S QDs served as PSs triggered by 808 nm near-infrared (NIR) light and also as carriers for high-efficiency delivery of drug molecules to the tumor site. Drug release experiments showed smart drug release under the acidic microenvironments (pH 5.5) in tumor cells. Additionally, the Ag2S QDs demonstrated outstanding PDT ability under NIR light, as confirmed by extracellular and intracellular reactive oxygen species generation. Significant treatment efficacy of the chemo-photodynamic synergistic therapy for cancer using the co-delivery system was demonstrated via in vitro and in vivo studies. These findings suggest that our system offers intelligent control of CHT and PDT, which will provide a promising strategy for constructing hybrid systems with synergistic effects for advanced applications in biomedicine, catalysis, and optoelectronics.
Introduction
Cancer treatment remains a significant challenge to human health, and the development of new therapeutic strategies is crucial to improving patient outcomes.1 While researchers have made progress with clinical treatments such as chemotherapy (CHT), radiotherapy, and surgery, the emergence of novel therapeutic modalities has occurred in recent years, including drug photodynamic therapy (PDT), photothermal therapy (PTT), magnetic hyperthermia, sonodynamic therapy (SDT), chemodynamic therapy (CDT), and immunotherapy.2 However, a major issue in clinical treatment is how to increase patient survival with the use of a single treatment method, as each modality has its limitations.3–7Combinatorial therapy, which involves using multiple treatment modalities, has been proposed as a method to enhance their complementarity and maximize their strengths to produce synergistic effects.8,9 Self-assembly approaches using supramolecular concepts are promising strategies for developing delivery nanosystems10–13 that can encapsulate quantum dots (QDs) and anticancer drugs for targeted treatment of tumor lesions. However, these approaches face significant challenges such as low stability, resulting in disassembly and payload leakage during the delivery process, uncontrollable drug release in the extracellular matrix of tumor tissue, and low efficiency in cancer treatment.14–18 This unmet situation has been rooted in the challenge of exploring suitable supramolecular host molecules to balance the noncovalent interaction between the multiple components in the complex chemical system.19,20
In this study, we employed cationic pillar[6]arene (CP6) columnar molecules capable of associating with Ag2S QDs modified with alkyl chain ligands through host–guest interactions to develop a stable and pH-responsive self-assembled co-delivery system for chemo-photodynamic cancer therapy. This system co-delivered semiconductor QDs (Ag2S QDs) and doxorubicin (DOX) to tumors, exhibiting strong reactive oxygen species (ROS) generation capability and stimuli-responsive release of drugs and QDs in acidic microenvironments (pH 5.5).21–23
Ag2S QDs were chosen for self-assembled nano-structure formation due to their ultra-small size of approximately 4 nm, which facilitated the formation of ordered structures driven by host–guest interactions.24 The surface of Ag2S QDs modified with a ligand possessing –COOH groups can interact with CP6, driving Ag2S QDs to form ordered self-assembled nanostructures. This interfacial engineering can produce ample hydrophobic spaces to accommodate DOX molecules due to the high surface area-to-volume ratio of the inorganic QDs, enabling the co-delivery system to load anticancer drugs. Ag2S QDs not only act as photosensitizers (PSs) under the excitation of 808 nm near-infrared (NIR) light, but also as nanocarriers that increase the efficiency of delivering anticancer drugs to the tumor site.15,25 A combination of phototherapy and CHT can reduce the likelihood of tumor recurrence after PDT, and the co-delivery system with satisfactory ROS generation ability and stimuli-response of payload release can be used to achieve intelligent control of drug release for CHT, offering a synergistic effect for chemo-photodynamic therapy.
Experimental
Materials
Silver nitrate (AgNO3) and ammonium sulfide ((NH4)2S) were purchased from Sigma-Aldrich. Doxorubicin (DOX) was purchased from Adamas Beta. N-Hydroxysuccinimide (NHS), α-lipoic acid (LA), 11-aminoundecanoic acid (11-AUA), and thiazolyl blue tetrazolium bromide (MTT) were obtained from TCI. Hoechst 33342, LysoTracker™ Deep Red and Dead Cell Apoptosis Kit with Annexin V FITC and propidium iodide (PI) for flow cytometry were purchased from Invitrogen™ and ThermoFisher Scientific. Cationic pillar[6]arene (CP6) was synthesized according to a previously reported protocol.24Synthesis of Ag2S QDs
Ag2S QDs were synthetized by two steps. First, Ag2S nanoparticles were prepared using a modified method.26,27 Briefly, 1.0 mL AgNO3 aqueous solution (20 mM) and 0.2 mL LA aqueous solution (20 mM) were mixed with pure water (8.3 mL) and then stirred for 10 min. Next, 0.5 mL (NH4)2S aqueous solution (20 mM) was added to the above mixture, which was stirred for 30 min, and then stored in the dark overnight at room temperature. The obtained nanoparticles were purified by ultrafiltration using Ultra-15 centrifugal filter units (Millipore, MWCO 3.0 kDa) and redispersed in 10.0 mL of Milli-Q water. Second, for 11-AUA modification on the Ag2S nanoparticles, NHS (0.25 mL, 20 mM) was added to the above Ag2S nanoparticles (10.0 mL), and then stirred for 8 h. Then, 11-AUA (1.0 mL, 5 mM) was added and reacted for another 16 h. The obtained Ag2S QDs were purified by ultrafiltration, redispersed in 5.0 mL of Milli-Q water, and stored at 4 °C in the dark.The preparation of the co-delivery system
For the self-assembled co-delivery system (Ag2S-DOX-CP6), 0.5 mL of Ag2S QDs was mixed with 1 mL of phosphate-buffered saline (PBS, pH 7.4). Next, CP6 (0.2 mL, 0.1 mM) and DOX (0.3 mL, 1 mM) were added to the mixture, which was stirred for 48 h in the dark. The obtained Ag2S-DOX-CP6 was collected by centrifugation (8000 rpm, 5 min) and redispersed in 1.0 mL H2O (the concentration was calculated as 1.0 mg mL−1).Additionally, Ag2S-DOX-CP6-783 nanoparticles were synthesized using a method similar to the DOX loading approach to study their performance in vivo. Briefly, 0.5 mL of Ag2S QDs was mixed with 1.0 mL of PBS (pH 7.4), CP6 (0.2 mL, 0.1 mM), DOX (0.3 mL, 1 mM), and IR-783 (2 μL, 20 mM) for 48 h reaction. The obtained Ag2S-DOX-CP6-783 was collected after centrifugation and redispersed in 1.0 mL H2O. The stability of Ag2S-DOX-CP6 was also estimated by dynamic light scattering (DLS) for different samples in PBS, Dulbecco's modified Eagle's medium (DMEM), and fetal bovine serum (FBS).
The details of characterization, drug release, ROS detection, biocompatibility, and chemo-photodynamic therapy methods in vitro and in vivo are provided in the ESI.†
Results and discussion
Design and synthesis of the co-delivery system
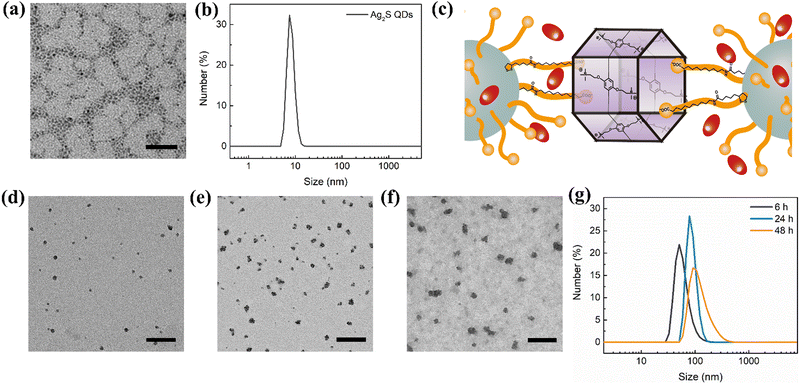
Zero-dimensional semiconductor nanomaterials, such as Ag2S QDs, have been shown to effectively generate ROS under NIR light energy and can be combined with anti-cancer drugs for co-delivery to tumor tissues.11–13 Thus, we selected Ag2S QDs as a component in our combinatorial therapeutic agent designed for chemo-photodynamic treatment of cancer. We developed a self-assembled co-delivery system (Ag2S-DOX-CP6), as illustrated in Scheme 1. First, we prepared Ag2S QDs by reducing AgNO3 with LA and (NH4)2S, followed by extending the alkyl chain of the surface ligand through the reaction between the –COOH and –NH2 groups of 11-AUA, to enable effective hydrophobic interaction with DOX molecules.28 The Ag2S QDs modified with a carboxyl (–COOH) group were adequately dispersed in an aqueous solution due to charge repulsion between the QDs, and transmission electron microscopy (TEM) analysis showed an average diameter of approximately 4 nm (Fig. 1a). The DLS results confirmed this size with a hydrodynamic diameter of approximately 8 nm (Fig. 1b).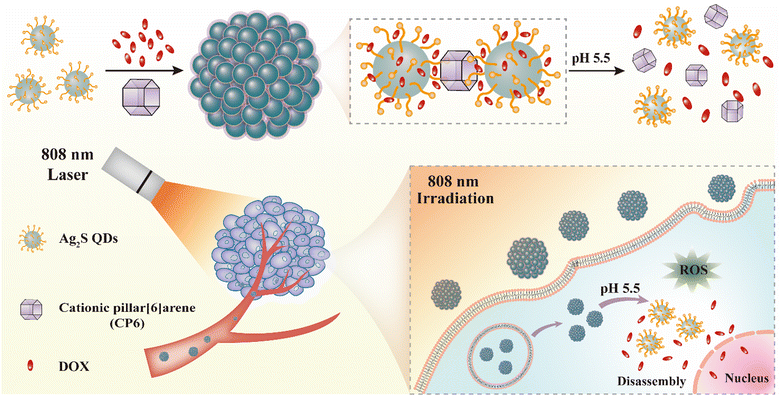 | ||
| Scheme 1 A representation of the self-assembled co-delivery system (Ag2S-DOX-CP6) based on host–guest interaction for chemo-photodynamic synergistic therapy of cancer. | ||
The negatively charged Ag2S QDs underwent host–guest interaction with CP6, which drove the self-assembly of Ag2S QDs into nanostructures. During the self-assembly process, DOX was loaded into the nanostructures through hydrophobic interaction between DOX and the alkyl chain on the surface of the Ag2S QDs (Fig. 1c). The key difference between our self-assembled approach and previous approaches is that the host–guest and hydrophobic interactions drive the self-assembly process based on small organic molecules.10 To evaluate the drug-loading and -release capacity of this self-assembled nanocarrier as a controlled-release drug delivery system, we used DOX as the anti-cancer drug to co-self-assemble with the Ag2S QDs and CP6 in PBS (pH 7.4). The encapsulation efficiency and loading efficiency of DOX were 50.7% and 10.2%, respectively. This self-assembled co-delivery system exhibited a high loading capacity for DOX, which was attributed to the hydrophobic interaction with DOX during self-assembly.
Unravelling the mechanism behind drug loading
To increase our understanding of the mechanism governing the drug loading directed by the co-assembled system, we investigated the morphological changes in the Ag2S-DOX-CP6 system using TEM and DLS. The co-delivery system was monitored during the drug-loading process using TEM and DLS. Fig. 1d–f shows TEM images of the co-delivery system for different reaction times of self-assembly, and the size of the nanocarriers increased from approximately 10 nm to 80 nm with increasing reaction time. The size distribution of hydrodynamic diameters from DLS (Fig. 1g) was consistent with the TEM results. This suggests that the self-assembled nanostructures of Ag2S-DOX-CP6 are highly size-dependent.The TEM analysis showed that the resulting Ag2S-DOX-CP6 nanocarriers were formed by the aggregation of several Ag2S QDs, driven by the noncovalent interaction between the CP6 macrocycle and ligands on the surface of Ag2S QDs. The noncovalent interaction responsible for the formation of Ag2S-DOX-CP6 was elucidated using nuclear magnetic resonance (NMR). Because the NMR signal of ligands on the QDs was poor, a model compound of 11-AUA was used to analyze the noncovalent interaction between the macrocycle and Ag2S QDs. The host–guest interaction between ligands on Ag2S QDs and CP6 (CP6⊃11-AUA) was studied by NMR spectra, and the signals of the methylene protons (H11) exhibited remarkable downfield shifts, indicating the noncovalent interaction between CP6 and 11-AUA (Fig. S1, ESI†). The noncovalent interaction was also evidenced from the significant decrease of the UV-Vis absorbance peak at 289 nm of a phosphate buffer solution of CP6 (0.1 mM) when various concentrations of 11-AUA were introduced (Fig. S2, ESI†). Finally, two-dimensional nuclear Overhauser effect (2D NOESY) experiments were conducted to examine the spatial conformation of the resulting inclusion complex (Fig. S3, ESI†). Nuclear Overhauser effect (NOE) correlation signals were observed between protons Ha and Hb on the macrocycles and the methylene protons H9, H10, or H11 of 11-AUA, confirming the aforementioned interaction. These results revealed that the host–guest interaction induced the self-assembly of Ag2S QDs.
DOX is typically buried in the hydrophobic pocket of liposomes, and we concluded that it was encapsulated by the alkyl chain of the ligands on the surface of the QDs during the noncovalent interaction between the macrocycle and the Ag2S QDs.29 The hydrodynamic diameter of the Ag2S-CP6 nanoparticles was approximately 100 nm and increased to approximately 110 nm of the Ag2S-DOX-CP6 nanoparticles from approximately 4 nm Ag2S QDs through host–guest interaction after 48 h of reaction (in Fig. S4a–e, ESI†). As shown in Fig. S4d (ESI†), the high-resolution transmission electron microscopy (HRTEM) imaging of the Ag2S-DOX-CP6 co-delivery system reveals that the resulting Ag2S-DOX-CP6 nanocarriers were formed through the aggregation of several Ag2S QDs. This aggregation was driven by the noncovalent interaction between the CP6 macrocycle and ligands on the surface of the Ag2S QDs. The encapsulation of DOX indicates that DOX can affect the self-assembly process and was helpful for the formation of a well-dispersed delivery system. These findings suggest that this self-assembly strategy based on small molecule organics provides kinetic control over the complex co-self-assembly process, representing an approach to preparing nanocarriers that contain QDs and drugs for cancer treatment.24
Drug release and ROS generation by the co-delivery system
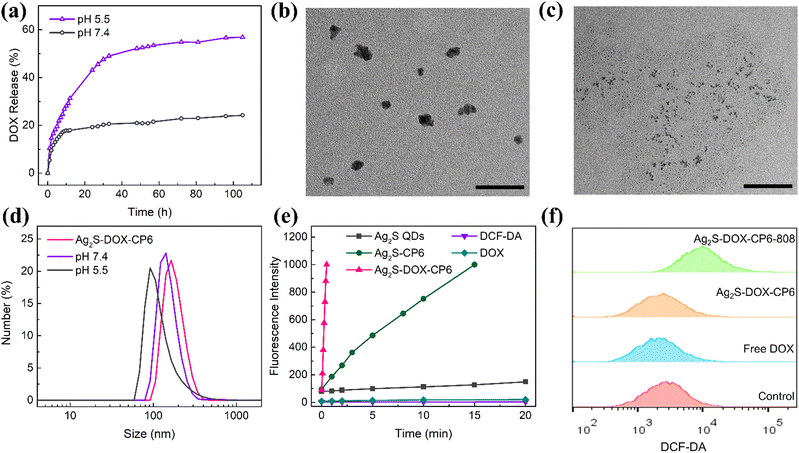
It was determined that the nanoparticles’ hydrodynamic diameter was approximately 110 nm (Fig. S4e, ESI†), which meets the criteria for the enhanced permeability and retention (EPR) effect in tumor cells according to a previous report.30 The stability of the co-delivery system in a physiological environment was tested using DLS (Fig. S4f, ESI†). The size distribution of Ag2S-DOX-CP6 increased when dispersed in FBS compared to that in PBS and DMEM due to the formation of a protein corona on the surface of the nanostructures.31 Nonetheless, the hydrodynamic diameter of Ag2S-DOX-CP6 remained in the range of 200–250 nm, which would be considered suitable for delivery to tumor tissue via the EPR effect. The zeta potentials of Ag2S QDs, Ag2S-CP6, and Ag2S-DOX-CP6 were −31.67 mV, −21.50 mV, and 5.16 mV, respectively (Fig. S4g, ESI†). The zeta potential of Ag2S QDs increased after interaction with CP6, which was affected by quaternary ammonium groups, and became slightly positive after drug loading due to changes in the self-assembly process for Ag2S QDs and CP6. This slightly positive electric charge is beneficial for nanoparticle uptake by cells.32To further investigate their therapeutic applications, we characterized the nanoparticles’ physicochemical properties (Fig. S5a and b, ESI†). The UV-Vis spectra of Ag2S QDs, Ag2S-CP6, DOX, and Ag2S-DOX-CP6 were analyzed, and it showed an obvious absorption peak at 808 nm after self-assembly for Ag2S-DOX-CP6. After loading DOX, the characteristic peak of DOX at 480 nm appeared, indicating successful drug loading in self-assembled nanostructures. Additionally, the fluorescence spectra of Ag2S QDs, Ag2S-CP6, DOX, and Ag2S-DOX-CP6 were tested, showing that Ag2S QDs emit no fluorescence under excitation by 480 nm light, which suggests that their photocatalytic performance may be excellent.
The release performance of Ag2S-DOX-CP6 was evaluated in PBS solutions at pH values of 7.4 and 5.5 to assess its drug release characteristics. As shown in Fig. 2a, the accumulated release of DOX was 19.26% and 20.9% in PBS with a pH of 7.4 after 24 and 48 h, respectively, while at pH 5.5, the accumulative release was 43.1% and 52.1% after the same time intervals. These results indicate that an acidic microenvironment can be used to control drug release from the co-delivery system, and pH-controlled drug release suggests its potential for cancer therapy, given the acidic environment of tumor cells.33 The size distribution of the self-assembled nanocarriers after drug release was also investigated using TEM and DLS, as shown in Fig. 2b–d. It was observed that the nanoparticle size sharply decreased after drug release at pH 5.5, suggesting that the co-delivery system disassembled into smaller sizes. This feature can enable intelligent control of chemotherapy to achieve more efficient drug delivery to tumor sites.21
Moreover, the ability of Ag2S-DOX-CP6 to generate ROS under 808 nm NIR laser irradiation was evaluated using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) as a fluorescence probe. As shown in Fig. 2e, the amount of ROS generated increased with the irradiation time, indicating that all Ag2S nanoparticles participated in photocatalytic activities under NIR excitation.34 Furthermore, the ability of self-assembled hybrid nanoparticles to generate ROS, especially Ag2S-DOX-CP6 after drug loading, was significantly enhanced due to the presence of CP6 and DOX. Simultaneously, various ROS were separately detected using different probes, as shown in Fig. S6 (ESI†). It was determined that singlet oxygen (1O2) was the primary ROS produced during the NIR irradiation process. This can be attributed to the fact that CP6 and DOX altered electronic transmission and separation during irradiation, leading to the trapping of unpaired electrons and increased ROS generation during the photocatalytic process.35
The enhanced photocatalytic activity of the Ag2S-DOX-CP6 nanoparticles suggested their potential as excellent photosensitizers in PDT. To further confirm this, intracellular ROS generation in MCF-7 breast cancer cells incubated with Ag2S-DOX-CP6 (50 μg mL−1) was investigated using flow cytometry and confocal laser scanning microscopy (CLSM), as shown in Fig. 2f and Fig. S7 (ESI†). The fluorescence of DCF increased in MCF-7 cells with the irradiation of an 808 nm NIR laser, indicating an increase in ROS generation. These results suggest that the co-delivery system could be used as an effective photosensitizer in PDT, enabling highly effective PDT under NIR light irradiation.
In vitro cytotoxicity and cellular uptake of co-delivery systems
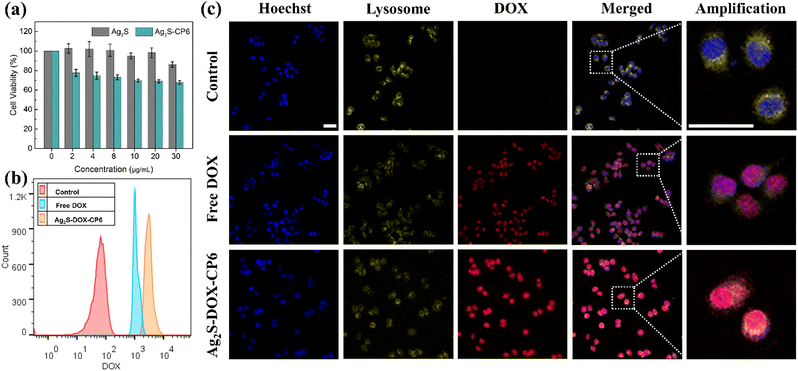
The biocompatibility of nanoplatforms is an essential consideration for any drug delivery system.25 To assess the cytotoxicity of the co-delivery nanocarriers, we performed the thiazolyl blue tetrazolium bromide (MTT) assay.33Fig. 3a shows the viabilities of MCF-7 cells after 24 h incubation with Ag2S QDs and Ag2S-CP6 nanoparticles at concentrations ranging from 0.5 to 30 μg mL−1. The viabilities of MCF-7 cells remained above 80% for Ag2S-CP6 nanoparticles, indicating low cytotoxicity. Based on the MTT results, the co-delivery system exhibited excellent biocompatibility for delivering drugs to tumor cells.DOX is known to inhibit nucleic acid synthesis, and thus it is an effective antitumor agent.29 Even after release into the cell nucleus, DOX continues to exert antitumor activity.36,37 In the current study, flow cytometry and CLSM were utilized to observe the cellular uptake capability of Ag2S-DOX-CP6 nanoparticles and their localization in MCF-7 cells. The uptake of Ag2S-DOX-CP6 nanoparticles in MCF-7 cells was evaluated using flow cytometry, as shown in Fig. 3b. The DOX fluorescence intensity in the Ag2S-DOX-CP6 group was found to be stronger than that of the free DOX group, indicating that the nanoparticles were more easily taken up and accumulated in the co-delivery system as compared to free DOX in MCF-7 cells. Additionally, CLSM was used to observe the process of cellular uptake of and drug release by Ag2S-DOX-CP6 nanoparticles in MCF-7 cells (DOX can easily enter the nuclei of MCF-7 cells) in Fig. S8 and S9 (ESI†). Additionally, the cellular uptake of Ag2S-DOX-CP6 increased with increasing incubation time for the samples exposed to Ag2S-DOX-CP6 nanoparticles. Consistent with the flow cytometry results, there was greater accumulation of Ag2S-DOX-CP6 nanoparticles in MCF-7 cells as compared to free DOX, as shown in Fig. 3c. These results demonstrated the superb potential of the self-assembled Ag2S-DOX-CP6 nanoparticles to become a co-delivery system candidate.
In vitro chemo-photodynamic therapy
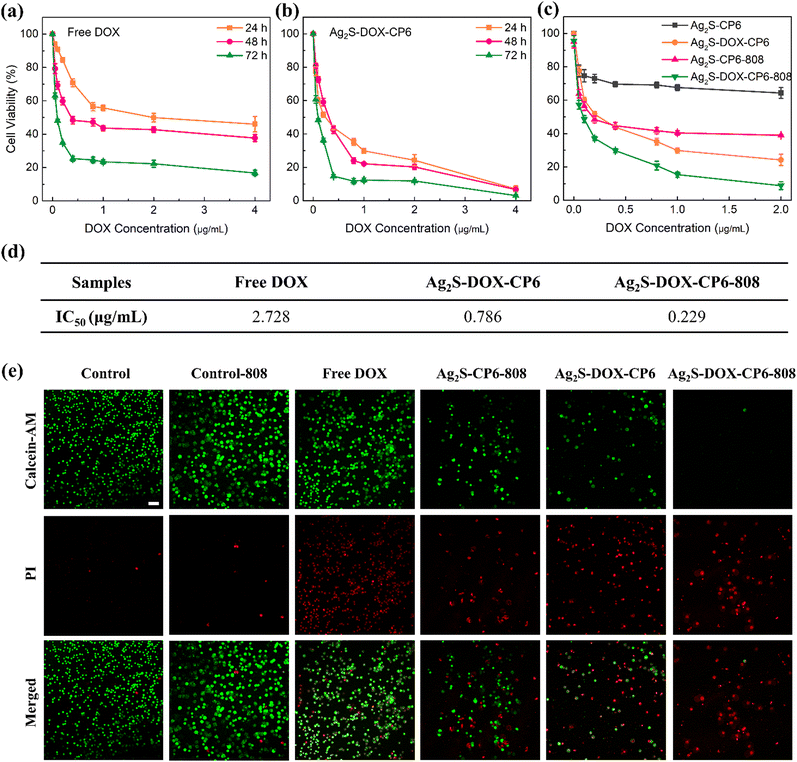
The Ag2S-DOX-CP6 nanoparticles were evaluated for their chemo-photodynamic synergistic therapy performance in vitro using the MTT assay.38 The CHT performance was evaluated first, and the viabilities of MCF-7 cells incubated with free DOX and Ag2S-DOX-CP6 nanoparticles were tested for 24, 48, and 72 h. As shown in Fig. 4a and b, the viabilities of MCF-7 cells incubated with Ag2S-DOX-CP6 nanoparticles were lower than those incubated with free DOX at a similar DOX concentration. The half maximal inhibitory concentration (IC50) values for free DOX and Ag2S-DOX-CP6 nanoparticles were 2.728 μg mL−1 and 0.786 μg mL−1 in MCF-7 cells (Fig. 4d), respectively, indicating that there was a greater chemotherapy effect against cancer by the co-delivery system of Ag2S-DOX-CP6.Furthermore, the combined chemo-photodynamic therapy performances of Ag2S-DOX-CP6 were studied, and the results are shown in Fig. 4c. Under NIR laser irradiation, the viability of MCF-7 cells for the Ag2S-DOX-CP6 group decreased to 8.8%, compared to 39.0% for cells incubated with Ag2S-CP6 and without DOX under the same laser conditions. The viability was 24.2% for MCF-7 cells exposed to Ag2S-DOX-CP6 nanoparticles without irradiation. In addition, the IC50 of Ag2S-DOX-CP6 nanoparticles under NIR light was approximately 11.9 times lower than that of free DOX in MCF-7 cells (Fig. 4d). These findings indicated that there was significant anti-tumor efficacy in chemo-photodynamic treatment for breast cancer.
Dead/live cell imaging was performed for cells treated with Ag2S-DOX-CP6 under 808 nm irradiation to evaluate their anti-tumor ability, as shown in Fig. 4e. In this experiment, MCF-7 cells were incubated with different samples (DOX concentration: 5 μg mL−1) for 8 h with or without 808 nm irradiation (1.0 W cm−2, 1 min). Low DOX concentration reduced the interference of DOX fluorescence on PI fluorescence in dead/live cell imaging tests. The amount of living cells can also be used to evaluate the anti-tumor ability of Ag2S-DOX-CP6 with NIR light. The number of living cells in the Ag2S-DOX-CP6-808 group was far less than that in other groups, which is consistent with the results from the MTT assay, and further proves the excellent chemo-photodynamic therapeutic ability of Ag2S-DOX-CP6 in MCF-7 cells. In addition, cell apoptosis in MCF-7 cells induced by Ag2S-DOX-CP6 nanoparticles was investigated by flow cytometry, as shown in Fig. S10 (ESI†). The early and later apoptotic cells constituted 9.08 ± 0.99% and 5.46 ± 0.67% when the MCF-7 cells were treated with free DOX and Ag2S-DOX-CP6, whereas they increased to 14.87 ± 2.03% when the cells were incubated with Ag2S-DOX-CP6 and treated with NIR light. Thus, there was a slightly lower amount of apoptotic cells from the Ag2S-DOX-CP6 group as compared to the group that was treated with free DOX, because most cells were already dead due to the cytotoxicity of Ag2S-DOX-CP6.
Thus, Ag2S-DOX-CP6 nanoparticles, when combined with NIR irradiation, exhibited superior anticancer activity compared to free DOX alone, resulting in apoptosis-induced inhibition of tumor cell proliferation. This demonstrates the successful design and development of a combined photocatalytic and drug delivery system for chemo-photodynamic synergistic therapy in MCF-7 cells. The promising potential of this approach highlights the possibility of a more effective method for inhibiting tumor proliferation.
In vivo biocompatibility and chemo-photodynamic therapy
To assess the potential of the self-assembled co-delivery system in cancer therapy, the in vivo biocompatibility of Ag2S-DOX-CP6 was evaluated in Institute of Cancer Research (ICR) mice. Following the tail vein injection of free DOX and Ag2S-DOX-CP6, the major organs of mice were subjected to hematoxylin-eosin (H&E) staining to examine organ pathology (Fig. S11, ESI†). In the group treated with free DOX, minor leukocyte infiltration was observed in the alveolar regions of the heart and lung. By contrast, no significant pathological lesions were found in the heart, liver, spleen, lung, or kidney of mice treated with Ag2S-DOX-CP6, indicating that this co-delivery system can mitigate the toxicity of DOX.39 Therefore, this stimuli-responsive co-delivery system showed excellent biocompatibility, suggesting its potential for cancer treatment.To monitor the accumulation of the co-delivery system, a NIR fluorescent dye IR-783 was loaded into the system. In vivo biodistribution and in vitro fluorescence images of tumors and major organs from MCF-7 tumor-bearing mice following injection of free IR-783 and Ag2S-DOX-CP6 labeled with IR-783 (Ag2S-DOX-CP6-783) are presented in Fig. S12 (ESI†). The fluorescence intensity of the tumor in the Ag2S-DOX-CP6-783 group increased over time, peaking at 24 h and remaining high for several days, as shown in Fig. S12a and b (ESI†). This indicated that Ag2S-DOX-CP6 exhibited exceptional tumor accumulation ability. As displayed in Fig. S12c and d (ESI†), in vitro fluorescence images of tumors and major organs at 24 h post-injection revealed that the tumor accumulation of Ag2S-DOX-CP6 nanoparticles was significantly higher compared to the free IR-783 group, further confirming their ability to accumulate in tumors. These findings demonstrate that the co-delivery system possessed excellent biocompatibility and strong tumor accumulation capability, and therefore it is a promising candidate for cancer treatment.
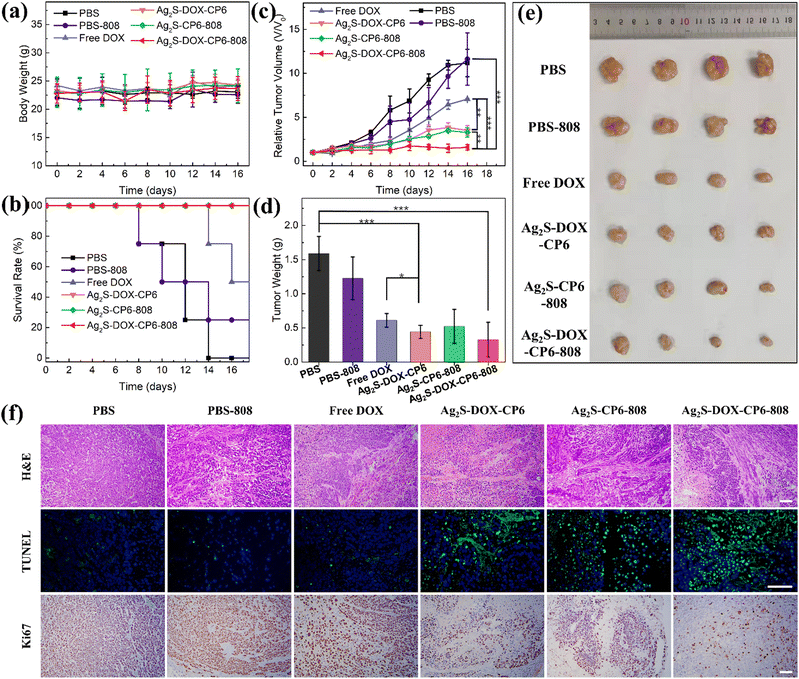
To further validate the excellent antitumor efficacy observed in vitro, the anti-tumor effect of the self-assembled co-delivery system was evaluated using a xenograft model of MCF-7 cells in BALB/c-nu mice (n = 4). The mice were treated with free DOX, Ag2S-DOX-CP6, or PBS three times on days 0, 4, and 8, and were then subjected to irradiation with an 808 nm laser on the day following tail vein injection. The mice were monitored every 2 days for changes in body weight and tumor volume, as shown in Fig. 5a–c. There was no significant evidence of weight loss or death of mice in the Ag2S-DOX-CP6 or Ag2S-DOX-CP6-808 groups during the treatment (Fig. 5b), indicating the excellent biocompatibility and safety of the nanoplatform during the treatment period.40 Moreover, it was observed that the relative tumor volumes in the mice treated with PBS sharply and uncontrollably increased, as shown in Fig. 5c. In contrast, those tumor-bearing mice treated with Ag2S-DOX-CP6 exhibited slower tumor growth as compared to the free DOX group, indicating the remarkable chemotherapeutic effect of this self-assembled co-delivery system. Under irradiation with the 808 nm laser, there was significantly enhanced anti-cancer efficacy for the group treated with Ag2S-DOX-CP6-808 as compared to the free DOX group and Ag2S-DOX-CP6 group. These results were further supported by tumor weights and images, as shown in Fig. 5d and e. Notably, the mice receiving Ag2S-DOX-CP6-808 treatment exhibited the strongest tumor inhibition capacity, confirming the synergism of chemo-photodynamic therapy.
Furthermore, to investigate the anti-tumor effects of Ag2S-DOX-CP6, we performed H&E staining, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining, and Ki-67 protein staining of tumor slices, as shown in Fig. 5f. The results showed that treatment with Ag2S-DOX-CP6 and 808 nm laser induced additional apoptosis and necrosis compared to other treatments, indicating the effectiveness of CHT and PDT in inhibiting tumor growth.41 These findings confirmed that the co-delivery system under NIR irradiation exhibited the strongest tumor inhibition ability, demonstrating the synergistic therapy of CHT and PDT for cancer.
To evaluate the potential systemic toxicity of Ag2S-DOX-CP6 in BALB/c-nu mice, we analyzed standard hematology markers, as shown in Fig. S13 (ESI†).42 The results revealed no significant differences in routine blood test indexes between the group treated with Ag2S-DOX-CP6 and 808 nm laser and the other treatment groups. Together, these results confirmed the excellent biocompatibility of the co-delivery system, and reinforced its potential as a drug delivery system for the chemo-photodynamic treatment of cancer.
Conclusions
In summary, we developed a self-assembled co-delivery system (Ag2S-DOX-CP6) to increase the efficiency of PDT and stimuli-responsive payload release for chemo-photodynamic therapy of cancer. By utilizing host-guest interactions, we were able to engineer the self-assembly of the PSs of Ag2S QDs and CP6 into ordered nanostructures. During this process, we took advantage of the high surface area-to-volume ratio of Ag2S QDs to produce ample hydrophobic spaces to accommodate DOX molecules with a drug-loading efficiency of 10.2%. In a PBS solution at pH 5.5, the amount of DOX released reached 43.1% and 52.1% after 24 h and 48 h, respectively, indicating the system's ability to intelligently release drugs in the acidic microenvironment of tumor cells. Furthermore, Ag2S QDs exhibited high catalytic activity under NIR light and demonstrated outstanding PDT ability based on the results of extracellular and intracellular ROS generation.Ag2S QDs were used as PSs to generate ROS under 808 nm NIR light, and they were also applied as nanocarriers to increase the efficiency of delivering hydrophobic anticancer drugs to the tumor. The uptake assessment of CLSM images and flow cytometry indicated that there was greater accumulation of Ag2S-DOX-CP6 nanoparticles in MCF-7 cells, where they were delivered and released DOX into the nucleus to achieve effective CHT. Moreover, when combined with NIR light, the Ag2S-DOX-CP6 treatment reduced the viability of MCF-7 cells to 8.8% (compared to the free DOX group of 49.91% with DOX concentration of 2 μg mL−1).
The anti-tumor effect and biocompatibility of Ag2S-DOX-CP6 were confirmed in vivo. In addition, H&E staining and hematology analysis of the major organs of mice revealed low side effects and satisfactory biocompatibility of the co-delivery system. These results suggest that there is great potential for the co-delivery system as a drug delivery system for chemo-photodynamic cancer treatment, and it could serve as a nanoplatform that co-delivers PSs and drugs to tumor sites while offering a synergistic effect of chemo-photodynamic therapy.
Author contributions
Concept, design, and research direction: J. L. and A. W. Material and nanostructure preparation: X. W. Experiments and characterization: X. W., J. Y., J. X., Y. L., R. Z., X. W., J. Y., D. Z., D. Q., and G. S. Manuscript writing: X. W. and J. L.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 22161016, 82202274, 82072032, 32025021, 52002380, 31971292), Ningbo 3315 Innovative Teams Program (Grant No. 2019A-14-C), the Sigrid Jusélius Foundation (senior researcher fellowship) and the Academy of Finland (318524, 355798), Fellowship of China Postdoctoral Science Foundation (2021M692872, 2022M723206), the member of Youth Innovation Promotion Association Foundation of CAS, China (2023310), and the Provincial Natural Science Foundation of Zhejiang (LQ23H180003). Markus Peurla (Laboratory Engineer, Institute of Biomedicine) and Cell Imaging and Cytometry Core (Turku Bioscience Centre, Turku, Finland) are acknowledged for their assistance with the TEM characterization and imaging/flow cytometry analysis.References
- R. Zheng, S. Zhang, H. Zeng, S. Wang, K. Sun, R. Chen, L. Li, W. Wei and J. He, J. Natl. Cancer Cent., 2022, 2, 1–9 CrossRef.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- S. Zhang, J. Wang, Z. Kong, X. Sun, Z. He, B. Sun, C. Luo and J. Sun, Biomaterials, 2022, 282, 121433 CrossRef CAS PubMed.
- N. Kwon, H. Kim, X. Li and J. Yoon, Chem. Sci., 2021, 12, 7248–7268 RSC.
- R. Keerthiga, Z. Zhao, D. Pei and A. Fu, ACS Biomater. Sci. Eng., 2020, 6, 5474–5485 CrossRef CAS PubMed.
- Z. Wang, N. Little, J. Chen, K. T. Lambesis, K. T. Le, W. Han, A. J. Scott and J. Lu, Nat. Nanotechnol., 2021, 16, 1130–1140 CrossRef CAS PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- X.-Y. Hu, J. Gao, F.-Y. Chen and D.-S. Guo, J. Controlled Release, 2020, 324, 124–133 CrossRef CAS PubMed.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Chem. Soc. Rev., 2021, 50, 4185–4219 RSC.
- Q. Qi, X. Zeng, L. Peng, H. Zhang, M. Zhou, J. Fu and J. Yuan, J. Biomater. Sci., Polym. Ed., 2020, 31, 1385–1404 CrossRef CAS PubMed.
- U. Badilli, F. Mollarasouli, N. K. Bakirhan, Y. Ozkan and S. A. Ozkan, TrAC, Trends Anal. Chem., 2020, 131, 116013 CrossRef.
- Y. W. Jiang and B. Z. Tian, Nat. Rev. Mater., 2018, 3, 473–490 CrossRef PubMed.
- H. Zhang, T. Wang, H. Liu, F. Ren, W. Qiu, Q. Sun, F. Yan, H. Zheng, Z. Li and M. Gao, Nanoscale, 2019, 11, 7600–7608 RSC.
- J. Wankar, N. G. Kotla, S. Gera, S. Rasala, A. Pandit and Y. A. Rochev, Adv. Funct. Mater., 2020, 30, 1909049 CrossRef CAS.
- K. Cheng, X.-Q. Yang, X.-S. Zhang, J. Chen, J. An, Y.-Y. Song, C. Li, Y. Xuan, R.-Y. Zhang, C.-H. Yang, X.-L. Song, Y.-D. Zhao and B. Liu, Adv. Mater., 2018, 28, 1803118 Search PubMed.
- D. Ling, W. Park, S.-J. Park, Y. Lu, K. S. Kim, M. J. Hackett, B. H. Kim, H. Yim, Y. S. Jeon, K. Na and T. Hyeon, J. Am. Chem. Soc., 2014, 136, 5647–5655 CrossRef CAS PubMed.
- D. Wu, B. L. Li, Q. Zhao, Q. Liu, D. Wang, B. He, Z. Wei, D. T. Leong, G. Wang and H. Qian, Small, 2020, 16, 1906975 CrossRef CAS PubMed.
- J. V. Rival, N. Nonappa and E. S. Shibu, ACS Appl. Mater. Interfaces, 2020, 12, 14569–14577 CrossRef CAS PubMed.
- C. Wang, H. Li, J. Dong, Y. Chen, X. Luan, X. Li and X. Du, Chem. – Eur. J., 2022, 28, e202202050 CAS.
- Q. Yang, W. Xu, M. Cheng, S. Zhang, E. G. Kovaleva, F. Liang, D. Tian, J.-A. Liu, R. M. Abdelhameed, J. Cheng and H. Li, Chem. Commun., 2022, 58, 3255–3269 RSC.
- C. B. Yang, Z. I. Lin, J. A. Chen, Z. R. Xu, J. Y. Gu, W. C. Law, J. H. C. Yang and C. K. Chen, Macromol. Biosci., 2021, 22, 2100349 CrossRef PubMed.
- A. S. Braegelman and M. J. Webber, Theranostics, 2019, 9, 3017–3040 CrossRef CAS PubMed.
- X. Liu, K. Jia, Y. Wang, W. Shao, C. Yao, L. Peng, D. Zhang, X.-Y. Hu and L. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 4843–4850 CrossRef CAS PubMed.
- G. Yu, J. Zhou, J. Shen, G. Tang and F. Huang, Chem. Sci., 2016, 7, 4073–4078 RSC.
- S. Chao, Z. Shen, Y. Pei, Y. Lv, X. Chen, J. Ren, K. Yang and Z. Pei, Chem. Commun., 2021, 57, 7625–7628 RSC.
- W. Jiang, Z. Wu, X. Yue, S. Yuan, H. Lu and B. Liang, RSC Adv., 2015, 5, 24064–24071 RSC.
- H. Jia, W. He, W. G. Wamer, X. Han, B. Zhang, S. Zhang, Z. Zheng, Y. Xiang and J.-J. Yin, J. Phys. Chem. C, 2014, 118, 21447–21456 CrossRef CAS.
- X. Wu, J. Liu, L. Yang and F. Wang, Colloids Surf., B, 2019, 175, 239–247 CrossRef CAS PubMed.
- S. Li, Y. Gao, Y. Ding, A. Xu and H. Tan, Chin. Chem. Lett., 2021, 32, 313–318 CrossRef CAS.
- Y. Zhang, M. Y. Li, X. M. Gao, Y. H. Chen and T. Liu, J. Hematol. Oncol., 2019, 12, 137 CrossRef PubMed.
- K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J. R. Lakowicz and C. D. Geddes, Curr. Opin. Biotech., 2005, 16, 55–62 CrossRef CAS PubMed.
- X. Wu, L. Luo, S. Yang, X. Ma, Y. Li, C. Dong, Y. Tian, L. E. Zhang, Z. Shen and A. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 9965–9971 CrossRef CAS PubMed.
- Y. Pan, L. e Zhang, L. Zeng, W. Ren, X. Xiao, J. Zhang, L. Zhang, A. Li, G. Lu and A. Wu, Nanoscale, 2016, 8, 878–888 RSC.
- D.-W. Zheng, B. Li, C.-X. Li, J.-X. Fan, Q. Lei, C. Li, Z. Xu and X.-Z. Zhang, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS PubMed.
- X. Wu, L. Yang, L. Luo, G. Shi, X. Wei and F. Wang, ACS Appl. Bio Mater., 2019, 2, 1998–2005 CrossRef CAS PubMed.
- R. K. Kankala, C. G. Liu, A. Z. Chen, S. B. Wang, P. Y. Xu, L. K. Mende, C. L. Liu, C. H. Lee and Y. F. Hu, ACS Biomater. Sci. Eng., 2017, 3, 2431–2442 CrossRef CAS PubMed.
- G. Lin, P. Mi, C. C. Chu, J. Zhang and G. Liu, Adv. Sci., 2016, 3, 1600134 CrossRef PubMed.
- J. Han, H. Xia, Y. Wu, S. N. Kong, A. Deivasigamani, R. Xu, K. M. Hui and Y. Kang, Nanoscale, 2016, 8, 7861–7865 RSC.
- Y. Cao, J. Yang, D. Eichin, F. Zhao, D. Qi, L. Kahari, C. Jia, M. Peurla, J. M. Rosenholm, Z. Zhao, S. Jalkanen and J. Li, Angew. Chem., Int. Ed., 2021, 60, 3062–3070 CrossRef CAS PubMed.
- Z. Shen, T. Chen, X. Ma, W. Ren, Z. Zhou, G. Zhu, A. Zhang, Y. Liu, J. Song, Z. Li, H. Ruan, W. Fan, L. Lin, J. Munasinghe, X. Chen and A. Wu, ACS Nano, 2017, 11, 10992–11004 CrossRef CAS PubMed.
- H. Li, K. Qian, H. Zhang, L. Li, L. Yan, S. Geng, H. Zhao, H. Zhang, B. Xiong, Z. Li, C. Zheng, Y. Zhao and X. Yang, Chem. Eng. J., 2021, 418, 129534 CrossRef CAS.
- X.-D. Zhang, D. Wu, X. Shen, P.-X. Liu, F.-Y. Fan and S.-J. Fan, Biomaterials, 2012, 33, 4628–4638 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb00592e |
| This journal is © The Royal Society of Chemistry 2023 |